Abstract
Glucagon-like peptide-1 (GLP-1) is released from endocrine L-cells lining the gut in response to food ingestion. However, GLP-1 is also produced in the nucleus of the solitary tract, where it acts as an anorectic neurotransmitter and key regulator of many autonomic and neuroendocrine functions. The expression and projections of GLP-1-producing neurons is highly conserved between rodent and primate brain, although a few key differences have been identified. The GLP-1 receptor (GLP-1R) has been mapped in the rodent brain, but no studies have described the distribution of GLP-1Rs in the nonhuman primate central nervous system. Here, we characterized the distribution of GLP-1R mRNA and protein in the adult macaque brain using in situ hybridization, radioligand receptor autoradiography, and immunohistochemistry with a primate specific GLP-1R antibody. Immunohistochemistry demonstrated that the GLP-1R is localized to cell bodies and fiber terminals in a very selective distribution throughout the brain. Consistent with the functional role of the GLP-1R system, we find the highest concentration of GLP-1R-immunoreactivity present in select hypothalamic and brainstem regions that regulate feeding, including the paraventricular and arcuate hypothalamic nuclei, as well as the area postrema, nucleus of the solitary tract, and dorsal motor nucleus of the vagus. Together, our data demonstrate that GLP-1R distribution is highly conserved between rodent and primate, although a few key species differences were identified, including the amygdala, where GLP-1R expression is much higher in primate than in rodent.
Glucagon-like peptide-1 (GLP-1), a posttranslational product of the preproglucagon gene, is a hormone released from gut endocrine L-cells upon meal ingestion. GLP-1 plays an important role as an incretin, enhancing glucose-stimulated insulin secretion in response to nutrient ingestion (1, 2). GLP-1 exerts its incretin action through the activation of the GLP-1 receptor (GLP-1R) expressed on pancreatic β-cells. The GLP-1R is a G protein-coupled receptor that predominately couples to a Gαs subunit, leading to the activation of adenylyl cyclase and subsequent accumulation of cAMP (3). GLP-1R agonism is an effective pharmacotherapy for treating type 2 diabetes mellitus (T2DM) in humans (4).
In addition to being expressed in peripheral tissues, preproglucagon and the GLP-1R are expressed in the central nervous system (CNS). Preproglucagon expression in the CNS is restricted to a small group of neurons in the brainstem, namely the caudal nucleus of the solitary tract (NTS) and the ventrolateral medulla (5). These neurons send projections to multiple hypothalamic areas that regulate energy balance, including the arcuate nucleus (ARC), paraventricular nucleus (PVN), and dorsomedial hypothalamus (DMH) (6–9). The expression pattern of preproglucagon neurons in the CNS is highly conserved between rodents and nonhuman primates (NHPs) (Macaca mulatta) (5, 10), but brainstem preproglucagon projections to the ARC are much more dense in the NHP (10) as compared with rodent (6, 7, 9, 11).
The GLP-1R mRNA and protein distribution has been mapped in the rodent brain, using in situ hybridization (ISH) and in situ ligand binding (ISLB), which has demonstrated that the GLP-1R is quite widespread in the CNS; however, the most abundant expression is in brain regions that control energy homeostasis (5, 6, 12–14). As its distribution would suggest, central GLP-1R activation regulates energy metabolism through the suppression of food intake (15–18). In addition to its well-known action on feeding, central GLP-1R signaling regulates many other physiological actions, including gastric emptying (19, 20), hepatic glucose production (21), heart rate (HR) and blood pressure (BP) (22), as well as certain neuroendocrine and behavioral responses to stress (23, 24). Studies in rodents demonstrate that GLP-1R agonists are able to enter into the brain, suggesting that they, when administered peripherally, can cross the blood brain barrier to activate GLP-1Rs in the CNS (25–27). Furthermore, GLP-1 has been demonstrated to bind directly to some of the circumventricular organs that contain the GLP-1R (14, 28, 29).
Although the distribution of the GLP-1R system has been mapped in the rodent, a thorough analysis of the GLP-1R distribution has not been documented in the NHP. It is critical to define the receptor distribution in higher species in order to identify specific brain regions that could be involved in mediating the multitude of actions of CNS GLP-1R signaling. However, a major factor that has limited the ability to clearly define GLP-1R distribution is the lack of reliable antibodies (30, 31). Using a novel GLP-1R monoclonal antibody (monoclonal antibody [MAb] 3F52) (31, 32) in combination with ISH and GLP-1 radioligand binding techniques, we mapped GLP-1R distribution in the NHP brain.
Materials and Methods
Animals
Young adult male Rhesus macaques (M. mulatta) were used. All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC) and Oregon Health and Science University and were approved by the ONPRC Institutional Animal Care and Use Committee. The ONPRC abides by the Animal Welfare Act and Regulations enforced by the United States Department of Agriculture and the Public Health Service Policy on Humane Care and Use of Laboratory Animals in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Tissue collection and preparation
For immunohistochemistry (IHC) (n = 4), animals were euthanized as previously described (10). Brains were perfused with ice-cold 0.9% saline containing 5000-U heparin/L, followed by phosphate-buffered paraformaldehyde (4%; pH7.4). The brains were then blocked into pieces containing the hypothalamus/thalamus/amygdala (AMGD), prefrontal cortex, temporal cortex (containing the hippocampus), parietal cortex, occipital cortex, cerebellum, midbrain, and hindbrain. The tissue blocks were postfixed in 4% paraformaldehyde solution overnight, followed by saturation in a 25% sucrose solution, and then frozen and stored at −80°C. The frozen tissue blocks were cut on a sliding microtome at 25 μm and then stored in cryoprotectant (glycerol/ethylene glycol solution) until used for IHC.
For in ISH (n = 3) and ISLB, unfixed tissue blocks were sectioned in a freezing cryostat into 12-μm-thick sections and then mounted directly onto slides. All solutions and handling for all experiments were under ribonuclease-free conditions.
Antibody characterization
Specific antibody information for all antibodies used in this study is provided in Supplemental Table 1. Specificity of the GLP-1R monoclonal antibody (MAb 3F52) was demonstrated by IHC in baby hamster kidney (BHK) cell lines that stably express the human GLP-1R using untransfected BHK cells as a negative control (31). Transfection of BHK cells with rabbit and mouse GLP-1R displayed no reactivity with MAb 3F52 confirming that this antibody is specific for the primate GLP-1R (32). In addition, extensive validation of MAb 3F52 specificity was performed in peripheral primate tissue (32).
In situ hybridization
ISH was performed using a 33P-RNA antisense probe directed against the human GLP-1R. The antisense probe was generated by in vitro transcription from linearized plasmid DNA containing a human GLP-1R cDNA clone (accession number NM_002062.2, insert from position 136 to 658). The ISH protocol has been previously described in detail (33). Two sections from each area were hybridized with the human GLP-1R probe. After hybridization, 1 section from each area was exposed to an autoradiographic film (Kodak, BIO Max MR for 9 d), whereas the other section was emulsion dipped (K5 Ilford for 21 d). The film and emulsion dipped sections were developed in Kodak D19 developer. The film was digitized using a flatbed scanner with a transparency unit (Epson perfection V700) and Epson's scanner software (Epson scan). The films were scanned as high-resolution images (6400 dpi in 8-bit gray-scale uncompressed TIF images). The scanner software exposure setting was set to maximum exposure and color management and automatic image enhancing features were disabled.
In situ ligand binding
The ISLB protocol for functional GLP-1R localization has previously been described in detail (34, 35). The tracer for these studies, 125I-GLP-1 (7-36) (NEX3080; PerkinElmer), was used at a concentration of 0.3nM, and specificity was assessed by the coincubation with 100nM unlabeled GLP-1.
