Abstract
This study presents the first reported measurements of stimulus frequency emissions (SFOAEs) in 15 human newborns and compares their magnitudes and phase-gradient delays to those reported in adults. SFOAEs in newborns were measured at stimulus levels as low as 15 dB sound pressure level (SPL). Responses were compared between adults and newborns at stimulus levels where SFOAEs in both age groups demonstrated approximately linear growth (<40 dB SPL for newborns, <25 dB SPL for adults). Neonates had adult-like SFOAE delays when compared in this fashion, which compensates for newborn middle ear inefficiencies.
1. Introduction
Stimulus-frequency otoacoustic emissions (SFOAEs) when evoked by low-level stimuli are thought to arise by a reflection mechanism that produces a backscattering of wavelets near the peak of the cochlear traveling wave. Although these reflection-source emissions may offer unique diagnostic advantages relative to other emission types (see Shera and Guinan, 1999), SFOAEs have not been recorded or defined in human newborns. Large-scale studies have shown that reflection-source emissions provide more sensitive detection of mild sensorineural hearing loss (SNHL) than distortion-product otoacoustic emissions (DPOAEs) (Gorga et al., 1993; Miller et al., 2004). Additionally, SFOAEs may eventually provide an objective measure of frequency selectivity (e.g., Bentsen et al., 2011; Keefe et al., 2008; Shera et al., 2002). Because loss of frequency tuning with SNHL has been demonstrated in adult humans (reviewed in Moore, 2007), an SFOAE-based objective measure of frequency tuning could enhance and expand the characterization of hearing deficits in infants and adults. Such advances in hearing diagnostics require a better understanding of developmental changes in OAEs.
Reflection-source OAEs—measured as either the reflection-component of the distortion-product OAE or a click-evoked (CE) OAE—are not adult-like in newborns; overall, they are larger in amplitude and show longer delays than in adult ears (e.g., Abdala and Dhar, 2010, 2012; Collet et al., 1993; Moleti et al., 2008). Theoretical and experimental evidence suggests that longer SFOAE delays correlate with sharper filters (e.g., Bentsen et al., 2011; Shera et al., 2002). One interpretation of longer OAE delays in newborns is that neonates have excessively sharp tuning at birth. However, previous work has suggested that cochlear immaturities (inferred from DPOAE measures) can mostly be accounted for by middle-ear inefficiencies in newborns, at least at high frequencies (Abdala and Keefe, 2006; Abdala et al., 2007). Whether larger reflection-emission magnitudes and longer delays in newborns can also be attributed to differences in middle-ear inefficiency deserves scrutiny using alternative methodology and a broader frequency range.
Existing comparisons of reflection-emission delays between adults and infants have relied on the reflection component of the DPOAE, which must be extracted through post hoc signal processing, or the CEOAE measured at fixed and moderate-to-high stimulus levels [∼80 dB peak sound pressure level (pSPL)]. However, forward transmission through the infant middle ear is less efficient than the adult middle ear by as much at 15 dB in some frequency regions (e.g., Keefe and Abdala 2007). Because cochlear filters are known to become broader with stimulus intensity (reviewed in Robles and Ruggero, 2001), making adult-neonate comparisons at the same fixed stimulus level produces differences in the effective stimulus levels at the cochlea and confounds the interpretation of the delays.
To make appropriate comparisons of tuning (or OAE delays) between age groups, our approach is to compare infant and adult SFOAEs at stimulus levels where the cochlea is operating linearly in both groups rather than at equal and fixed SPLs. By equating SFOAEs in this way and pushing into a low-level linear regime, we will compare the most-sharply tuned filters for each age group regardless of probe level. Additionally, this study represents the first step in characterizing basic features of SFOAEs in newborns, a necessary task to probe their potential application as a clinical screen of hearing in the future.
2. Methods
2.1. Experimental protocol
Stimuli were digitally generated and recorded using standard suppression-based discrete-tone SFOAE measurement protocols on the Mimosa Acoustics measurement system (Kalluri and Shera, 2007a).
