Abstract
Humans live, eat, and become overweight/obese in complex surroundings where there are many available food choices. Prenatal exposure to poor food choices predisposes offspring to increased negative health risks, including obesity. Many animal experiments have analyzed intergenerational body weight parameters in an environment without food choices, which may not be directly translatable to the human food environment. In this study, offspring from mothers with a defined high-fat diet (HFD) or low-fat diet (LFD) were arbitrarily assigned to either an exclusively LFD or HFD or to a diet where they have a choice between LFD and HFD (choice diet). Offspring displayed negative outcomes of increased body weight, body fat, serum leptin, and blood glucose levels when given the choice diet compared with offspring on the LFD. Conversely, improved energy expenditure was found for offspring given the choice diet compared with offspring from HFD dams given LFD. In addition, maternal diet-specific influences on offspring metabolic parameters were identified, especially in offspring from HFD dams, including positive outcomes of reduced leptin in LFD offspring, reduced corticosterone and cholesterol levels in HFD offspring, and increased exercise levels in choice offspring, as well as the negative outcome of increased calorie intake in LFD offspring from HFD dams. This defined model can now be used as the basis for future studies to characterize the cycle of inter- and intragenerational obesity and whether more realistic diet environments, especially those including choice, can mitigate phenotype.
The incidence of chronic noncommunicable disease has grown exponentially in recent years and has been attributed to the collective influences of genetic predisposition and adult lifestyle. More recently, perinatal environment has been studied as a third and critical factor that influences the proportion of fat/lean mass, metabolism, appetite, and lifelong susceptibility to certain chronic diseases (1–3). Early studies uncovered an association between an adverse intrauterine environment, determined primarily by low birth weight, and an increased risk of coronary heart disease later in life (4). Further studies found low birth weight to also be associated with impaired glucose tolerance, type 2 diabetes, and permanent, transgenerational changes in insulin and glucose metabolism (1, 5–7). In working to explain such associations, this body of research established the fetal origins of adult disease hypothesis and launched the concepts of developmental plasticity, predictive adaptive response, mismatch, and the thrifty phenotype hypothesis (6, 8). More recently, altered programming of the hypothalamus and the hypothalamic-pituitary-adrenal axis has been explored as yet another potential mechanism behind the fetal origins of adult disease (9, 10).
There appears to be several windows in which developmental pathways exhibit plasticity (ie, the ability of an organism to change its phenotype in response to changes in the environment): during gestation, early in postnatal development, at puberty, and in the geriatric period (11). These 4 life periods represent windows of time in which the epigenome may be unstable and hence more vulnerable to environmental influences. The predictive adaptive response refers to adaptations to perceived environmental cues during the early windows of vulnerability, which can alter the trajectory of growth and development. In most cases, such a response is beneficial for the survival of the organism, as when prenatal adaptations correctly anticipate the postnatal environment resulting in positive lifelong health outcomes. However, the problem of mismatch occurs when individuals developmentally adapted to one environment are exposed to another (5). Mismatch is thought to be involved in the current epidemics of type 2 diabetes and cardiovascular disease in countries such as India that are undergoing swift economic and nutritional transitions (4, 12).
The hypothalamus plays a critical role in the regulation of appetite and body composition. A series of studies, primarily in rodents, have explored the possibility that maternal nutrition during pregnancy may alter the level of energy intake in the offspring through induction of changes in the hypothalamic circuitry and in the expression, localization, and action of specific neuropeptides (10, 13).
Although previous experimental studies in animals have examined the effects of maternal high-fat diet (HFD) and low-fat diet (LFD) on offspring phenotypes, such studies have been done in the absence of choice, which is a central element of the human environment. Humans live, eat, and experience changes in body weight in a complex environment where there are many available food choices. Although ignoring choice (one food type analysis) is extremely important for understanding the biological pathways for a specific food type, a single food analysis may be misleading in terms of drawing accurate conclusions on diet, nutrition, and health in a choice environment. Diet and nutrition are both aggregate and compositional concepts. Diet quality is defined by total food intake and the composition of that total food intake, not by the intake of one food. As a consequence, the beneficial (or harmful) attributes of one food can be either mitigated or exacerbated by attributes of another food, so focusing on the consumption of a single food can give a very misleading indication of nutrition and consequently health in an environment of choice.
This study examines the role of food choice in offspring, which can both model the human condition, and allow an examination of whether offspring diet can alter maternal diet-induced effects. Offspring from dams given either HFD or LFD were presented with LFD alone, HFD alone, or a choice of both LFD and HFD, ad libitum. Offspring phenotypes including body weight, body composition, metabolic performance, and blood glucose, hormone, and lipid levels, were measured to determine the effect of diet, both in early development and adulthood, and how altering the diet in adulthood and providing choice would mitigate or exacerbate such parameters. The results provide some understanding to the contribution of pre- and postnatal dietary factors on lifelong health outcomes.
Materials and Methods
Animals and diets
The Virginia Tech Animal Care and Use Committee approved the study. Mice were identified by a unique 4-digit ear tag (National Band and Tag Company). Four-week-old male and female C57BL/6 mice (The Jackson Laboratory) were acclimated for 1 week at 22.0°C ± 1°C, 40% to 60% humidity, and a 12-hour light, 12-hour dark cycle with 4 mice per cage. During acclimation, mice were provided LFD, considered a standard rodent diet (Harlan Teklad Global Diet 2018) and water ad libitum.
After acclimation, the breeder males were continued on LFD for the duration of the project and the females were arbitrarily assigned to 1 of 2 dietary groups: LFD (Harlan Teklad Irradiated Diet 2018; n = 13) or HFD (Harlan Teklad Global Diet TD.06414; n = 17). Both diets were in pellet form, containing 18% kcal from protein as well as all of the nutritional requirements of mice. LFD contained 6.2% fat, whereas HFD contained 60.3% fat. Dietary treatments were continued for 5 weeks before breeding to establish chronic consumption of the specific diet. During this time, female mice were housed together with a maximum of 4 mice per cage.