IHC using nickel-diaminobenzidine
Sections were removed from cryoprotectant and rinsed in potassium phosphate-buffered saline (KPBS) (pH 7.4). Sections were incubated in 1% hydrogen peroxide in KPBS for 30 minutes, rinsed, and then blocked with 3% BSA in KPBS for 60 minutes. Sections were then rinsed, incubated in Avidin Block for 20 minutes (1 drop/mL KPBS; Vector kit SP2001), rinsed, and then incubated in Biotin Block for 20 minutes (1 drop/mL KPBS; Vector kit SP2001). Sections were rinsed, incubated with 2% normal donkey serum in 0.4% Triton X-100/KPBS for 30 minutes, incubated in primary antibody (mouse-anti-GLP-1R [MAb 3F52]; 1 μg/mL in KPBS/Triton X-100 with 2% normal donkey serum) for 1 hour at room temperature, then overnight incubation at 4°C. For visualization of GLP-1R-immunoreactivity (ir), sections were rinsed and then incubated in biotinylated donkey-antimouse antibody (1:600; Jackson catalog 715-066-150) for 60 minutes. Sections were rinsed, incubated in avidin/biotin solution (Vector, catalog PK-6200) for 30 minutes, rinsed, and then incubated with Biotinylated Tyramide (PerkinElmer catalog SAT700; 5 μL/mL in KPBS + 3.3 μL of 3% hydrogen peroxide/mL) for 10 minutes. Sections were then rinsed, incubated in nickel-diaminobenzidine solution (Vector catalog SK-4100) for 30 minutes, rinsed again, mounted onto gel-subbed slides, and left to dry. Sections were dehydrated through a series of increasing concentrations of ethyl alcohol and then xylene and then cover slipped with Permount (Fisher catalog SP15-500). For analysis, the slides were scanned into digital images on an Olympus Slide scanner at ×20 magnification. All acquired images were enhanced for brightness and contrast in Adobe Photoshop 13. Line drawings illustrating the distribution of GLP-1R-ir in the NHP brain were made in Adobe Illustrator 16. The images were imported into Adobe Illustrator and the line-drawing tool was used to accurately trace GLP-1R-ir. For the delineation of nuclei and areas in the NHP brain the atlas by Paxinos et al (36) was used as a reference.
IHC using fluorescence
The same mouse-anti-GLP-1R antibody was used at 1 μg/mL as above and was cocktailed with rabbit antioxytocin (1:8000, Immunostar catalog 20068), rabbit antivasopressin (1:16 000; Immunostar catalog 20069), or rabbit anticocaine and amphetamine-regulated transcript (CART) (1:5000; Phoenix Pharmaceuticals catalog H-003-62). GLP-1R-ir was detected with the same amplification steps as above; however, after the avidin-biotin complex, tissues were incubated in streptavidin-Alexa Fluor 568 (1:1000; Invitrogen catalog S11226) for 2 hours at 37°C. Oxytocin, vasopressin, and CART-ir were detected by incubating for 1 hour at room temperature with donkey antirabbit Alexa Fluor 488 (1:1000; Invitrogen catalog A21206). Images were acquired on a Leica Confocal microscope using a ×20 immersion objective.
Results
Confirmation of GLP-1R antibody specificity in the NHP using ISH and ISLB
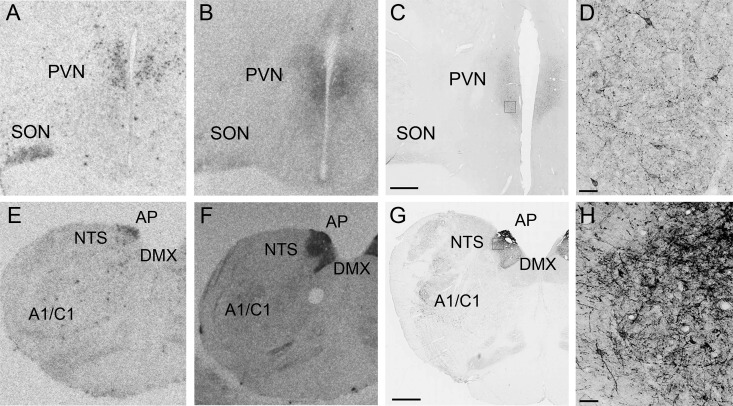
Although the gold standard of antibody validation traditionally is lack of staining in a mouse gene specific knockout model, due to the characteristic of the current antibody (species specific) this technique could not be applied. Therefore, we used multiple neuroanatomical techniques to validate the antibody specificity, including ISLB, ISH, and IHC. The overlap of these techniques is demonstrated in representative areas at 2 different levels of the brain: the PVN and area postrema (AP)/NTS (Figure 1). Strong GLP-1R-ir is demonstrated at the level of the PVN (Figure 1, C and D), which is consistent with the distribution of GLP-1R mRNA (Figure 1A) and ISLB (Figure 1B). The most intense GLP-1R immunostaining was in the brainstem, particularly in the NTS and the AP, where staining of cell bodies and a dense network of fibers was observed (Figure 1, G and H). Figure 1, E and F, represents ISH and ISLB at the level of the AP/NTS. Table 1 contains a summary of brain areas in the NHP brain that contain GLP-1R mRNA (ISH), GLP-1R binding (ISLB), and GLP-1R-ir (IHC). These data are compared with the expression in rats that has been previously published (5, 14, 28). The consistent overlap of GLP-1R mRNA and GLP-1R binding with the GLP-1R-ir strongly supports the specificity of this antibody.
Figure 1. Confirmation of GLP-1R antibody specificity using ISH and ISLB.
GLP-1R distribution using ISH (A and E), ISLB (B and F), and IHC (C and D and G and H) in the hypothalamus at the level of the PVN (A–D) and AP/NTS (E–H). D, High-power magnification of C. H, High-power magnification of G. Scale bars, 1000 μm (C and G) and 50 μm (D and H).
Table 1.
Summary of GLP-1R mRNA, GLP-1 Radioligand Binding, and GLP-1R-ir in the Nonhuman Primate CNS
| Area | Monkey ISH | Monkey ISLB | Monkey IHC (cells) | Monkey IHC (fibers) | Rat ISH | Rat ISLB |
|---|---|---|---|---|---|---|
| Cortex | ||||||
| Prefrontal cortex | − | − | − | − | − | − |
| Parietal cortex | − | − | − | − | − | − |
| Temporal cortex | − | − | − | − | − | − |
| Occipital cortex | − | − | − | − | − | − |
| Forebrain | ||||||
| Hippocampus | + | − | − | − | + | − |
| NAc | ++ | ++ | ++ | ++ | + | ++ |
| Caudate-Putamen | − | − | − | − | + | − |
| Striatum | ++ | ++ | ||||
| Globus pallidus (internal) | + | + | + | + | NA | NA |
| Globus pallidus (external) | − | − | − | − | NA | NA |
| Islands of Calleja | − | +++ | − | − | − | ++ |
| Diagonal Band of Broca | + | + | ++ | ++ | + | ++ |
| Central nucleus of the AMGD | +++ | +++ | ++ | ++++ | − | ++ |
| Medial nucleus of the AMGD | ++ | ++ | + | ++ | − | ++ |
| Bed nucleus of the stria terminalis | ++ | ++ | +++ | +++ | + | + |
| Lateral septum | + | + | ++ | ++ | ++ | +++ |
| Medial septum | + | + | ++ | ++ | + | + |
| Thalamus | ||||||
| Anterodorsal nucleus | − | − | − | − | − | ++ |
| Centromedial nucleus | − | − | − | − | − | ++ |
| Laterodorsal nucleus | − | − | − | − | + | ++ |
| PVN | ++ | + | + | + | ++ | + |
| Posterior nucleus | − | − | − | − | +++ | ++ |
| Zona incerta | − | − | − | − | + | ++ |
| Hypothalamus | ||||||
| POA | + | ++ | ++ | ++ | +++ | + |
| Supraoptic nucleus | +++ | ++ | +++ | +++ | +++ | NA |
| PVN | +++ | +++ | +++ | +++ | ++++ | ++ |
| Lateral hypothalamus | ++ | + | + | + | + | ++ |
| Dorosmedial nucleus | ++ | ++ | ++ | ++ | + | ++ |
| Arcuate nucleus | ++++ | ++++ | ++++ | ++++ | ++++ | +++ |
| Ventromedial nucleus | − | − | − | − | − | + |
| Midbrain | ||||||
| VTA | ++ | ++ | ++ | ++ | ++ | ++ |
| Substantia nigra | + | ++ | ++ | ++ | NA | NA |
| DTg | ++++ | ++++ | ++++ | ++++ | +++ | +++ |
| PAG | + | ++ | + | +++ | +++ | ++ |
| DR | + | ++ | + | ++ | + | ++ |
| Pontine reticular nucleus | + | + | − | ++ | + | ++ |
| Lateral reticular formation | ++ | ++ | ++ | +++ | + | NA |
| LC | + | ++ | + | + | + | NA |
| Cerebellar lobule 9–10 | ++ | ++ | − | +++ | NA | NA |
| Medial cerebellar nuclei | +++ | +++ | +++ | +++ | NA | NA |
| Hindbrain | ||||||
| Parabrachial nucleus | ++ | ++ | ++ | +++ | + | + |
| NTS | ++ | ++++ | ++++ | ++++ | +++ | ++++ |
| AP | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| DMX | + | ++++ | ++ | ++++ | NA | NA |
| A1/C1 area | + | ++ | − | ++ | NA | NA |
| Gi | + | ++ | + | ++ | NA | NA |
| Inferior olivary nucleus | + | ++ | − | + | +++ | +++ |
| Spinal cord | ||||||
| Dorsal horn | − | ++++ | − | ++++ | NA | NA |
Overview of areas in the monkey brain containing GLP-1R mRNA (ISH), GLP-1R binding (ISLB), and GLP-1R-ir (IHC) from low (+) to high (++++) concentrations. To facilitate comparison with the rodent, our data are compared with previously published data in the rat. Rat ISH data from Merchenthaler et al (5). Rat ISLB data from Goke et al (14). NA, data not available.