2.2. Newborns
SFOAEs were successfully recorded in 15 of 24 human newborns tested within 48 h of term birth (i.e., 37–41 wk gestation). All newborns passed a click-evoked auditory brain stem response screening conducted at 35 dB hearing level (HL). SFOAEs were measured in an acoustic isolette providing between 25 and 40 dB of attenuation (Eckels ABC-100). Due to limited recording time with newborns, measures were attempted in 100–500 Hz segments with at least 50 Hz step resolution to allow for adequate phase unwrapping. Recording segments were: 1.3–1.5, 1.7–2, 2.4–2.7, and 3–3.5 kHz. Pure tone probes ranged from 15 to 40 dB SPL in 5–10 dB steps. A fixed-level suppressor tone was presented 50 Hz below the probe frequency at 60 dB SPL. SFOAEs for at least one probe level and frequency segment were measurable in 15 of 24 newborns tested. SFOAEs at two or more levels were measurable in 10 newborns, and complete SFOAE input/output functions were measurable in five newborns.
2.3. Adults
SFOAEs were measured at a probe tone level of 40 dB SPL between 0.5 and 4 kHz in 11 adult subjects aged 18–45 yr with normal hearing by report and audiometric evaluation. Additionally, to facilitate comparisons of SFOAE phase-gradient delays across stimulus levels, we supplemented the adult data by reanalyzing delay trends extracted from previously published SFOAE data (Bergevin et al., 2012; Kalluri and Shera, 2007b; Shera and Guinan, 2003).
To make meaningful comparison between the two age groups, we computed the ratio between the emission and stimulus pressures to create an effective transfer function TSF (Kalluri and Shera, 2007b) and compared SFOAE delays at stimulus levels where the transfer functions in both age groups were level invariant (indicative of behavior approximating that of a linear system). Once this was achieved, it was not necessary to match stimulus levels but rather to identify a level range where the SFOAEs were within their region of linear growth. SFOAE data were included if the noise floor was less than −10 dB SPL and two adjacent frequency points had ≥6 dB signal to noise ratio (SNR). Although this was the minimum allowable SNR, actual SNRs ranged from 6 to 33 dB across frequency and level in newborn subjects and averaged approximately 18 dB for the data set as a whole.
3. Results
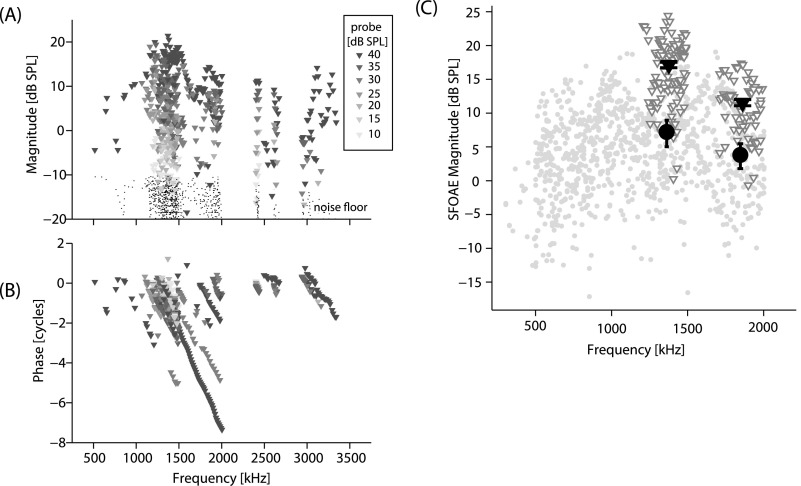
Figures 1(A) and 1(B) display SFOAE magnitudes and phase measured in 15 newborns. The SPL of the probe varied from 15 to 40 dB SPL and is indicated by a gradation of shading with lighter shading for lower stimulus levels. The phase of the SFOAE in newborns [Fig. 1(B)] varies rapidly with frequency as is characteristic of reflection-source emissions. Figure 1(C) compares the SFOAE magnitudes evoked with 40 dB SPL probe tones in adults (n = 11, gray circles) and newborns (n = 13, triangles). Large filled triangles (newborn SFOAEs) and circles (adult SFOAEs) display the mean and standard errors of the mean (SEM) averaged over two frequency segments. (Not all subjects contributed data at all frequencies.) Overall SFOAEs are larger in newborns than adults by 8–10 dB across frequency when compared at the 40 dB SPL probe levels. Larger SFOAEs in newborns are consistent with previous findings that CEOAEs and the reflection-component of DPOAEs are larger in infants than in adults (Abdala and Dhar, 2010, 2012; Norton and Widen, 1990; Prieve et al., 1997).