For breeding, females were placed overnight with males in a 2:1 ratio and checked the following morning for the presence of a vaginal plug, which indicated a successful mating event. Plug-positive females were designated as gestation day 0 and were then singly housed while remaining on their assigned diet throughout gestation and lactation. At the time of delivery, pup number was determined by gentle nondisruptive inspection of the cage, and pup age was designated as postnatal day 1. Litter size was established by counting the number of pups delivered. Offspring mortality rate was calculated as number of pups that died at any point after delivery divided by number of pups delivered. The sex of the pups was determined by 3 weeks of age, and the number of males and females in each litter was documented. At postnatal day 25, pups were weaned and female offspring were kept in the study and continued on the diet of their dam until 7 weeks of age. At 7 weeks of age, offspring were assigned, via stratified random sampling, to 1 of 6 treatment groups (Figure 1A) that allowed them to continue on the diet of their dam, be placed on the opposing diet, or be given a choice between the 2 diets. There were 14 dams on HFD that gave birth to 78 pups, of which 27 were female, and 26 of these females were used in the offspring analysis. There were 12 dams on LFD that gave birth to 80 pups, 33 of which were females, with all 33 of these females used in the offspring analysis. In summary, 7 to 9 females gave rise to the 8 to 11 female pups in each group.
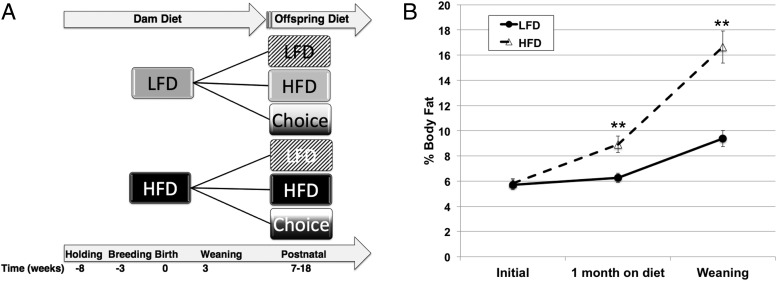
Figure 1. Experimental design and confirmation of fat gain in dams.
A, Diagram showing experimental design, with offspring from HFD or LFD dams continuing on the same diet from weaning to 7 weeks of age and then switched to one of three diets until the end of the study. B, Body fat in LFD (filled circles) and HFD (open triangles) dams at diet start, after 4 weeks on diet just before breeding, and at offspring weaning. *, P ≤ .05; **, P ≤ .01.
For the choice groups, equal amounts of LFD and HFD were placed in the same style top-feeder used by all the mice in the experiment, with a metal plate separating the 2 diets (free choice of either diet). The sample size for each treatment group was set at 10, and randomization was used to assign pups at weaning. The final groups based on this random assignment were as follows: 1) LFD→LFD, n = 11; 2) LFD→HFD, n = 11; 3) LFD→choice, n = 11; 4) HFD→LFD, n = 8; 5) HFD→HFD, n = 9; and 6) HFD→choice, n = 9. The 6 treatment groups were continued until 18 weeks of age, marking the end of the study.
Body weight, body composition, and food consumption
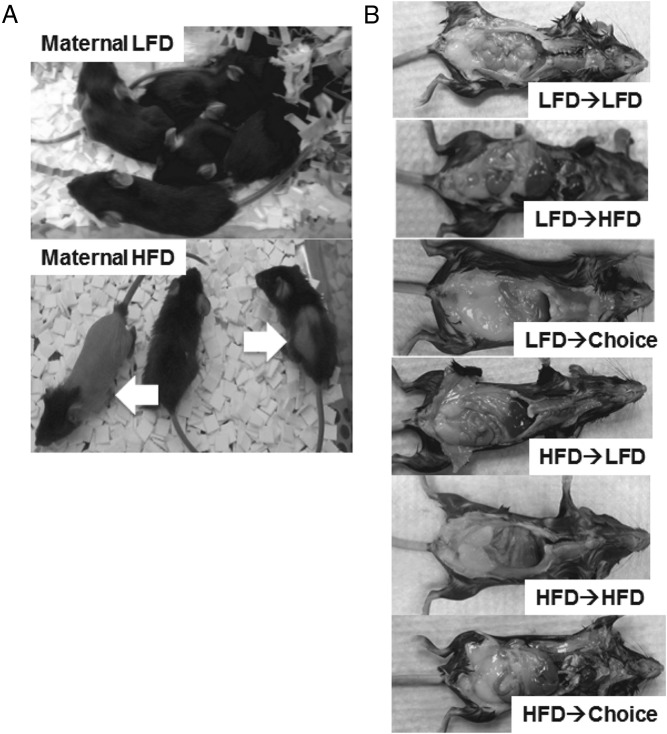
Body weight and food consumption were measured using an electronic scale (catalog item 80104002; OHAUS Scout Pro). Body composition was determined via nuclear magnetic resonance (Bruker LF90 NMR Analyzer). For the dams, body weight and food consumption were measured weekly during the 5 weeks of dietary treatment before breeding. Body composition was determined at 3 time points: 1) before starting the diet, 2) after 1 month on the diet, and 3) at weaning. For the offspring, body weight was measured weekly from 1 to 18 weeks of age. Food consumption was measured weekly from 7 to 18 weeks of age (ie, once they entered their treatment group), but weeks 8 and 9 were not analyzed and considered the washout period as the mice adjusted to the diet of their treatment group, which may or may not have been different from their previous diet. Body composition was determined at 7 and 18 weeks of age. The amount of intra-abdominal fat was visually assessed immediately after euthanasia at 18 weeks of age, with representative pictures taken for each treatment group.
Glucose tolerance test
Blood glucose levels (milligrams per deciliter) were determined by performing a glucose tolerance test on offspring at 7 and 18 weeks of age. Mice underwent an overnight 8- to 10-hour fast. The following morning, mice were placed into a restraining device that allows easy access to the tail. Blood from the lateral tail vein was taken via insertion and removal of a 25-gauge needle to produce a drop of blood, which was then placed on a hand-held glucometer (FreeStyle Lite glucose meter; Abbott Diabetes Care Inc). After determining fasted blood glucose levels (time 0), a glucose load equaling 2 g/kg was injected IP and tail blood glucose was obtained at 15, 30, 60, 90, and 120 minutes after the glucose load. Blood glucose levels were compared among the treatment groups including LFD controls. The area under the curve (AUC) for each animal was calculated in STATA using the cubic spline approach (14, 15).