GLP-1R-ir in the NHP CNS
Cerebral cortex
GLP-1R-ir was assessed in 4 different regions of the cerebral cortex. No evidence of GLP-1R-ir was detected in the parietal, temporal, frontal or occipital cortices (data not shown).
Forebrain
In the forebrain, a large quantity of GLP-1R-ir fibers and cell bodies were detected in the diagonal band of Broca (Figures 2A and 3A), nucleus accumbens shell (Figures 2A and 3B), bed nucleus of the stria terminalis (Figure 2A), and substantia innominata (Figure 2A). In the basal ganglia, a lack of a positive signal was noted in caudate nucleus, putamen, and ventral pallidum (Figure 2A). The preoptic area (POA) (Figures 2B and 3C) also contained a significant number of GLP-1R-ir cell bodies and fibers. A strong GLP-1R immuno-positive signal was detected in the AMGD (Figures 2, B–E, and 3E), where both fiber-like staining and cell body staining was identified in the medial and central nuclei (CeA) of the AMGD. In the hippocampus, we detected very low GLP-1R mRNA expression, which is likely why we failed to detect the presence of GLP-1R-ir (data not shown). The thalamus contained low levels of GLP-1R mRNA, GLP-1 binding, and GLP-1R-ir, primarily in the paraventricular thalamic nucleus (data not shown), which is in contrast to much higher levels that have been reported in the rodent (5, 14). GLP-1R-ir in the supraoptic nucleus was less pronounced and mostly fibers were detected (Figure 2, B and C). Several hypothalamic nuclei expressed high levels of GLP-1R-ir. The strongest staining was observed in the PVN (Figures 1, C and D, and 3C), the DMH (Figures 2D and 3D), and the ARC (Figures 2, E and F, and 3F). The hypothalamic nucleus containing the most dense IHC signal was the ARC, which contained both small diameter neurons and a dense fiber plexus that spanned the entire rostrocaudal extent of the nucleus. The magnocellular nucleus of the lateral hypothalamus (Figure 2F) also contained strong GLP-1R labeling. The ventromedial hypothalamic nucleus was devoid of GLP-1R-ir (data not shown).
Figure 2. Schematic representation of GLP-1R-ir distribution in the NHP forebrain.
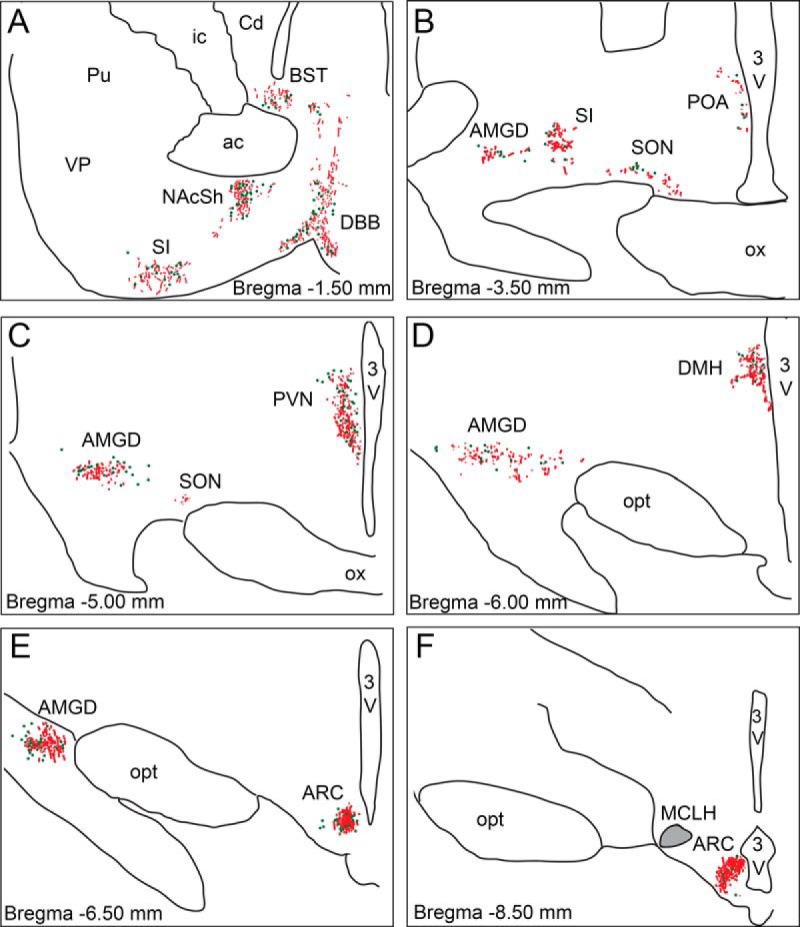
Depiction of GLP-1R-ir cell bodies (green dots) and GLP-1R-ir fibers (red lines) in forebrain sections arranged from rostral (A) to caudal (F). Gray shaded area in the magnocellular nucleus of the lateral hypothalamus (MCLH) indicates dense fiber network. 3V, third ventricle; ac, anterior commissure; BST, bed nucleus of the stria terminalis; Cd, caudate; DBB, diagonal band of Broca; DMH, dorsomedial nucleus of the hypothalamus; ic, internal capsule; NAcSh, nucleus accumbens shell; opt, optic tract; ox, optic chiasm; Pu, putamen; SI, substantia innominata; SON, supraoptic nucleus; VP, ventral pallidum.
