Abstract
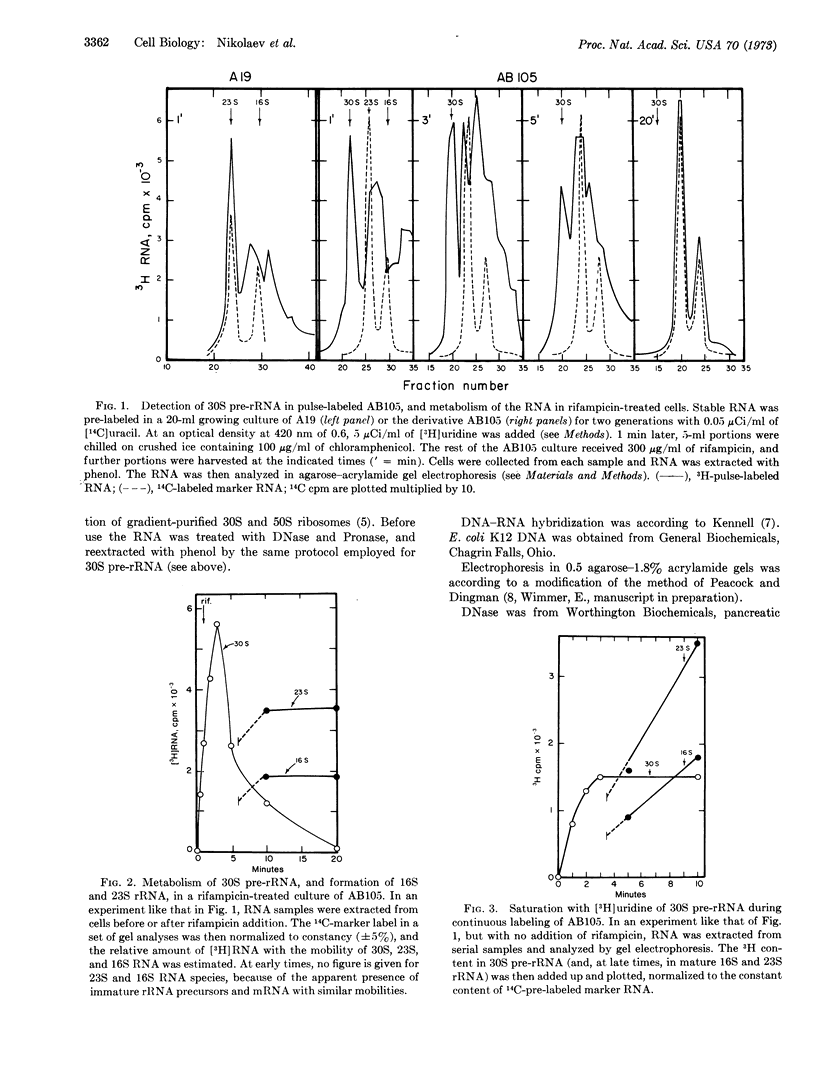
A mutant of E. coli, isolated by Kindler and Hofschneider as a strain defective in RNase III activity, forms a 30S precursor of ribosomal RNA (“30S pre-rRNA”). The half-life of the 30S pre-rRNA in growing cells at 30°, estimated by the rate of specific 3[H]uridine incorporation, is about 1 min. In rifampicin-treated cells, the RNA is metabolized to mature rRNA with a half-life of about 2 min.