Serum insulin, leptin, and corticosterone levels
ELISA kits were used to determine serum insulin (catalog item 80-INSMSU-E01; ALPCO Diagnostics), leptin (catalog item 90030; Crystal Chem Inc), and corticosterone (Arbor Assays) concentrations of offspring at 7 and 18 weeks of age. At both ages, blood was collected after an 8- to 10-hour fast. Blood, approximately 200 μL, was collected at 7 weeks of age via the lateral saphenous vein into Microvette CB 300 capillary tubes (catalog item NC9059691; Fisher Scientific). Blood, approximately 700 to 900 μL, collected at 18 weeks of age was attained via cardiac puncture while under isoflurane anesthesia using a 1-mL syringe with a 27-gauge, 0.5-inch needle. For both ages, blood was allowed to clot for 30 minutes to 1 hour at room temperature and centrifuged for 20 minutes, after which the serum was collected. Serum aliquots of 20 μL were stored at −80°C until the time of performing the ELISAs.
Serum lipid levels
At 18 weeks of age, serum aliquots of 300 to 500 μL from each animal were submitted to the Clinical Pathology Laboratory at the Virginia-Maryland College of Veterinary Medicine for determination of triglyceride and cholesterol levels using an Olympus AU480 Chemical Analyzer. For this analysis, most samples were submitted within hours of blood collection; however, samples attained over the weekend, when the Clinical Pathology Laboratory was closed, were stored at 2°C to 8°C and submitted within 48 hours. Lipid levels were compared among the various treatment groups including the LFD controls as well as evaluated based on published reference ranges for adult female C57BL/6 mice for cholesterol (76.1–107.9 mg/dL) and triglycerides (65.8–90.2 mg/dL) (16).
Metabolic chambers
For offspring at 18 weeks of age the PhenoMaster/LabMaster (Technical Scientific Equipment Systems) was employed to simultaneously measure respiratory exchange ratio (RER), energy expenditure, and physical activity over a 48-hour period. For this test, mice remained in their home cage, which limited stress, and thereby allowed for an accurate reflection of typical metabolic performance. The home cage was outfitted with monitoring devices, and the data generated were recorded and analyzed by integrated hardware and software programs. Indirect calorimetry, which calculates energy produced by measuring O2 consumption and CO2 production, was used to determine the RER and energy expenditure. As an index of physical activity, infrared light-beam frames surrounding the home cage measured activity in 3 dimensions (x, y, and z axes).
Statistical analysis
The experimental design is a standard 2-factor design, with factor A being the dam's diet and consisting of 2 levels (LFD or HFD) and factor B being the offspring food environment consisting of 3 levels (LFD, HFD, or choice). For the baseline or before the pups were placed on their experimental diets, a one-way ANOVA was used to test differences, because the only factor at that point that differed was the maternal diet. For the experimental period, a two-way ANOVA, complemented with a multiple regression model, was used to test the hypotheses of interest, which controls for interaction effects between maternal and pup diets (STATA version 11). All variance estimates were repeated measurement corrected. Statistical significance is assumed to be P < .05. For ease in communication, factor A effects are called dam diet or intergenerational diet effects and factor B effects are called offspring diet or intragenerational diet effects.
Results
Female dams on HFD show body fat gain; offspring show alopecia
The initial body fat percentage of all female dams used for generation of offspring was comparable at the start of the study (Figure 1B). As expected, those dams on HFD showed significant body fat gain, compared with those dams on LFD both by the time of initial mating (1 month after diet start; LFD, 6.2% ± 0.37%, compared with HFD, 8.9% ± 0.65%, P < .01) and at the time of weaning (LFD, 9.3% ± 0.64% compared with HFD, 16.6% ± 1.28%, P < .01). Interestingly, there was no significant difference in total body weight between HFD and LFD dams at any measured time point (data not shown).
In comparing litters born to LFD and HFD dams, no statistical differences in litter size, offspring mortality rate, or in the number of males and females in each litter were found. Notably, many of the pups in litters born to dams on HFD displayed alopecia (Figure 2A), accompanied by oily fur. Alopecia was most likely due to excessive grooming by the dams as it was corrected in all affected pups after weaning.
Figure 2. Physical appearance of offspring at weaning and study end.
A, Offspring from HFD or LFD dams at weaning. Arrows point to HFD pups showing prominent alopecia. B, Representative necropsy findings subjectively assessing the amount of intra-abdominal fat at study end for each of the 6 offspring treatment groups.