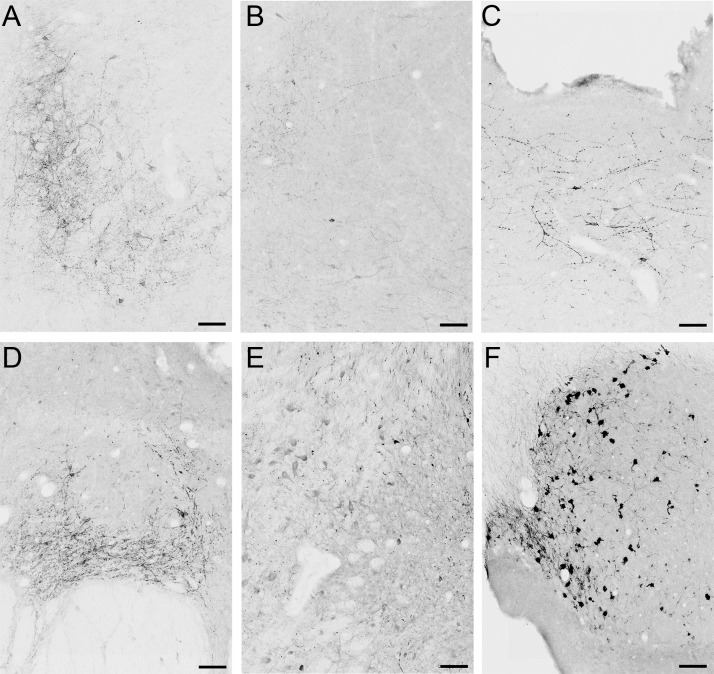
Figure 3. Photomicrographs of GLP-1R-ir in the NHP forebrain.
GLP-1R-ir was detected in the diagonal band of Broca (DBB) (A), nucleus accumbens shell (NAcSh) (B), POA (C), DMH (D), AMGD (E, image at the level of the ARC), and ARC (F). 3V, third ventricle; DMH, dorsomedial nucleus of the hypothalamus. Scale bars, 100 μm.
To determine which neuronal cell types express the GLP-1R in the PVN, one of the main nuclei involved in mediating the effects of intracerebroventricularly injected GLP-1 on homeostatic feeding, we applied double-labeled immunofluorescence with a number of anorexigenic neuropeptides. Sections containing the PVN were labeled with GLP-1R and oxytocin, CART, or arginine vasopressin (AVP). We failed to detect GLP-1R colocalization with oxytocin-ir (Figure 4A) or AVP-ir cell bodies (Figure 4B). We determined that CART-ir is abundant in the PVN around GLP-1R-ir cells, but we did not detect any colocalization of CART-ir fibers with GLP-1R-ir (Figure 4, C and D).
Figure 4. Distribution of GLP-1R immunofluorescence with anorexogenic neuronal populations in the NHP PVN.
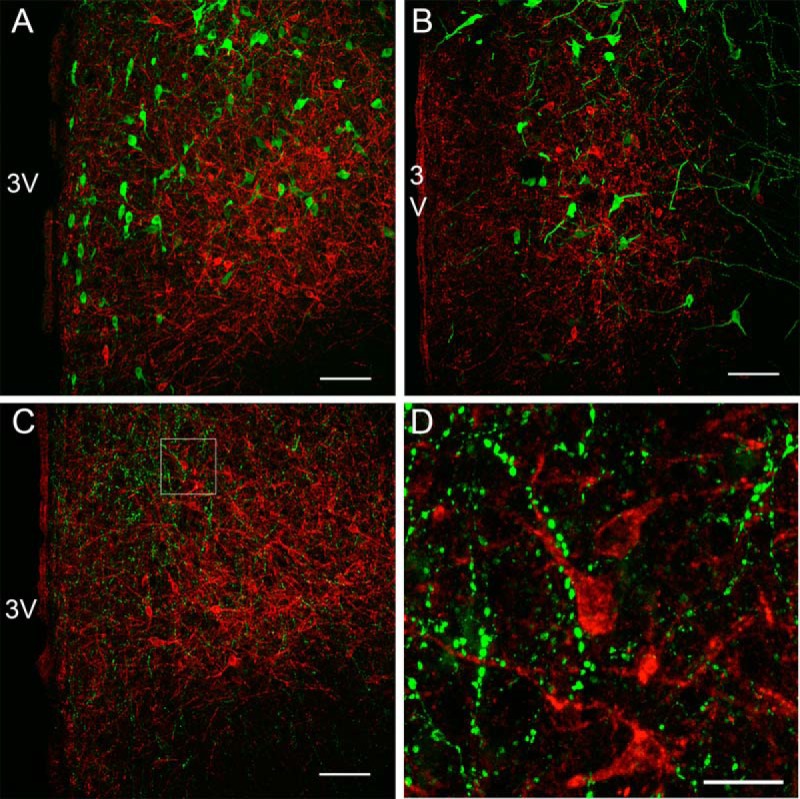
Confocal microscopic images showing GLP-1R-ir (red) with oxytocin-ir (A; green), AVP-ir (B; green) or CART-ir (C; green). D, High-power magnification of C. 3V, third ventricle. Scale bars, 100 μm (A–C) 25 μm (D).
Midbrain
In the midbrain, IHC in the ventral tegmental area (VTA) revealed staining of both cell bodies and fiber-like structures (Figure 5A). The microcellular tegmental nucleus contained both GLP-1R-positive cells and fibers (Figures 5A and 6A). Moderate staining was observed in the periaqueductal gray (PAG) (Figures 5, A and B, and 6B). In both the PAG and in the underlying dorsal raphe (DR) (Figures 5, A and B, and 6C) most staining was of fiber-like structures. The dorsal tegmental nucleus (DTg) was also intensely labeled using the monoclonal GLP-1R antibody (Figures 5C and 6D) and contained numerous heavily labeled cell bodies and fiber-like structures. The lateral parabrachial nucleus (LPBN) at this level primarily contained GLP-1R immuno-positive fibers (Figure 5C). The locus coeruleus (LC) contained both GLP-1R-positive fibers and cells (Figures 5D and 6E). Fiber-like staining was present in the pontine reticular nucleus (Figure 5C). GLP-1R-ir in the cerebellum was detected in the molecular layer of the ninth and 10th lobules and appeared to be confined to cell bodies (Figures 5D and 6F).
Figure 5. Schematic representation of GLP-1R-ir distribution in the NHP midbrain.
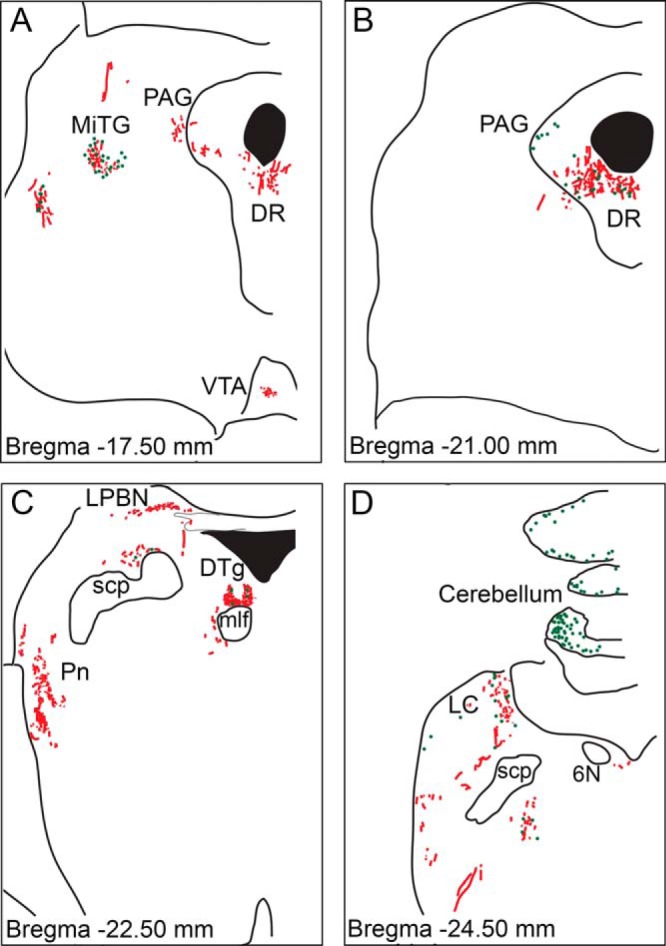
Depiction of GLP-1R-ir cell bodies (green dots) and GLP-1R-ir fibers (red lines) in midbrain sections arranged from rostral (A) to caudal (D). 6N, abducens nucleus; MiTG, microcellular tegmental nucleus; mlf, medial longitudinal fasciculus; Pn, pontine reticular nucleus; scp, superior cerebellar peduncle.