Fig. 1.
SFOAEs in newborns and adults. (A) and (B) SFOAE magnitudes and phase in 15 newborns evoked at probe levels from 10 to 40 dB SPL (darker symbols indicate larger stimulus levels). (C) SFOAE magnitudes evoked by 40 dB SPL probe tones in newborns (triangles) and adults (circles). Large triangles and circles show average (±SEM) SFOAE magnitudes in newborns and adults in two frequency regions (1.3–1.5 and 1.7–2.0 kHz).
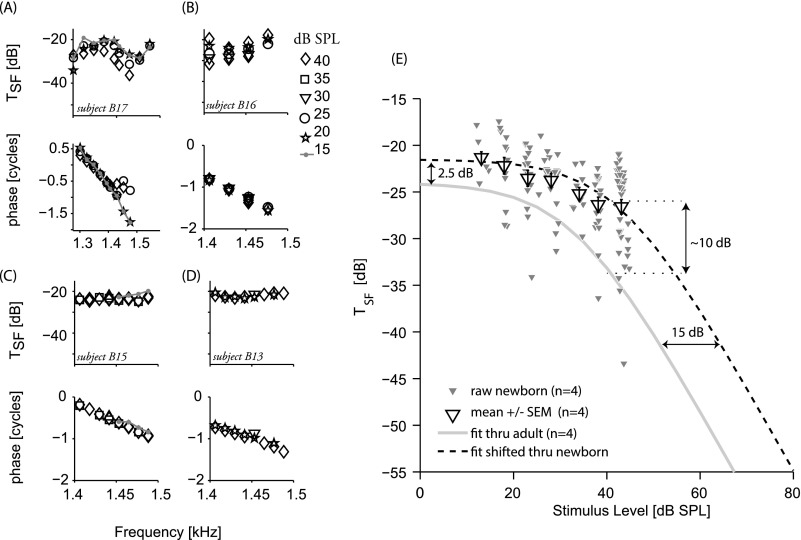
Figures 2(A)–2(D) show the frequency dependence of SFOAE magnitude and phase in four of five newborns in whom we recorded a complete level series; stimulus level is indicated by symbol. We chose to highlight the data from these four subjects because their datasets were most complete over comparable frequency ranges and were collected without interruption or probe refit. The SFOAEs in Fig. 2 are displayed as effective transfer functions by computing the ratio between the emission and stimulus pressures. In subjects B13 and B15, who have little spectral structure within the measured frequency band, the transfer functions are nearly identical at all levels [Figs. 2(C) and 2(D)]. In subjects B16 and B17, who have greater spectral structure, the overlap among transfer functions is less perfect, perhaps reflecting sensitivity to changes in stimulus level near spectral notches. Figure 2(E) shows the level dependence of the transfer function magnitudes measured over a 100 Hz bandwidth in the four newborns. In addition, Fig. 2(E) shows a fit to adult group data (gray line) derived in a previous study (see Kalluri and Shera, 2007b for details), and this same fit shifted to approximate newborn data (dashed line). The fit was shifted (1) slightly upward on the magnitude axis to accommodate the larger neonatal SFOAE amplitudes (approximately 10 dB at 40 dB SPL as estimated from Fig. 2) and (2) rightward on the stimulus level axis by 15 dB, the amount of middle-ear attenuation in forward transmission estimated by Abdala and Keefe (2006) in newborn ears. The SFOAE in newborns appears to continue growing linearly beyond levels at which the adult response has begun to compress. Whereas compression in adult SFOAE transfer functions begins at stimulus levels of approximately 20–30 dB SPL, the rightward shift of the newborn data suggests that compression begins at higher levels in this age group. Although these middle-ear age effects had been estimated for high-frequency signals only (f2 = 6 kHz), one can see that the shifted fit approximates the newborn data at these mid-frequencies as well and indicates an expanded region of linear growth (∼15 dB) in this age group. When transfer function magnitudes are compared between adults and newborns at low stimulus levels where both functions are in the linear range (flat segment of transfer function), the age differences are reduced from 8–10 dB noted at 40 dB SPL to 2–4 dB.