Maternal diet influences response to offspring diet for body weight and fat
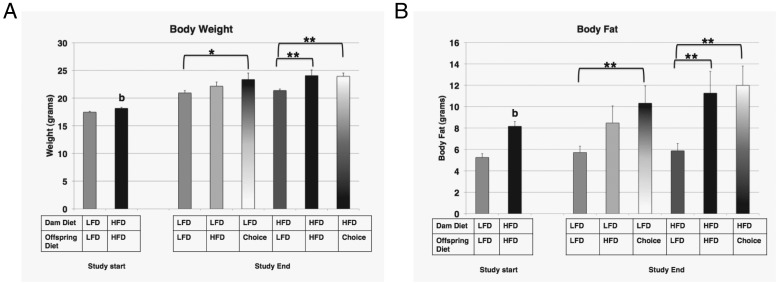
By the start of the study at 7 weeks of age, offspring from HFD dams had a significantly higher body weight (Figures 2B, 3A; LFD, 17.4 ± 0.17 g, compared with HFD, 18.1 ± 0.19 g, P < .01) and body fat (Figure 3B; LFD, 5.3 ± 0.35 g, compared with HFD, 8.1 ± 0.45 g, P < .01), suggesting that pups of HFD dams went through a period of catch-up growth to reach their trajectory for body habitus, as they were significantly smaller than pups from LFD dams 2 weeks postnatally (Supplemental Figure 1). By study end at 18 weeks of age, regardless of dam diet, there was no difference in body weight or percent body fat between either group of offspring on LFD (LFD→LFD vs HFD→LFD). However, for offspring on LFD→choice, there was a significant increase in weight (Figure 3A, LFD→LFD, 20.9 ± 0.41 g, compared with LFD→choice, 23.9 ± 1.15 g, P < .05) and percent body fat (Figure 3B; LFD→LFD, 5.7% ± 0.58%, compared with LFD→choice, 10.3% ± 1.62%, P < .01) compared with offspring that remained on the LFD. For HFD offspring, both HFD and choice led to significant increases in body weight and body fat compared with offspring that were switched to LFD (Figure 3A: HFD→LFD, 21.4 ± 0.28 g, compared with HFD→HFD, 24.1 ± 1.02 g, and HFD→choice, 23.9 ± 0.58 g, P < .01; Figures 2B and 3B: HFD→LFD, 5.8 ± 0.67 g, compared with HFD→HFD, 11.3 ± 2.0 g, and HFD→choice, 11.9 ± 1.8 g, P < .01). Offspring from dams on HFD showed increased body weight gain over the 11 weeks when maintained on HFD, but not if switched from LFD (LFD→HFD, 4.1 ± 0.33 g gained, compared with HFD→HFD, 6.2 ± 0.47 g gained, P < .01), whereas choice led to significant gains over starting weight for both offspring groups (LFD→choice, 5.3 ± 0.87 g gained, compared with HFD→choice, 5.1 ± 0.62 g gained, P < .05), with a similar trend for body fat (Supplemental Figure 2). Interestingly, of all groups, only HFD→LFD offspring showed an average body fat loss during that time period. These data support both an inter- and intragenerational effect of maternal diet on offspring phenotypes of body weight and fat.
Figure 3. Body weight and fat in offspring.
Body weight (grams) (A) and body fat (grams) (B) for offspring from the 2 groups at study start or the 6 groups at study end. The diets for each group are indicated. For the effect of offspring diet (identical maternal diet): *, P ≤ .05; **, P ≤ .01; For the effect of maternal diet (identical offspring diet).
Caloric intake can be influenced by intergenerational diet type switching
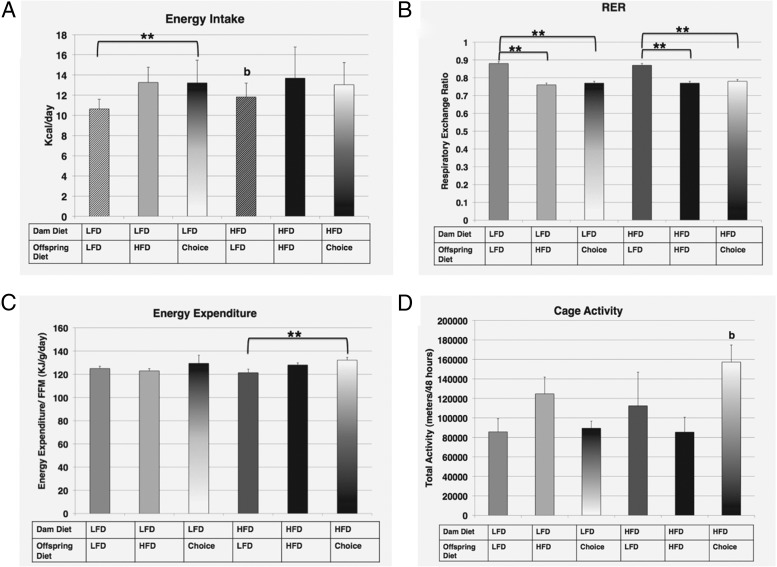
Offspring from HFD dams on LFD showed a significantly higher caloric intake compared with offspring from LFD dams who were also on LFD (an intergenerational diet switching effect), suggesting a maternal effect of calorie intake (Figure 4A; HFD→LFD, 11.8 ± 1.37 kcal/mouse/d, compared with LFD→LFD, 10.6 ± 0.95 kcal/mouse/d, P < .01). There was also a significantly higher caloric intake for offspring from LFD dams given the choice diet (LFD→choice, 13.2 ± 2.24 kcal/mouse/d, P < .01), implicating food choice effect on feeding behavior/amount of energy consumed. This result would not have been captured in the absence of food choice. No other significant effects of caloric intake were detected. In addition, there was no significant difference in HFD intake between the choice groups, with each consuming approximately 95% of their diet as HFD. Specifically, the LFD→choice group consumed 12.9 ± 2.24 g/wk HFD vs 0.36 ± 0.41 g/wk LFD, whereas the HFD→choice group consumed 12.7 ± 2.14 g/wk HFD vs 0.30 ± 0.18 g/wk LFD, with Student's t test indicating no significant differences in intake.
Figure 4. Energy intake and expenditure.
Calorie intake (kcal/mouse/d) (A), respiratory exchange ratio (B), energy expenditure values (kilojoules per gram per day) (C), and activity (meters per 48 hours) (D) measured for the 6 groups at study end. The diets for each group are indicated. For the effect of offspring diet (identical maternal diet): *, P ≤ .05; **, P ≤ .01; for the effect of maternal diet (identical offspring diet): b, P ≤ .01.
Choice diet affects both energy expenditure and physical activity levels
All effects on RER and energy expenditure were influenced by offspring diet (intragenerational diet effect), with no intergenerational diet effects (Figure 4, B and C). Offspring, from either dam group, on HFD or choice diets had significantly lower RER as compared with offspring on LFD, consistent with a higher fat intake and use of fat as an energy source (Figure 4B; LFD→LFD, 0.88 ± 0.01 g, compared with LFD→HFD, 0.76 ± 0.01 g, and LFD→choice, 0.77 ± 0.01 g, P < .01; HFD→LFD, 0.87 ± 0.01 g, compared with HFD→HFD, 0.77 ± 0.01 g, and HFD→choice, 0.78 ± 0.01 g, P < .01). There was little effect on energy expenditure, regardless of dam or offspring diet, with the exception of offspring from HFD dams on choice. These animals showed significantly higher energy expenditure compared with HFD→LFD offspring (Figure 4C; HFD→LFD, 121.39 ± 3.05 kJ/g/d, compared with HFD→choice, 132.1 ± 2.38 kJ/g/d, P < .01).