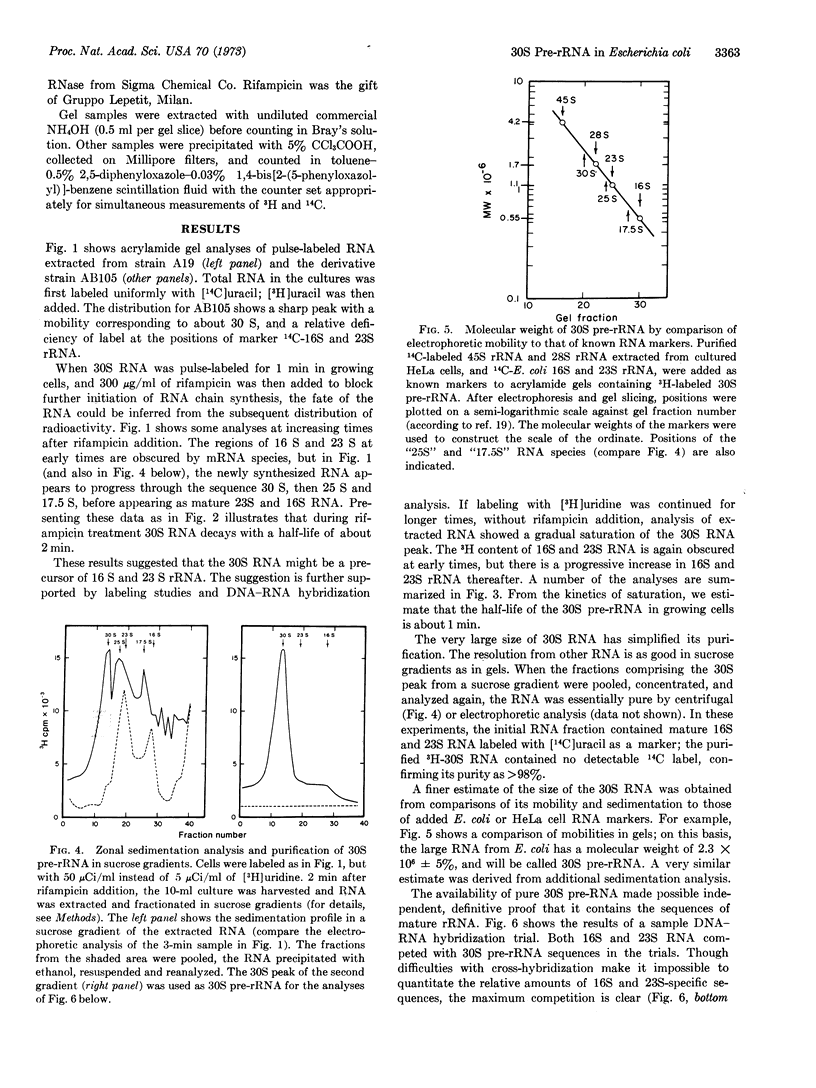
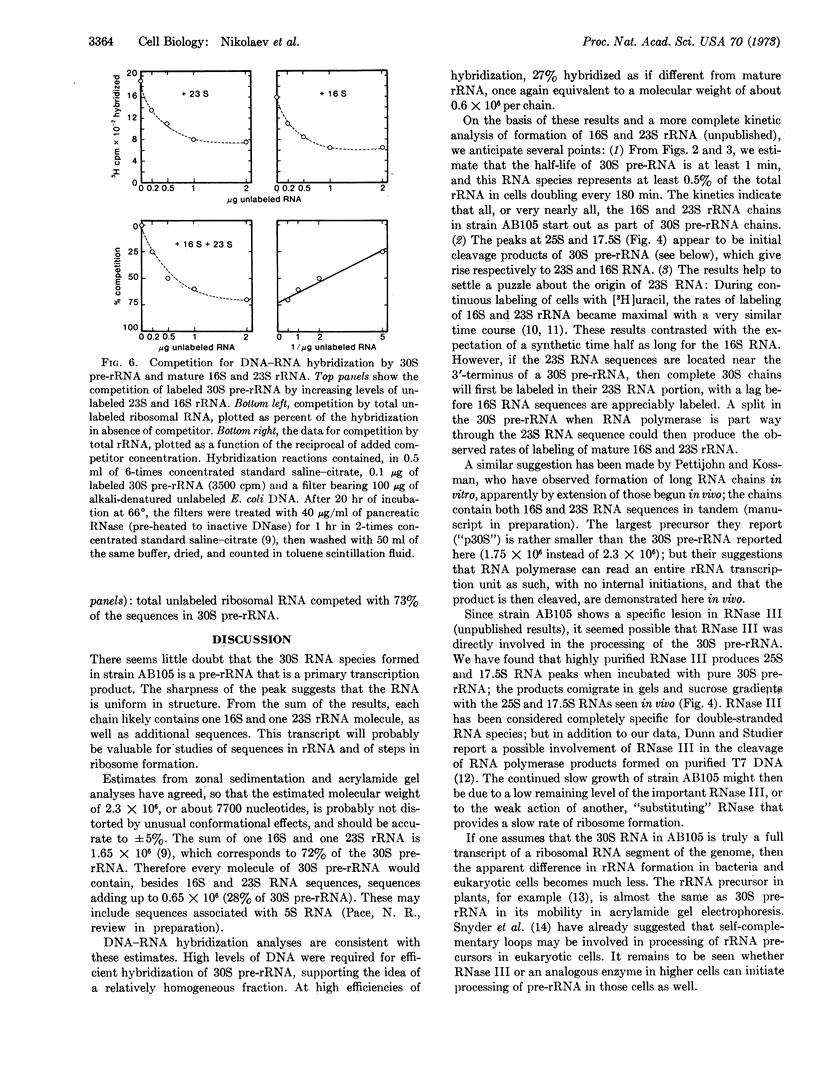
The 30S pre-rRNA has been highly purified. DNA-RNA hybridization tests demonstrate that it contains both 16S and 23S rRNA sequences. Also, in cultures treated with rifampicin, the cleavage products of radioactive 30S pre-rRNA include 25S and 17.5S RNA species, destined to becomes 23S and 16S rRNA. Thus, each 30S chain probably contains one 16S and one 23S RNA sequence, as well as additional sequences. Two independent techniques indicate that the additional portions account for about 27% of the total lenght: (1) By comparison to the sedimentation rate and electrophoretic mobility of marker RNAs, the 30S pre-RNA has an apparent molecular weight of 2.3 × 106 ± 5%, or 28% more than the sum of 16S and 23S rRNA; (2) 27% of the 30S pre-rRNA is not competed away from hybridization by mature 16S and 23S rRNA.
Thus, bacteria appear to make a pre-rRNA similar in some respects to that observed in eukaryotes; though in normal E. coli cells, the pre-rRNA is ordinarily cleaved endonucleolytically during its formation.
Keywords: ribonuclease III, DNA-RNA hybridization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Levinthal C. Synthesis and maturation of ribosomal RNA in Escherichia coli. J Mol Biol. 1969 Dec 14;46(2):281–303. doi: 10.1016/0022-2836(69)90422-7. [DOI] [PubMed] [Google Scholar]
- Bleyman M., Kondo M., Hecht N., Woese C. Transcriptional mapping: functional organization of the ribosomal and transfer ribonucleic acid cistrons in the Bacillus subtilis genome. J Bacteriol. 1969 Aug;99(2):535–543. doi: 10.1128/jb.99.2.535-543.