Figure 6. Photomicrographs of GLP-1R-ir in the NHP midbrain.
GLP-1R-ir was detected in the microcellular tegmental nucleus (MiTG) (A), PAG (B), DR (C), DTg (D), LC (E), and cerebellum (F). Scale bars, 100 μm.
Hindbrain
In the caudal brainstem, the A1/C1 area contained GLP-1R-ir fibers and cell bodies (Figures 1G and 7, A–E). The LPBN at this level contained substantial GLP-1R-positive cells and fibers (Figure 7, A and F). The most intense staining in the brainstem was observed in the NTS and the AP (Figures 1, G and H, and 7, B–D). The staining in this region included staining of cell bodies and a dense network of fibers. The dorsal motor nucleus of the vagus (DMX) also contained a significant number of GLP-1R-ir fibers (Figures 1G and 7, B and C). In the gigantocellular nucleus (Gi) (Figure 7, A, B, and G) and dorsal inferior olivary nucleus (Figure 7, A–C), most of the staining was fiber-like structures.
Figure 7. Schematic representation and photomicrographs of GLP-1R-ir distribution in the NHP hindbrain.
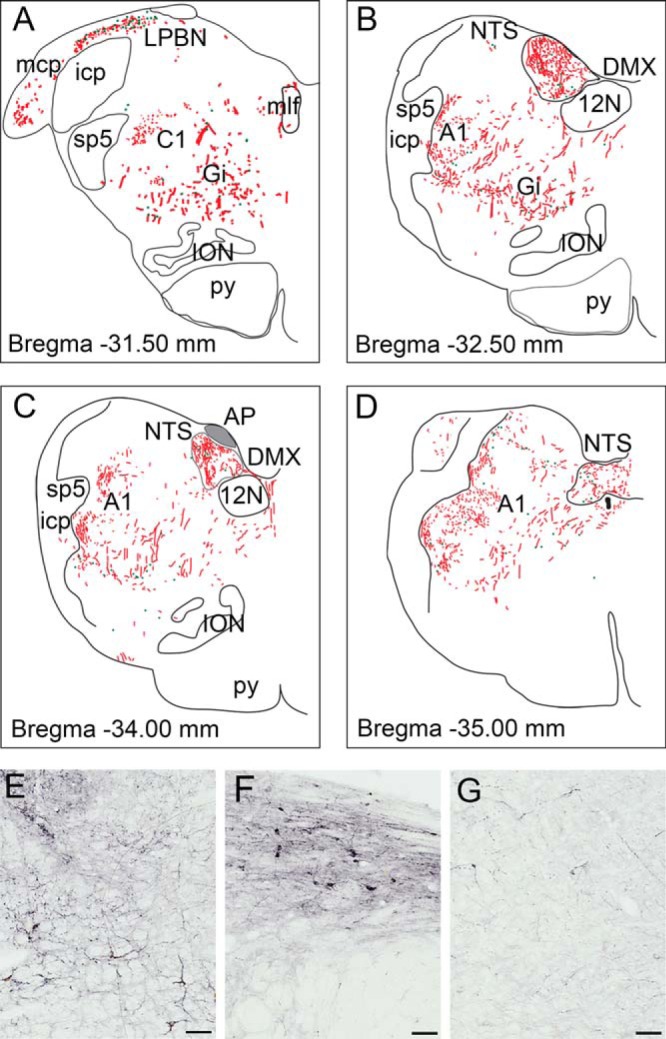
Depiction of GLP-1R-ir cell bodies (green dots) and GLP-1R-ir fibers (red lines) in hindbrain sections arranged from rostral (A) to caudal (D). Gray shaded area in the AP indicates dense fiber network. Photomicrographs of GLP-1R-ir are shown in the A1 area (E), LPBN (F), and Gi (G). 12N, hypoglossal nucleus; A1, noradrenergic cell group; C1, adrenergic cell group; icp, inferior cerebellar peduncle; ION, inferior olivary nucleus; mcp, middle cerebellar peduncle; mlf, medial longitudinal fasciculus; py, pyramidal tract; sp5, spinal trigeminal tract. Scale bars, 100 μm.
Discussion
These data provide the first detailed mapping of the GLP-1R in the entire NHP primate brain using a recently characterized monoclonal GLP-1R antibody (32). Controversy over acceptable GLP-1R antibodies has arisen (30, 31). However, our novel GLP-1 antibody is specific and detects only GLP-1R protein in the NHP and human (32). The specificity of the antibody used is supported in the current study by the selective overlap between GLP-1R mRNA, GLP-1R binding sites and immunoreactivity. Furthermore, the location of the GLP-1R that we have identified in this study is consistent with the location of GLP-positive fibers that have previously been described in this species (10). Together, these data indicate that a large number of CNS GLP-1Rs are targets of preproglucagon-expressing neurons that originate in the NTS. These data highlight that GLP-1R distribution in the NHP brain is similar to that of the rodent with the most abundant expression in hypothalamic areas that control feeding, including the PVN, DMH, and ARC. One interesting species mismatch that we identified was in the amygdaloid body where a much higher level of GLP-1R-ir and mRNA was noted in the NHP as compared with the rodent. Another difference was in the hippocampus where we failed to detect GLP-1Rs in the NHP, whereas GLP-1R expression has been reported in the rodent (5). An interesting point to note is that some areas of the brain contain abundant fibers and ligand binding but low levels of mRNA expression and immunoreactive cell bodies, which is particularly prominent in the dorsal horn (Table 1). These data suggest that GLP-1 may not only be acting directly on cell bodies to regulate neuronal function, but may be acting on dendrites or axon terminals to regulate synaptic inputs or neuropeptide release from axons. Further analysis of whether these fibers are dendrites or axons will be required to elucidate this action of GLP-1R signaling.
Functional considerations for GLP-1R signaling in mesolimbic areas
The presence of GLP-1R-ir and binding in mesolimbic areas, including the nucleus accumbens (NAc) and VTA, suggests that GLP-1R signaling may play a role in reward and addictive behavior in the NHP. Studies in rats support a role for GLP-1R action in the regulation of food preference as peripheral administration of the GLP-1 analog, liraglutide, decreased preference for highly palatable foods (37, 38). Moreover, direct injection of the GLP-1 analog, exendin-4, into the NAc or VTA reduces food reward without causing visceral illness (39, 40). Exendin-4 was also shown to reduce alcohol consumption in mice (41) and rats (42), which was likely due to a reduction in dopamine release from the NAc (41). Studies in humans investigating the effects of GLP-1 analogs on reward and addictive behavior are limited. A Japanese population of T2DM patients treated chronically with liraglutide had a decreased preference for fat consumption (43), suggesting that GLP-1R agonism decreases the rewarding value of highly palatable foods. Interestingly, a study comparing gastric bypass surgery with gastric banding showed that gastric bypass patients, who have increased GLP-1 levels, report a change in food preference and decreased their consumption of candy, cookies, and chocolate while increasing their consumption of fruits and vegetables (44). To further support a role for GLP-1R signaling in regulating food reward, recent functional magnetic resonance imaging (fMRI) studies in obese and obese T2DM patients have demonstrated that iv infusion of exenatide decreases the activation of brain regions involved in mediating food reward (45). Our current data in the NHP provide neuroanatomical evidence that GLP-1Rs are present in mesolimbic structures involved in mediating food reward and, therefore, strengthen this novel role of GLP-1R signaling. However, more extensive behavioral studies are necessary to fully understand the role of the GLP-1 system in regulating reward-related behavior in higher species.