Interestingly, home cage activity, as measured by beam breaks over a 48-hour period, showed significantly higher levels only in offspring from HFD dams on choice, as compared with LFD→choice offspring (Figure 4D; LFD→choice, 89 393.6 ± 37 273.4 rotations/48 hours, compared with HFD→choice, 157 285.8 ± 17 476.5 rotations/48 hours, P < .01). This represents an intergenerational or maternal diet effect, because there was no significant difference in activity for offspring choice if they came from LFD dams.
Cholesterol, but not triglyceride, levels show both inter- and intragenerational effects
Serum cholesterol levels for offspring from LFD dams showed an intragenerational diet switching effect, as the levels were significantly higher when offspring were switched to HFD or choice, as compared with offspring staying on LFD (Figure 5A; LFD→LFD: 77.3 ± 3.73 mg/dL compared with LFD→HFD: 103.6 ± 4.96 mg/dL and LFD→choice: 106.7 ± 7.72 mg/dL, P < .01). Interestingly, for offspring from HFD dams, there was no significant difference in serum lipid levels for offspring on any diets. Of special note is that HFD→HFD offspring showed an apparent maternal protective effect granted by the early exposure to a high fat diet, with this group having significantly lower cholesterol levels than the LFD→HFD offspring (LFD→HFD: 103.6 ± 4.96 mg/dL compared HFD→HFD: 83.9 ± 7.66 mg/dL, P < .05). There were no intergenerational (maternal) or intragenerational (offspring) effects for serum triglyceride levels (Figure 5B).
Figure 5. Serum lipid profiles A and B, Total serum cholesterol (milligrams per deciliter) (A) and total serum triglycerides (milligrams per deciliter) (B) measured for the 6 groups at study end.
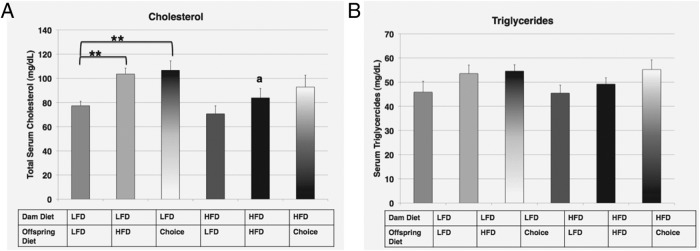
The diets for each group are indicated. For the effect of offspring diet (identical maternal diet): *, P ≤ .05; **, P ≤ .01; for the effect of maternal diet (identical offspring diet): a, P ≤ .05.
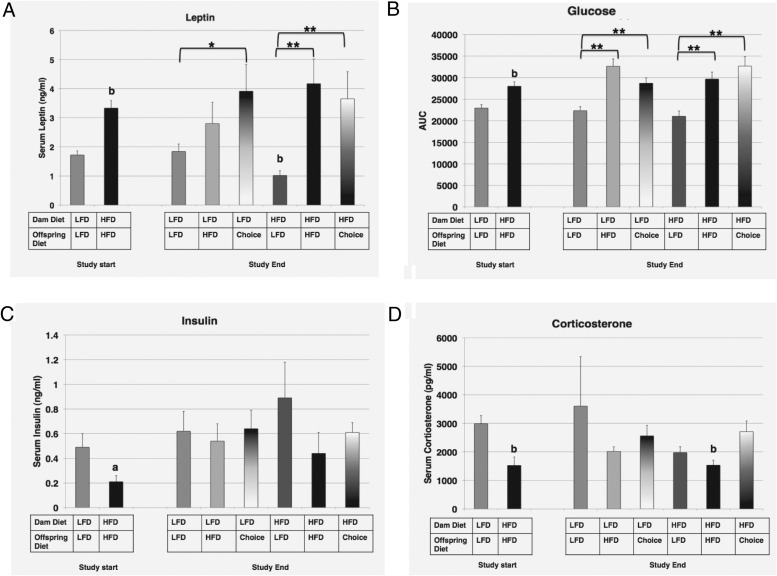
Glucose, insulin, and corticosterone levels are affected by maternal and offspring diet type
At 7 weeks of age, before adult diet group assignment, offspring from HFD dams had significantly higher levels of glucose (as assessed by AUC) and leptin, with significantly decreased levels of serum insulin and corticosterone, as compared with offspring from LFD dams (Figure 6; glucose: LFD, 22 933.6 ± 800.58, compared with HFD, 28 009.38 ± 992.69, P < .01; leptin: LFD: 1.7 ± 0.14 ng/mL, compared with HFD, 3.3 ± 0.26 ng/mL, P < .01; insulin: LFD, 0.5 ± 0.11 ng/mL, compared with HFD, 0.2 ± 0.05 ng/mL, P < .05; corticosterone: LFD, 2986.2 ± 287.5 pg/mL, compared with HFD, 1525.1 ± 295.3 pg/mL, P < .01). These are all maternal diet effects.
Figure 6. Blood glucose and serum metabolic hormones A–D, Serum leptin (nanograms per milliliter) (A), AUC (calculated from glucose tolerance curves, arbitrary units) (B), serum insulin (nanograms per milliliter) (C), and serum corticosterone (picograms per milliliter) (D) calculated for offspring from the 2 groups at study start or the 6 groups at study end.
The diets for each group are indicated. For the effect of offspring diet (identical maternal diet): *, P ≤ .05; **, P ≤ .01; for the effect of maternal diet (identical offspring diet): a, P ≤ .05; b, P ≤ .01.