Fig. 2.
SFOAE level dependence. (A)–(D) SFOAE magnitudes and phase (represented as transfer functions) in four newborns. SFOAEs were measured with stimulus levels ranging from 15 to 40 dB SPL (different symbols for different levels). (E) Transfer function magnitudes in the same four newborns plotted against probe level (small triangles); across-subject averages are shown with their SEMs (large triangles). The gray line is a fit representing the level dependence of adult SFOAEs recording in a previous study (Kalluri and Shera, 2007b). The dashed line is the same fit shifted along the x axis by 15 dB SPL [to account for effects of middle-ear immaturities (Abdala and Keefe, 2006)] and along the y axis by10 dB (to account for the differences in emission amplitudes between newborns and adults at probe levels of 40 dB SPL).
Figure 3 compares SFOAE delays measured in infants to those in adults. The raw SFOAE delays [Fig. 3(A)] were computed as the negative of the phase gradient. The scatter evident in the adult and newborn delays in Fig. 3(A) is an intrinsic feature of SFOAE delays and originates from the random scattering process that underlies the generation of all reflection emissions. Whereas adult group delays are shown only for 40 dB SPL, the newborn data measured with probe levels ranging from 15 to 40 dB SPL were pooled. (The apparent linear behavior in level dependence of newborn SFOAEs below 40 dB SPL supports our decision to pool these neonatal data; delays are expected to be level independent in the linear region.)
Fig. 3.
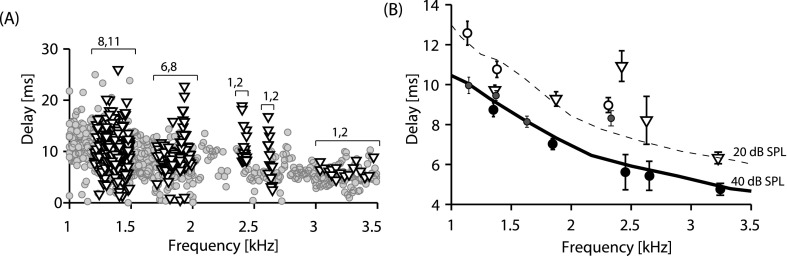
SFOAE delays compared. (A) SFOAE phase-gradient delays computed from SFOAE phase data [e.g., Fig. 1(B)]. Gray circles show delay data pooled from 11 adults measured at 40 dB SPL probe tones; triangles show delays measured in 15 newborns and pooled across probe levels (15–40 dB SPL). Brackets show frequency bands over which delays were averaged to present the consolidated plot in (B). The numbers above the bracket indicate the number of newborn and adult subjects contributing to each band. (B) SFOAE delays across age groups and stimulus levels. Large open triangles (newborn) and closed circles (adult) show mean delays (±SEM) from the newborn and adult ears tested in the present study. Data were averaged over the bandwidths shown by brackets in (A). Small open and closed circles show average delays (±SEMs) measured at both 20 and 40 dB SPL from four adult subjects (Kalluri and Shera, 2007a), illustrating the level dependence of delays in the same group of individuals. Dashed and solid lines show the delay trends at 20 and 40 dB SPL reported in two published studies for larger groups of adult subjects (trend lines extracted from Bergevin et al., 2012). Newborn delays (large open triangles) are most similar to adult delays at 20 dB SPL.