In this study, we find a limited number of hippocampal cells that express GLP-1R mRNA and were unable to detect any binding or GLP-1R-ir in the NHP hippocampus, which is in contrast with the rodent where much greater expression has been reported (5, 46). Many studies suggest that activation of GLP-1R signaling enhances learning and memory in rodents (47–52). Furthermore, studies in rodents have demonstrated that GLP-1R agonists have neuroprotective properties in hippocampal cells (53) and can reduce the development of amyloid-β plaques, a common characteristic of Alzheimer's disease (52). Whether this is a direct action on hippocampal cells or whether these improvements are secondary to improvements in inflammation, glycemic control or insulin signaling in the brain warrants further clarification. Together, rodent data in the literature suggest that GLP-1R agonists are promising pharmacotherapeutics for diseases associated with cognitive dysfunction and neurodegeneration of hippocampal cells. However, we did not detect the presence of GLP-1R-ir or GLP-1R binding in the hippocampus of the NHP, and therefore, our data suggest that GLP-1R signaling may not play as prominent of a role in memory formation or hippocampal neuroprotection in higher species. GLP-1R agonists may act indirectly to prevent hippocampal cell degeneration in higher species by improving glucose homeostasis and enhancing weight loss, which could lead to a reduction in glucotoxicity in the hippocampus. Clinical studies that are currently underway (54) as well as studies using positron emission tomography and MRI neuroimaging techniques to evaluate the effects of GLP-1 therapeutics on the progression of neurodegenerative diseases (reviewed in Ref. 55) will clarify whether GLP-1R agonists improve learning and memory in humans with diseases such as Alzheimer's.
One of the key differences that our data reveals is that there is a higher expression of GLP-1R in the AMGD of the NHP compared with the rodent. This may indicate an important species-specific difference in the functional activity of GLP-1R signaling in this brain region. The CeA is an area that is involved in regulating learning and memory associated with conditioned taste aversion (56, 57), and it has been shown that injection of GLP-1 directly into the CeA of rats results in conditioned taste aversion (58). GLP-1R agonists have shown promising effects on weight reduction in humans that is mainly a result of a decrease in food intake (59; and reviewed in Refs. 60, 61). It has been suggested that the decreased food intake and subsequent weight reduction is partially due to increased nausea from GLP-1R analog therapeutics (62, 63). However, studies with liraglutide have not consistently confirmed this. An analysis across 7 large trials of diabetic patients treated with GLP-1R agonists only showed a minor difference in body weight loss between patients reporting nausea compared with patients that do not report nausea which indicates that nausea is not the sole cause for weight loss induced by GLP-1R agonists (64). In obese subjects treated with an investigational dose of liraglutide (3.0 mg) for 52 weeks, those who reported at least one episode of nausea/vomiting, lost significantly more weight compared with those who did not report nausea/vomiting (9.2 vs 6.3 kg, 1-year treatment); however, both groups lost significantly more weight than placebo treated controls (65). Those subjects who experience visceral illness from liraglutide report that the gastrointestinal side effects are usually mild or moderate and transient in nature, occurring mainly in the first 1–6 weeks of treatment during dose escalation to the maintenance dose (65). It is unclear why a subpopulation of patients has a higher susceptibility to nausea induced by GLP-1R agonists and why this effect is transient. Further characterization of the GLP-1R-expressing neuronal populations in the CeA may help us to distinguish neural circuits involved in mediating GLP-1 induction of visceral illness vs neural circuits that mediate homeostatic feeding. This would provide crucial data for the development of novel therapeutics that can promote weight loss without the risk of inducing nausea.
The AMGD is critically involved in coordinating behavioral responses to stressors, and interestingly, GLP-1R signaling has been shown to play an important role in regulating anxiety-like behavior in response to psychogenic stressors in rodents (reviewed in Ref. 66). Our data indicate that the AMGD contains a substantially larger amount of GLP-1R in the NHP, which may suggest that GLP-1R signaling in higher species plays a more prominent role in the pathophysiology of psychological disorders involving amygdalar dysfunction. A limited number of studies have tested the psychological effects of GLP-1 in higher species. Intravenous infusion of GLP-1 did not induce panic attacks in patients with panic disorder, suggesting that GLP-1 does not increase the risk of panic episodes in patients at high risk for these reactions (67). Another study compared the effects of insulin vs the GLP-1 agonist, exenatide on quality of life in T2DM patients. Compared with insulin, chronic treatment with exenatide led to improved psychological parameters, which was independent of changes in body mass index (68). Currently, the functional role of GLP-1R signaling in regulating psychological function is unclear. The dense population on GLP-1R in the AMGD that we report in the NHP highlights a species-specific difference between the NHP and rodent. Therefore, further investigation of the effects of GLP-1R agonists on psychological stress in larger patient populations is warranted.
Functional considerations for GLP-1R signaling in the hypothalamus
We describe GLP-1R distribution in the NHP throughout key hypothalamic areas that control feeding, including the ARC, PVN, DMH, and lateral hypothalamic areas. This receptor distribution is consistent with the functional role of GLP-1 on food intake regulation in rodents, primates and humans (reviewed in Ref. 69). In rodents, injection of GLP-1 directly into the PVN causes a reduction in food intake (21, 70). In order to understand which neuronal populations are involved in mediating the anorexigenic action of GLP-1 in the PVN, we investigated the colocalization of GLP-1R-ir with neuropeptides that inhibit feeding. In the rat, GLP-1-ir fibers form close apposition with oxytocin neurons in the PVN (71). Moreover, pharmacological blockade of GLP-1Rs ablates the anorexigenic action of oxytocin (72). Together these data suggest that oxytocin may mediate the effects GLP-1 on feeding. However, we did not detect GLP-1R colocalization with oxytocin neurons, suggesting that GLP-1 does not act directly on oxytocin neurons in the PVN of the NHP. AVP has been implicated to regulate feeding and acute activation of AVP neurons in the PVN of mice causes an inhibition of feeding (73). Again, we did not detect colocalization of GLP-1R-ir cell bodies with AVP-ir cell bodies, and therefore, AVP in the PVN may not be a direct mediator of the anorectic action of GLP-1R activation in the NHP. CART is another anorectic neuropeptide with abundant nerve terminals in the PVN (74). Furthermore, intracerebroventricular administration of a GLP-1R antagonist blocks the ability of CART to inhibit food intake (75), suggesting that GLP-1R signaling regulates CART activation. We hypothesized that CART-ir terminals would come in close contact with GLP-1R-ir cells in the PVN of the NHP, which would provide a neuroanatomical evidence for GLP-1R activation to cause a suppression of feeding. We do find an abundance of CART-ir fibers in the PVN around GLP-1R-ir cells, but we failed to detect colocalization of CART-ir fibers with GLP-1R-ir neurons. Based on these data, it is unclear whether or not GLP-1R signaling regulates CART action in the PVN of the NHP. In the future, more thorough colocalization studies in other areas of the brain, especially the ARC where CART and GLP-1R cell bodies are abundant, will give us a better understanding of CART and GLP-1R interactions. Corticotropin-releasing hormone (CRH) causes a significant reduction in food intake (76), and activation of CRH neurons may be responsible for mediating the anorexigenic action of GLP-1 in rodents (23). Unfortunately, we were not able to characterize GLP-1R-ir on CRH neurons in these studies due to technical difficulties with the lack of CRH accumulation within cell bodies.
In addition to regulating feeding, a large body of literature demonstrates that GLP-1R signaling in the PVN is involved in mediating the neuroendocrine responses to stress in rodent models (reviewed in Ref. 66). In humans, iv administration of GLP-1 to healthy subjects as well as type 1 diabetic patients increases plasma cortisol levels indicating that GLP-1R signaling regulates hypothalamic-pituitary-adrenal function in higher species (77). We demonstrate that the PVN of the NHP is densely populated with GLP-1R-ir cell bodies and fibers. Further characterization of the phenotype of GLP-1R-ir neurons in the PVN could give us a better understanding of which neurons regulate GLP-1 modulation of hypothalamic-pituitary-adrenal function.