For leptin, LFD→choice offspring showed a significantly higher serum level as compared with LFD→LFD offspring (Figure 6A; LFD→LFD, 1.8 ± 0.26 ng/mL, compared with LFD→choice, 3.9 ± 0.92 ng/mL, P < .05). Comparatively, offspring from HFD dams who were switched to choice or remained on HFD had significantly higher serum leptin levels compared with HFD→LFD offspring (HFD→LFD, 1.0 ± 0.16 ng/mL, compared with HFD→HFD, 4.2 ± 0.85 ng/mL, and HFD→choice, 3.7 ± 0.93 ng/mL, P < .01). Interestingly, this HFD→LFD group also showed a significant protective maternal effect of diet, with lower leptin levels than LFD→LFD offspring (HFD→LFD, 1.0 ± 0.16 ng/mL, compared with LFD→LFD, 1.8 ± 0.26 ng/mL, P < .01). This finding could be due to diet switching in the HFD group. Fasting serum glucose levels appeared to reflect caloric intake of diets, with offspring from both maternal diets showing significantly higher fasting glucose levels for those on HFD or choice, as compared with LFD, indicating offspring-only effects on glucose levels, which is different from the maternal effects seen at 7 weeks (Figure 6B; LFD→LFD, 22 333.2 ± 924.10, compared with LFD→HFD, 32 642.2 ± 1684.44, and LFD→choice, 28 692.9 ± 1234.74, P < .01; HFD→LFD, 21 041.8 ± 1227.60, compared with HFD→HFD, 29 666.2 ± 1632.31, and HFD→choice, 32 685.5 ± 2167.25, P < .01). At study end, there was no effect of offspring or maternal diet on serum insulin levels (Figure 6C). Corticosterone levels in offspring at study end reflected no differences between offspring diets. However, the maternal effect of HFD on lowering corticosterone at study start is maintained in the HFD→HFD offspring as compared with the LFD→HFD group (Figure 6D; LFD→HFD, 2016.8 ± 160.28 pg/mL, compared with HFD→HFD, 1533.6 ± 173.18 pg/mL, P < .01).
Discussion
Our results demonstrate that different maternal and offspring diets can either exacerbate or mitigate offspring body habitus and metabolic parameters. Stated alternatively, intergenerational diet effects can be exacerbated or mitigated by intragenerational diet environments. Furthermore, we show that exposure to a choice environment for offspring can result in worsening of effects compared with either diet alone. This is the first demonstration of the effect of a choice diet in offspring, after diet switching between maternal and offspring diets. Collectively, the data establish that exposure to a diet rich in fat is capable of inducing obesity, elevated lipid levels, and hyperglycemia as well as other useful markers for the diagnosis of the metabolic syndrome and that choice diets appear to either exacerbate or protect against obesity-related phenotypes, depending on maternal diet.
Dams exposed to HFD showed higher body fat and caloric intake relative to dams given LFD; however, they did not have a significantly higher body weight (data not shown), suggesting that when assessing body physique and fitness, body weight is often not the best indicator of health or body fat levels. There was no significant difference in litter size or in offspring mortality rate (data not shown), indicating that general birth rate parameters were not affected by diet and that diets were equivalent in regard to meeting the nutritional needs of pregnancy.
Exposure of HFD affected not only the dams; this diet also influenced body habitus and metabolism of the offspring. Early in the postnatal period, offspring fur appeared greasy, with significant patchy alopecia, which could be attributed to excess grooming by the dam. Although this effect was found before weaning, it resolved upon separation. Additionally, before 7 weeks of age, pups born from HFD dams were smaller/weighed less than those born from LFD dams; however, by 7 weeks of age, offspring body weight and fat were significantly higher in offspring from HFD dams than those from LFD dams, and this occurred before diet switching. This finding suggests that the HFD pups underwent a period of catch-up growth, and as such, these data are consistent with similar studies on offspring from dams on HFD, for example (17–19). It is also noteworthy that pups of HFD dams who were then fed LFD from 7 weeks to the end of the study achieved body weight and fat levels equivalent to the pups of LFD dams who remained on LFD throughout the study. This observation demonstrates that adverse effects of maternal diet on body habitus may be negated through healthy eating postnatally. Also at 7 weeks of age, offspring of HFD dams had significantly higher glucose and leptin levels concurrent with significantly lower insulin and corticosterone levels as compared with offspring of LFD dams. The apparent hyperglycemia in the HFD offspring by 7 weeks of age, accompanied by hypoinsulinemia at the same time suggests a prediabetic state. Higher leptin levels in this group reflect the linear association with percent body fat and is consistent with established data that leptin, an adipocyte-derived hormone, is positively correlated to body adiposity (20). Interestingly, 7-week-old HFD offspring display lower serum corticosterone levels, and as suggested by previous studies, this may reflect a lower overall anxiety level (21).
In this study, female offspring were exclusively studied. Much of the work using mice has been done on male offspring; however, recent work suggests significant differences in gender responses to HFD with several recent articles concluding that females are resistant to metabolic effects of HFD (22–25). However, other studies suggest that epigenetic inheritance may be occurring through the female line, specifically in obesity-related genes (26). This initial study using females was designed to help us to understand any metabolic or other changes that might occur in the first generation of female offspring in response to switched diets and diet choice as a foundation for future studies using second-generation offspring. It is important to note that estrous cycle synchronicity was not included in this study, and diet-induced obesity can alter the estrous cycle of mice (27). However, to our knowledge, the effect of stage of cycle has not been shown to affect serum leptin, corticosterone, glucose, or insulin levels.
There have been only a few studies of diet switching and, specifically, diet choice in offspring from HFD dams, with all 3 mentioned below done in rats rather than mice. In rats, offspring from HFD dams show increased preference for HFD and/or high sugar, when given a diet choice (25, 28, 29). But with mice being the preferred model to study the contributions of specific genes to feeding behavior, it was important to develop this model, because differences in the genetic contribution to feeding behavior and choice might be teased out more readily with knockout and transgenic animals. For example, the melanocortin 4 receptor-knockout mouse actually shows a reduced preference for a high-sucrose diet when presented with diet choice, unlike normal or heterozygous mice that preferred that diet over standard chow (30). In one of our previous studies, female mice with a mutation of the tubby gene, presented with diet choice through a food choice and cost paradigm, preferred HFD when the cost (set by lever presses in an operant chamber) was low but lost weight and chose LFD as the price of the HFD rose (31). In humans, polymorphisms of the TUB gene are associated with increased carbohydrate intake (32). Because humans live in a natural choice environment, studies to understand the link between genes and food preferences are needed.