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corte G., Schlessinger D., Longo D., Venkov P. Transformation of 17 s to 16 s ribosomal RNA using ribonuclease II of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):325–338. doi: 10.1016/0022-2836(71)90297-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J Mol Biol. 1966 Jul;18(2):356–371. doi: 10.1016/s0022-2836(66)80253-x. [DOI] [PubMed] [Google Scholar]
- Keil T. U., Hofschneider P. H. Secondary structure of RNA phage M12 replicative intermediates in vivo. Biochim Biophys Acta. 1973 Jun 23;312(2):297–310. doi: 10.1016/0005-2787(73)90375-4. [DOI] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Titration of the gene sites on DNA by DNA-RNA hybridization. I. Problem of measurement. J Mol Biol. 1968 May 28;34(1):71–84. doi: 10.1016/0022-2836(68)90235-0. [DOI] [PubMed] [Google Scholar]
- Kossman C. R., Stamato T. D., Pettijohn D. E. Tandem synthesis of the 16S and 23S ribosomal RNA sequences of Escherichia coli. Nat New Biol. 1971 Nov 24;234(47):102–104. doi: 10.1038/newbio234102a0. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D., Silengo L. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry. 1968 Jan;7(1):456–472. doi: 10.1021/bi00841a058. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Snyder A. L., Kann H. E., Jr, Kohn K. W. Inhibition of the processing of ribosomal precursor RNA by intercalating agents. J Mol Biol. 1971 Jun 14;58(2):555–565. doi: 10.1016/0022-2836(71)90371-8. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- Yuki A. Tentative identification of a "maturation enzyme" for precursor 16 s ribosomal RNA in Escherichia coli. J Mol Biol. 1971 Dec 14;62(2):321–329. doi: 10.1016/0022-2836(71)90430-x. [DOI] [PubMed] [Google Scholar]