The ARC has been implicated in regulating the anorectic action of GLP-1 as chemical lesion of the ARC in rats ablates the inhibitory action of GLP-1 on feeding (78). Activation of proopiomelanocortin (POMC) neurons in the ARC causes a suppression of feeding, and interestingly, colocalization of GLP-1R and POMC neurons has been demonstrated in the ARC of rats (21). Moreover, electrophysiological evidence demonstrates that GLP-1 directly stimulates ARC POMC neurons in rodents (29). Taken as a whole, much data supports the involvement of ARC POMC neurons in mediating the reduction in food intake induced by GLP-1. We attempted to determine GLP-1R colocalization with POMC neurons of the NHP. However, due to the high background signal of the GLP-1R antibody in the ARC we were unable to distinguish GLP-1R-ir cell bodies using immunofluorescence. As data in the literature highlight that ARC POMC neurons are critical mediators of GLP-1R actions in rodents (79, 80), future studies are needed to reveal GLP-1R/POMC interactions in the ARC of the NHP.
Functional considerations for GLP-1R signaling in the brainstem
We have identified dense GLP-1R mRNA expression, binding and protein in brainstem regions that regulate feeding, including the AP, NTS, and DMX. The GLP-1Rs in the AP and underlying the NTS can be activated by peripherally administered GLP-1 (28). Therefore, it is possible that these receptors are also involved in mediating the suppression of food intake of peripherally administered GLP-1 agonists. We have not identified the neuronal phenotypes of hindbrain GLP-1R-expressing neurons, but most likely they are not found on the preproglucagon-expressing (GLP-1 containing) neurons. At least, it has been shown in rodents that the GLP-1-containing neurons do not respond to GLP-1 nor do they express the GLP-1R (81).
In addition to food intake regulation, the brainstem is also involved in regulating the autonomic response to stress. GLP-1 causes an acute increase in HR and BP, and this effect is blocked with central administration of a GLP-1R antagonist as well as with vagotomy in rodents (82). In the monkey brain, we identified GLP-1R expression in a number of brainstem areas involved in controlling cardiovascular function, including the AP, NTS, and DMX. In humans, acute peripheral administration of GLP-1 produces a much smaller effect on HR and BP as compared with rodents (83–85). A recent report highlights species-specific differences of GLP-1 action on cardiovascular function (86). Although our current data in the NHP indicate that there is a similar pattern of expression of brainstem GLP-1Rs in areas that control cardiovascular function, it is unclear as to why the cardiovascular effects of GLP-1 do not directly translate from rodents to higher species. Further characterization of neuronal phenotypes that express the GLP-1R in the brainstem may reveal species-specific differences and would enable us to elucidate the underlying mechanism for the disparate functional role of GLP-1R action on cardiovascular function in higher species. The GLP-1R is also expressed in the heart of rodents (87) as well as the NHP and humans (32), and differential action of GLP-1 directly on cardiac tissue may also contribute to species differences. Taken together, future studies are necessary to reveal the mechanism responsible for mediating species-specific differences in GLP-1 action on cardiovascular function.
Conclusion
In summary, we employed 3 different techniques to map the GLP-1R in the CNS of the NHP. GLP-1R distribution is highly conserved when compared with rodents with key differences in the AMGD, hippocampus and thalamus. These species-specific differences highlight possible differences in the functional role of CNS GLP-1Rs in these brain regions. Therefore, interpretation of rodent studies involving these brain regions and their translation to effects in higher species should be made with careful consideration. A further analysis of the phenotype of the neuronal populations that contain GLP-1Rs that we have identified is necessary to give us a more complete understanding of the plethora of GLP-1R mediated actions in the CNS. Identifying the phenotype of these neurons can aid in the development of therapeutics that target desired GLP-1R-expressing neuronal populations and avoid activating GLP-1R in other regions which may ultimately limit unwanted side effects of GLP-1-based therapies.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Grant P51 OD011092 (to K.L.G. and Oregon National Primate Research Center), the NIH Grant RC4 DK090956 (to K.L.G.), and the Novo Nordisk Grant SRA-11-061 (to K.L.G.).
Disclosure Summary: K.L.G. is a member of the Novo Nordisk SAB. C.P., A.S., and L.B.K. are full time employees of Novo Nordisk, who markets liraglutide for the treatment of T2DM. K.M.H., M.K., S.J.P., R.B., and N.V. have nothing to disclose.
Footnotes
- AMGD
- amygdala
- AP
- area postrema
- ARC
- arcuate nucleus
- AVP
- arginine vasopressin
- BHK
- baby hamster kidney
- BP
- blood pressure
- CART
- cocaine and amphetamine-regulated transcript
- CeA
- central nucleus of the AMGD
- CNS
- central nervous system
- CRH
- corticotropin-releasing hormone
- DMH
- dorsomedial hypothalamus
- DMX
- dorsal motor nucleus of the vagus
- DR
- dorsal raphe
- DTg
- dorsal tegmental nucleus
- Gi
- gigantocellular nucleus
- GLP-1
- glucagon-like peptide-1
- GLP-1R
- GLP-1 receptor
- HR
- heart rate
- IHC
- immunohistochemistry
- ir
- immunoreactivity
- ISLB
- in situ ligand binding
- ISH
- in situ hybridization
- KPBS
- potassium phosphate-buffered saline
- LC
- locus coeruleus
- LPBN
- lateral parabrachial nucleus
- MAb 3F52
- monoclonal antibody 3F52
- NAc
- nucleus accumbens
- NHP
- nonhuman primate
- NTS
- nucleus of the solitary tract
- ONPRC
- Oregon National Primate Research Center
- PAG
- periaqueductal gray
- POA
- preoptic area
- POMC
- proopiomelanocortin
- PVN
- paraventricular nucleus
- T2DM
- type 2 diabetes mellitus
- VTA
- ventral tegmental area.
References
- 1. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304. [DOI] [PubMed] [Google Scholar]
- 2. Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79(2):616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Willard FS, Sloop KW. Physiology and emerging biochemistry of the glucagon-like peptide-1 receptor. Exp Diabetes Res. 2012;2012:470851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards KL, Stapleton M, Weis J, Irons BK. An update in incretin-based therapy: a focus on glucagon-like peptide-1 receptor agonists. Diabetes Technol Ther. 2012;14(10):951–967. [DOI] [PubMed] [Google Scholar]
- 5. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280. [DOI] [PubMed] [Google Scholar]
- 6. Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271(4):519–532. [DOI] [PubMed] [Google Scholar]
- 7. Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77(1):257–270. [DOI] [PubMed] [Google Scholar]
- 8. Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118–126. [DOI] [PubMed] [Google Scholar]
- 9. Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS. Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol. 2013;521(10):2235–2261. [DOI] [PubMed] [Google Scholar]
- 10. Vrang N, Grove K. The brainstem preproglucagon system in a non-human primate (Macaca mulatta). Brain Res. 2011;1397:28–37. [DOI] [PubMed] [Google Scholar]
- 11. Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137(11):5159–5162. [DOI] [PubMed] [Google Scholar]
- 13. Shimizu I, Hirota M, Ohboshi C, Shima K. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology. 1987;121(3):1076–1082. [DOI] [PubMed] [Google Scholar]
- 14. Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7(11):2294–2300. [DOI] [PubMed] [Google Scholar]
- 15. Turton MD, O'Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. [DOI] [PubMed] [Google Scholar]
- 16. Tang-Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271(4 pt 2):R848–R856. [DOI] [PubMed] [Google Scholar]
- 17. Meeran K, O'Shea D, Edwards CM, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology. 1999;140(1):244–250. [DOI] [PubMed] [Google Scholar]
- 18. Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31(10):3904–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273(4 pt 1):G920–G927. [DOI] [PubMed] [Google Scholar]
- 20. Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006;1111(1):117–121. [DOI] [PubMed] [Google Scholar]
- 21. Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57(8):2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138(10):4445–4455. [DOI] [PubMed] [Google Scholar]
- 24. Kinzig KP, D'Alessio DA, Herman JP, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23(15):6163–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152(8):3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27(3):313–318. [DOI] [PubMed] [Google Scholar]