Consistent with previous studies on the effect of diet switching on offspring phenotypes (25), LFD→HFD offspring showed no significant difference in weight or fat relative to LFD→LFD offspring. However, the LFD→choice diet resulted in significantly increased weight and fat relative to LFD→LFD offspring. These findings are interesting given the food choice environment experienced by humans. Based on these data, a mother who chooses a LFD during gestation would not protect her offspring from developing adult overweight or obesity in a food environment consisting of choice, but could protect them in a food environment consisting of high-fat foods with no choice. These results may be similar to a 2013 study in which weight outcomes of children born to mothers before or after bariatric surgery were examined. In that study, there was no overall significant effect of surgery to weight outcomes in the younger ages (3 and 6 years), but 10-year-old female offspring showed higher body mass indices after surgery than female offspring born before surgery (33). However, these data are in contrast to earlier work showing that maternal bariatric surgery before pregnancy could protect children of these mothers from overweight and obesity as young adults (34). Because both of these studies were in humans, who were in a food choice environment, it may be that maternal LFD can protect later in life. Indeed, in our results, LFD→choice body fat levels appear reduced compared with HFD→choice body fat levels, although this difference was not significant.
Leptin levels also were influenced by diet switching in offspring, notably in offspring from dams on HFD who were switched to LFD. Serum leptin levels in HFD→LFD animals were significantly lower than in offspring from LFD dams also fed LFD (LFD→LFD), suggesting a maternal diet effect on serum leptin levels. Differential methylation of the leptin gene promoter, and leptin gene expression, as well as lower serum leptin levels have previously been associated with obesity or HFD in several studies, including those involving humans with HFD resulting in increased methylation and reduced expression of leptin (26, 35, 36). In one study (26), differential methylation and leptin gene expression was observed only in female offspring. What is interesting about the data presented in our study is that neither HFD nor choice after maternal HFD led to this change in leptin levels, suggesting a clear protective effect in the LFD offspring exposed to HFD in utero and through weaning. Previously, studies in this area have not included choice or diet switching, making our finding unique and suggesting future analysis of promoter methylation changes in these conditions are warranted.
Levels of cholesterol and corticosterone showed a maternal diet protective effect. For both, offspring in the HFD→HFD treatment group displayed significantly lower levels of total serum cholesterol and corticosterone compared with offspring in the LFD→HFD group. This suggests that maternal HFD actually protects offspring, at least within the time frame tested, from stress and high cholesterol. Differential promoter methylation resulting from maternal diet could again account for these differences. For cholesterol, approximately 25% of the total cholesterol production occurs in liver, with methylation status of certain enzymes in the lipoprotein synthesis pathway, linked to the variability of lipid profile in humans (37). For corticosterone, food restriction leads to promoter methylation of the CRH gene in hypothalamic tissue and increased corticosterone in mice (38). Because both cholesterol and corticosterone levels are not significantly different with choice diet, future studies to examine the promoters of these genes could yield interesting new data on the role of food choice in mitigating maternal diet influences.
As noted previously, food choice is the norm for humans, but rodent models are rarely given food choice in obesity or food intake studies. In studies of choice between HFD, chow, and sucrose water, la Fleur and colleagues (28) found that diet choice increased overall caloric intake. Our data show a similar trend and include the addition of maternal diet effects on offspring phenotype. Likewise, we found that although higher body weight and body fat were observed with offspring choice diets for either maternal diet, increased calorie intake could explain increased weight and fat only in the LFD→choice group. This is consistent with the effects of diet switching between the maternal diet and offspring diet, because there was not a significant caloric increase in the LFD→HFD offspring, suggesting that the addition of choice diet exacerbated the increase. There was no significant difference in calorie intake between offspring with a maternal HFD.
Interestingly, choice diet in the HFD→choice group led to an unexpected increase in energy expenditure, via cage activity levels, relative to the HFD→LFD group, suggesting that access to a varied diet can actually have a beneficial effect on weight management. However, although increased energy expenditure should have resulted in reduced body weight, the increased weight and fat in the HFD→choice treatment group is not explained by this finding. To our knowledge, and using the search terms diet choice, exercise, and rodent, we found no other studies examining the effects of diet choice on exercise levels in mice. In humans, exercise has been shown to stimulate visual preference for low-fat foods (39), suggesting a link between exercise and food choice.
In conclusion, these data examined the role of maternal-offspring diet, especially food choice, in offspring body weight and metabolic parameters and present unique findings related to both maternal-offspring diet switching and diet choice. The findings suggest that food choice can have both negative and positive effects on offspring metabolic parameters. Because humans live, eat, and reproduce in an environment with complex food choice parameters, it is important to study the effects of food choice and maternal-offspring diet switching on body weight phenotypes. This is the first study to do so, and using this model, the unique contributions of a food choice diet on body weight outcomes, including increased calorie consumption and serum leptin levels with choice diets were identified. In addition, maternal-offspring diet switching has allowed identification of maternal diet-specific influences, including those on leptin, corticosterone, calorie intake, exercise levels, and cholesterol. Use of this model can now be expanded to examine the possibility of tissue-specific epigenetic changes, and effects of diet restrictions on obesity outcomes, to identify whether the cycle of intergenerational obesity can be mitigated through altering the choice environment in addition to exercise or other factors initiated by an individual.
Acknowledgments
We thank members of the Animal Care Staff in the Integrated Life Sciences Building for providing exceptional animal care. This paper is dedicated to Ms Pam Mohr, an animal care technician who recently lost her battle with cancer.
This work was supported by internal institutional grants from the Institute for Society, Culture, and Environment and the Fralin Life Sciences Institute, Intergenerational Obesity Initiative, at Virginia Tech, and pilot study funds from National Institutes of Health Grant 5R13HD068062.
Current address for B.B.: Department of Pathology, Campbell University School of Osteopathic Medicine, Lillington, NC, 27546.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AUC
- area under the curve
- HFD
- high-fat diet
- LFD
- low-fat diet.