To visualize differences between the adult and newborn delays that may be obscured by the scatter in Fig. 3(A), Fig. 3(B) shows averaged delay values over the small frequency bands denoted with brackets in Fig. 3(A). Newborn delays are shown as open triangles. Adult delays are compiled from a variety of sources: (1) Data collected at 40 dB SPL in the present study, (2) delay trend lines at 20 and 40 dB SPL from two groups of subjects in two previous studies (Bergevin et al., 2012; Shera and Guinan, 1999), and (3) data from a previous study in which SFOAEs were measured at 20 and 40 dB SPL in the same four adults during the same testing session (Kalluri and Shera, 2007b). Figure 3(B) shows that SFOAE delays measured at 40 dB SPL are longer in newborns than in adults. Also, collectively, the adult delays at 20 dB SPL are longer than those at 40 dB SPL. The data from the four adult subjects collected at both stimulus levels during the same test session provide evidence that level dependence of SFOAE delays does not result from different measurement conditions across different studies. Although newborn SFOAEs have longer delays than those in adults when compared at 40 dB SPL, newborn-adult delays are similar when equated by the region of SFOAE linear growth and compared at lower stimulus levels.
4. Discussion
To our knowledge, this study provides the first published report of SFOAEs in newborns and represents a first step in their full characterization in this challenging population. Although SFOAEs may be simpler to interpret than other OAEs because they elicit an emission at a single frequency and at a relatively focal cochlear site, their application has largely been limited to the research laboratory. SFOAEs can be more difficult to measure than DPOAEs because they require more averaging to improve SNR; consequently, SFOAE protocols can be lengthy, and this is not always feasible with human newborns. However, even with a less than ideal discrete-tone probe presentation, we were able to measure robust SFOAEs in newborns at multiple levels, down to 15 dB SPL in some ears. This allowed us to compare adult and newborn SFOAEs in the linear region of the growth function rather than at a fixed stimulus level. This initial effort bodes well for future attempts to define more fully the basic features of the newborn SFOAE and to assess their clinical utility as a tool for newborn hearing screening.
We found that SFOAEs were larger in magnitude in newborns than adults by approximately 10 dB overall when compared at the same 40 dB SPL probe level. The result of overall larger SFOAEs is consistent with observations for click-evoked OAEs in infant ears (Norton and Widen, 1990; Prieve et al., 1997) and more recently, for the reflection component of the 2f1–f2 DPOAE in newborns (Abdala and Dhar, 2010, 2012). Because reflection-source emissions are linked to cochlear amplifier gain and tuning, the observation of robust SFOAEs could be interpreted to indicate an atypically strong cochlear amplifier and sharper cochlear filters in the newborn ear. Indeed, neonatal DPOAE suppression tuning curves are also sharper and narrower than adult tuning curves (Abdala, 1998). However, if the inefficiencies in forward transmission through the immature middle ear combined with a gain in reverse transmission (due to both middle ear and ear-canal factors) are considered, the age effects of DPOAE suppression tuning curves can largely be accounted for by conductive not cochlear factors (Abdala and Keefe, 2012). These conductive factors, predict an effective attenuation of approximately 15 dB in the stimulus levels driving the newborn cochlea for high-frequency stimuli.