- 28. Orskov C, Poulsen SS, Møller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes. 1996;45(6):832–835. [DOI] [PubMed] [Google Scholar]
- 29. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Panjwani N, Mulvihill EE, Longuet C, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(−/−) mice. Endocrinology. 2013;154(1):127–139. [DOI] [PubMed] [Google Scholar]
- 31. Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor–or not? Endocrinology. 2013;154(1):4–8. [DOI] [PubMed] [Google Scholar]
- 32. Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue; novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014:155(4):1280–1290. [DOI] [PubMed] [Google Scholar]
- 33. Paulsen SJ, Christensen MT, Vrang N, Larsen LK. The putative neuropeptide TAFA5 is expressed in the hypothalamic paraventricular nucleus and is regulated by dehydration. Brain Res. 2008;1199:1–9. [DOI] [PubMed] [Google Scholar]
- 34. Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48(5):736–743. [DOI] [PubMed] [Google Scholar]
- 35. Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151(4):1473–1486. [DOI] [PubMed] [Google Scholar]
- 36. Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Sterotaxic Coordinates. London, UK: Academic Press; 2000. [Google Scholar]
- 37. Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56(1):8–15. [DOI] [PubMed] [Google Scholar]
- 38. Hansen G, Jelsing J, Vrang N. Effects of liraglutide and sibutramine on food intake, palatability, body weight and glucose tolerance in the gubra DIO-rats. Acta Pharmacol Sin. 2012;33(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32(14):4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31(41):14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013;38(8):1259–1270. [DOI] [PubMed] [Google Scholar]
- 42. Shirazi RH, Dickson SL, Skibicka KP. Gut peptide GLP-1 and its analogue, exendin-4, decrease alcohol intake and reward. PLoS One. 2013;8(4):e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inoue K, Maeda N, Kashine S, et al. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olbers T, Björkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244(5):715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Bloemendaal L, RG IJ, Ten Kulve JS, et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63(12):4186–4196. [DOI] [PubMed] [Google Scholar]
- 46. Alvarez E, Roncero I, Chowen JA, Thorens B, Blázquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996;66(3):920–927. [DOI] [PubMed] [Google Scholar]
- 47. During MJ, Cao L, Zuzga DS, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173–1179. [DOI] [PubMed] [Google Scholar]
- 48. Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: interaction between type 2 diabetes and Alzheimer's disease. Behav Brain Res. 2009;205(1):265–271. [DOI] [PubMed] [Google Scholar]
- 49. Oka JI, Goto N, Kameyama T. Glucagon-like peptide-1 modulates neuronal activity in the rat's hippocampus. Neuroreport. 1999;10(8):1643–1646. [DOI] [PubMed] [Google Scholar]
- 50. McClean PL, Gault VA, Harriott P, Holscher C. Glucagon-like peptide-1 analogues enhance synaptic plasticity in the brain: a link between diabetes and Alzheimer's disease. Eur J Pharmacol. 2010;630(1–3):158–162. [DOI] [PubMed] [Google Scholar]
- 51. Han WN, Hölscher C, Yuan L, et al. Liraglutide protects against amyloid-β protein-induced impairment of spatial learning and memory in rats. Neurobiol Aging. 2013;34(2):576–588. [DOI] [PubMed] [Google Scholar]
- 52. McClean PL, Parthsarathy V, Faivre E, Hölscher C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci. 2011;31(17):6587–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McIntyre RS, Powell AM, Kaidanovich-Beilin O, et al. The neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res. 2013;237:164–171. [DOI] [PubMed] [Google Scholar]
- 54. Egefjord L, Gejl M, Moller A, et al. Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimer s disease - protocol for a controlled, randomized double-blinded trial. Dan Med J. 2012;59(10):A4519. [PubMed] [Google Scholar]
- 55. Femminella GD, Edison P. Evaluation of neuroprotective effect of glucagon-like peptide 1 analogs using neuroimaging. Alzheimers Dement. 2014;10(1 suppl):S55–S61. [DOI] [PubMed] [Google Scholar]
- 56. Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci. 1997;17(21):8443–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lasiter PS, Glanzman DL. Cortical substrates of taste aversion learning: involvement of dorsolateral amygdaloid nuclei and temporal neocortex in taste aversion learning. Behav Neurosci. 1985;99(2):257–276. [DOI] [PubMed] [Google Scholar]
- 58. Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22(23):10470–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(6):784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tong J, Sandoval DA. Is the GLP-1 system a viable therapeutic target for weight reduction? Rev Endocr Metab Disord. 2011;12(3):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Campbell RK. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clin Ther. 2011;33(5):511–527. [DOI] [PubMed] [Google Scholar]
- 62. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. [DOI] [PubMed] [Google Scholar]
- 63. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. [DOI] [PubMed] [Google Scholar]
- 64. Davidson JA. Incretin-based therapies: focus on effects beyond glycemic control alone. Diabetes Ther. 2013;4(2):221–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lean ME, Carraro R, Finer N, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(5):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Strawn JR, D'Alessio DA, Keck PE, Jr, Seeley RJ. Failure of glucagon-like peptide-1 to induce panic attacks or anxiety in patients with panic disorder. J Psychiatr Res. 2008;42(9):787–789. [DOI] [PubMed] [Google Scholar]
- 68. Grant P, Lipscomb D, Quin J. Psychological and quality of life changes in patients using GLP-1 analogues. J Diabetes Complications. 2011;25(4):244–246. [DOI] [PubMed] [Google Scholar]
- 69. Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7(9):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998;274(1 pt 2):R23–R29. [DOI] [PubMed] [Google Scholar]
- 71. Tauchi M, Zhang R, D'Alessio DA, Stern JE, Herman JP. Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat. 2008;36(3–4):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R99–R106. [DOI] [PubMed] [Google Scholar]
- 73. Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol Metab. 2014;3(2):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–76. [DOI] [PubMed] [Google Scholar]
- 75. Aja S, Ewing C, Lin J, Hyun J, Moran TH. Blockade of central GLP-1 receptors prevents CART-induced hypophagia and brain c-Fos expression. Peptides. 2006;27(1):157–164. [DOI] [PubMed] [Google Scholar]
- 76. Arase K, York DA, Shimizu H, Shargill N, Bray GA. Effects of corticotropin-releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am J Physiol. 1988;255(3 pt 1):E255–E259. [DOI] [PubMed] [Google Scholar]
- 77. Gil-Lozano M, Pérez-Tilve D, Alvarez-Crespo M, et al. GLP-1(7-36)-amide and exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology. 2010;151(6):2629–2640. [DOI] [PubMed] [Google Scholar]
- 78. Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide 1(7-36) amide's central inhibition of feeding and peripheral inhibition of drinking are abolished by neonatal monosodium glutamate treatment. Diabetes. 1998;47(4):530–537. [DOI] [PubMed] [Google Scholar]
- 79. Dalvi PS, Nazarians-Armavil A, Purser MJ, Belsham DD. Glucagon-like peptide-1 receptor agonist, exendin-4, regulates feeding-associated neuropeptides in hypothalamic neurons in vivo and in vitro. Endocrinology. 2012;153(5):2208–2222. [DOI] [PubMed] [Google Scholar]
- 80. Seo S, Ju S, Chung H, Lee D, Park S. Acute effects of glucagon-like peptide-1 on hypothalamic neuropeptide and AMP activated kinase expression in fasted rats. Endocr J. 2008;55(5):867–874. [DOI] [PubMed] [Google Scholar]
- 81. Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like peptide 1 neurons. Diabetes. 2010;59(8):1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277(5 pt 1):E784–E791. [DOI] [PubMed] [Google Scholar]
- 83. Gill A, Hoogwerf BJ, Burger J, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bharucha AE, Charkoudian N, Andrews CN, et al. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R874–R880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vilsboll T. Liraglutide: a new treatment for type 2 diabetes. Drugs Today (Barc). 2009;45(2):101–113. [DOI] [PubMed] [Google Scholar]
- 86. Goodwill AG, Mather KJ, Conteh AM, Sassoon DJ, Noblet JN, Tune JD. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev Endocr Metab Disord. 2014;15(3):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19(5):567–575. [DOI] [PubMed] [Google Scholar]