References
- 1. Demmelmair H, von Rosen J, Koletzko B. Long-term consequences of early nutrition. Early Hum Dev. 2006;82(8):567–574. [DOI] [PubMed] [Google Scholar]
- 2. Lillycrop KA. Effect of maternal diet on the epigenome: implications for human metabolic disease. Proc Nutr Soc. 2011;70(1):64–72. [DOI] [PubMed] [Google Scholar]
- 3. Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92(2):287–298. [DOI] [PubMed] [Google Scholar]
- 4. Hanson MA, Gluckman PD. Developmental processes and the induction of cardiovascular function: conceptual aspects. J Physiol. 2005;565(Pt 1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bateson P, Barker D, Clutton-Brock T, Deb D, et al. Developmental plasticity and human health. Nature. 2004;430(6998):419–421. [DOI] [PubMed] [Google Scholar]
- 6. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. [DOI] [PubMed] [Google Scholar]
- 7. Hales CN, Ozanne SE. The dangerous road of catch-up growth. J Physiol. 2003;547(Pt 1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo ZC, Fraser WD, Julien P, et al. Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med Hypotheses. 2006;66(1):38–44. [DOI] [PubMed] [Google Scholar]
- 9. Beck B, Kozak R, Moar KM, Mercer JG. Hypothalamic orexigenic peptides are overexpressed in young Long-Evans rats after early life exposure to fat-rich diets. Biochem Biophys Res Commun. 2006;342(2):452–458. [DOI] [PubMed] [Google Scholar]
- 10. Fahrenkrog S, Harder T, Stolaczyk E, et al. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. 2004;134(3):648–654. [DOI] [PubMed] [Google Scholar]
- 11. Dolinoy DC, Das R, Weidman JR, Jirtle RL. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr Res. 2007;61(5 Pt 2):30R–37R. [DOI] [PubMed] [Google Scholar]
- 12. Norris SA, Osmond C, Gigante D, et al. Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care. 2012;35(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146(10):4211–4216. [DOI] [PubMed] [Google Scholar]
- 14. Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–250. [DOI] [PubMed] [Google Scholar]
- 15. Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6(1):79–98. [DOI] [PubMed] [Google Scholar]
- 16. Fox JG. The Mouse in Biomedical Research. 2nd ed Amsterdam, The Netherlands: Elsevier; 2007. [Google Scholar]
- 17. Mingrone G, Manco M, Mora ME, et al. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care. 2008;31(9):1872–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuelsson AM, Matthews PA, Argenton M, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383–392. [DOI] [PubMed] [Google Scholar]
- 19. Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R528–538. [DOI] [PubMed] [Google Scholar]
- 20. Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. [DOI] [PubMed] [Google Scholar]
- 21. Sasaki A, de Vega WC, St-Cyr S, Pan P, McGowan PO. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience. 2013;240:1–12. [DOI] [PubMed] [Google Scholar]
- 22. Hwang LL, Wang CH, Li TL, et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity. 2010;18(3):463–469. [DOI] [PubMed] [Google Scholar]
- 23. Liu H, Choi JW, Yun JW. Gender differences in rat plasma proteome in response to high-fat diet. Proteomics. 2012;12(2):269–283. [DOI] [PubMed] [Google Scholar]
- 24. Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS One. 2012;7(9):e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Treesukosol Y, Sun B, Moghadam AA, Liang NC, Tamashiro KL, Moran TH. Maternal high-fat diet during pregnancy and lactation reduces the appetitive behavioral component in female offspring tested in a brief-access taste procedure. Am J Physiol Regul Integr Comp Physiol. 2014;306(7):R499–R509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ge ZJ, Luo SM, Lin F, et al. DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity. Environ Health Perspect. 2014;122:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bermejo-Alvarez P, Rosenfeld CS, Roberts RM. Effect of maternal obesity on estrous cyclicity, embryo development and blastocyst gene expression in a mouse model. Hum Reprod. 2012;27(12):3513–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. la Fleur SE, Luijendijk MC, van der Zwaal EM, Brans MA, Adan RA. The snacking rat as model of human obesity: effects of a free-choice high-fat high-sugar diet on meal patterns. Int J Obes (Lond). 2014;38(5):643–649. [DOI] [PubMed] [Google Scholar]
- 29. Nakashima Y, Tsukita Y, Yokoyama M. Preferential fat intake of pups nursed by dams fed low fat diet during pregnancy and lactation is higher than that of pups nursed by dams fed control diet and high fat diet. J Nutr Sci Vitaminol. 2008;54(3):215–222. [DOI] [PubMed] [Google Scholar]
- 30. Panaro BL, Cone RD. Melanocortin-4 receptor mutations paradoxically reduce preference for palatable foods. Proc Natl Acad Sci U S A. 2013;110(17):7050–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davis GC, Jacob J, Good DJ. Effects of price and genetics on food choices and obesity-related phenotypes in mice. Open Neuroendocrinol J. 2012;5:13–21. [Google Scholar]
- 32. van Vliet-Ostaptchouk JV, Onland-Moret NC, Shiri-Sverdlov R, et al. Polymorphisms of the TUB gene are associated with body composition and eating behavior in middle-aged women. PloS One. 2008;3(1):e1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Willmer M, Berglind D, Sorensen TI, Naslund E, Tynelius P, Rasmussen F. Surgically induced interpregnancy weight loss and prevalence of overweight and obesity in offspring. PloS One. 2013;8(12):e82247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94(11):4275–4283. [DOI] [PubMed] [Google Scholar]
- 35. Marchi M, Lisi S, Curcio M, et al. Human leptin tissue distribution, but not weight loss-dependent change in expression, is associated with methylation of its promoter. Epigenetics. 2011;6(10):1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Milagro FI, Campión J, García-Díaz DF, Goyenechea E, Paternain L, Martínez JA. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 2009;65(1):1–9. [DOI] [PubMed] [Google Scholar]
- 37. Guay SP, Brisson D, Lamarche B, et al. DNA methylation variations at CETP and LPL gene promoter loci: new molecular biomarkers associated with blood lipid profile variability. Atherosclerosis. 2013;228(2):413–420. [DOI] [PubMed] [Google Scholar]
- 38. Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci. 2010;30(48):16399–16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crabtree DR, Chambers ES, Hardwick RM, Blannin AK. The effects of high-intensity exercise on neural responses to images of food. Am J Clin Nutr. 2014;99(2):258–267. [DOI] [PubMed] [Google Scholar]