The level dependence of SFOAEs recorded in newborns here is consistent with the notion that middle-ear factors influenced our results. Though tested in only a small group of newborns, SFOAEs appear to grow linearly to approximately 40 dB SPL in newborns, whereas adult SFOAEs begin to saturate between 20 and 30 dB SPL. This estimated 15 dB difference in the linear range of SFOAE growth agrees well with the predicted effective 15 dB attenuation imposed by the immature middle ear between 4 and 6 kHz (Abdala and Keefe, 2006). It may be that comparable middle-ear inefficiencies exist in the newborn ear at mid-frequencies where most of these SFOAE measurements were made. Once the SFOAEs were equated by their region of linear growth, the newborn OAEs were larger by only 2–4 dB overall. This effect is probably accounted for by the smaller newborn ear canal diameter, which also effectively boosts the outgoing signal. Although outer and middle ear factors appear to be the dominant factor accounting for these SFOAE magnitude and delay effects in neonates, the pristine condition of the neonatal cochlea (with its ample complement of sensory cells and optimal mechanics) may combine with these conductive effects to produce robust cochlear reflection.
Our data suggest that when SFOAE delays are compared between age groups at stimulus levels where the emission grows linearly (rather than at a fixed probe level), the SFOAE delays are similar in adults and newborns. This finding supports the idea that immaturities in the neonatal cochlea do not include sharper tuning and that longer SFOAE delays can mostly be explained my middle-ear factors. However, these conclusions are confined to mid-frequency cochlear regions, and it is not known whether SFOAE delays are mature in newborns below 1.5 kHz. DPOAE phase and cochlear scaling in the apical half of the newborn cochlea are not adult-like and these immaturities cannot be easily explained by middle-ear factors (Abdala et al., 2011).
Acknowledgments
We thank Mahnaz Ahmadi, Lucy Gharibian, and Srikanta Mishra for data collection. This work was supported by grants from the NIH, National Institute of Deafness and Communication Disorders (R01 DC03552 and P30 DC010743) and the Capita Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References and links
- 24.Abdala, C. (1998). “ A developmental study of DPOAE 2f1-f2 suppression in humans,” Hear. Res. 121, 125–138. [DOI] [PubMed] [Google Scholar]
- 1.Abdala, C. , and Dhar, S. (2010). “ Distortion product otoacoustic emission phase and component analysis in human newborns,” J. Acoust. Soc. Am. 127, 316–325 10.1121/1.3268611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdala, C. , and Dhar, S. (2012). “ Maturation and aging of the human cochlea: A view through the DPOAE looking glass,” J. Assoc. Res. Otolaryngol. 13, 403–421 10.1007/s10162-012-0319-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdala, C. , Dhar, S. , and Mishra, S. (2011). “ The breaking of cochlear scaling symmetry in human newborns and adults,” J. Acoust. Soc. Am. 129, 1584–1594 10.1121/1.3569737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdala, C. , and Keefe, D. H. (2006). “ Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears,” J. Acoust. Soc. Am. 120, 3832–3842 10.1121/1.2359237 [DOI] [PubMed] [Google Scholar]
- 5.Abdala, C. , and Keefe, D. H. (2012). “ Morphological and functional development of the ear,” in Springer Handbook of Auditory Research in Human Auditory Development, edited by Werner L., Popper A., and Fay R. ( Springer; New York: ), pp. 19–60. [Google Scholar]
- 6.Abdala, C. , Keefe, D. H. , and Oba, S. I. (2007). “ Distortion product otoacoustic emission suppression tuning and acoustic admittance in human infants: Birth through 6 months,” J. Acoust. Soc. Am. 121, 3617–3627 10.1121/1.2734481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentsen, T. , Harte, J. M. , and Dau, T. (2011). “ Human cochlear tuning estimates from stimulus-frequency otoacoustic emissions,” J. Acoust. Soc. Am. 129, 3797–3807 10.1121/1.3575596 [DOI] [PubMed] [Google Scholar]
- 8.Bergevin, C. , Fulcher, A. , Richmond, S. , Velenovsky, D. , and Lee, J. (2012). “ Interrelationships between spontaneous and low-level stimulus-frequency otoacoustic emissions in humans,” Hear. Res. 285, 20–28 10.1016/j.heares.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Collet, L. , Gartner, M. , Veuillet, E. , Moulin, A. , and Morgon, A. (1993). “ Evoked and spontaneous otoacoustic emissions: A comparison of neonates and adults,” Brain Dev. 15, 249–252 10.1016/0387-7604(93)90018-4 [DOI] [PubMed] [Google Scholar]
- 10.Gorga, M. P. , Neely, S. T. , Bergman, B. M. , Beauchaine, K. L. , Kaminski, J. R. , Peters, J. , Schulte, L. , and Jestead, W. (1993). “ A comparison of transient-evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects,” J. Acoust. Soc. Am. 94, 2639–2648 10.1121/1.407348 [DOI] [PubMed] [Google Scholar]
- 11.Kalluri, R. , and Shera, C. A. (2007a). “ Comparing stimulus-frequency otoacoustic emissions measured by compression, suppression, and spectral smoothing,” J. Acoust. Soc. Am. 122, 3562–3575 10.1121/1.2793604 [DOI] [PubMed] [Google Scholar]
- 12.Kalluri, R. , and Shera, C. A. (2007b). “ Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions,” J. Acoust. Soc. Am. 121, 2097–2110 10.1121/1.2435981 [DOI] [PubMed] [Google Scholar]
- 13.Keefe, D. H. , and Abdala, C. (2007). “ Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears,” J. Acoust. Soc. Am. 121, 978–993 10.1121/1.2427128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keefe, D. H. , Ellison, J. C. , Fitzpatrick, D. F. , and Gorga, M. P. (2008). “ Two-tone suppression of stimulus frequency otoacoustic emissions,” J. Acoust. Soc. Am. 123, 1479–1494 10.1121/1.2828209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, J. A. L. , Marshall, L. , and Heller, L. M. (2004). “ A longitudinal study of changes in evoked otoacoustic emissions and pure-tone thresholds as measured in a hearing conservation program,” Int. J. Audiol. 43, 307–322 10.1080/14992020400050040 [DOI] [PubMed] [Google Scholar]
- 16.Moleti, A. , Sisto, R. , Paglialonga, A. , Sibella, F. , Anteunis, L. , Parazzini, M. , and Tognola, G. (2008). “ Transient evoked otoacoustic emission latency and estimates of cochlear tuning in preterm neonates,” J. Acoust. Soc. Am. 124, 2984–2994 10.1121/1.2977737 [DOI] [PubMed] [Google Scholar]
- 17.Moore, B. C. J. (2007). Cochlear Hearing Loss, 2nd ed. ( Wiley and Sons, New York: ). [Google Scholar]
- 18.Norton, S. , and Widen, J. (1990). “ Evoked otoacoustic emissions in normal hearing infants and children: Emerging data and issues,” Ear Hear. 11, 121–127 10.1097/00003446-199004000-00006 [DOI] [PubMed] [Google Scholar]
- 19.Prieve, B. A. , Fitzgerald, T. S. , and Schulte, L. E. (1997). “ Basic characteristics of click-evoked otoacoustic emissions in infants and children,” J. Acoust. Soc. Am. 102, 2860–2870 10.1121/1.420341 [DOI] [PubMed] [Google Scholar]
- 20.Robles, L. , and Ruggero, M. A. (2001). “ Mechanics of the mammalian cochlea,” Physiol. Rev. 81, 1305–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shera, C. A. , and Guinan, J. J. (1999). “ Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs,” J. Acoust. Soc. Am. 105, 782–798 10.1121/1.426948 [DOI] [PubMed] [Google Scholar]
- 22.Shera, C. A. , and Guinan, J. J. (2003). “ Stimulus-frequency-emission group delay: A test of coherent reflection filtering and a window on cochlear tuning,” J. Acoust. Soc. Am. 113, 2762–2772 10.1121/1.1557211 [DOI] [PubMed] [Google Scholar]
- 23.Shera, C. A. , Guinan, J. J. , and Oxenham, A. J. (2002). “ Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements,” Proc. Natl. Acad. Sci. U.S.A. 99, 3318–3323 10.1073/pnas.032675099 [DOI] [PMC free article] [PubMed] [Google Scholar]