Abstract
Phenotypic diversity may play an adaptive role by providing graded biological responses to fluctuations in environmental stimuli. We used single-cell imaging of the metabolizable fluorescent fatty acid analog 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY)-C12 and fluorescent 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG) to explore cellular heterogeneity in nutrient uptake in white adipose tissue (WAT) explants of rhesus macaques. Surprisingly, WAT displayed a striking cell size-independent mosaic pattern, in that adjacent adipocytes varied with respect to insulin-stimulated BODIPY-C12 and 2-NBDG uptake. Relative free fatty acid (FFA) transport activity correlated with the cellular levels of FFA transporter protein-1 and the scavenger receptor CD36 in individual adipocytes. In vitro incubation of WAT explants for 24 hours caused partial desynchronization of cellular responses, suggesting that adipocytes may slowly alter their differential nutrient uptake activity. In vitro-differentiated human adipocytes also exhibited a mosaic pattern of BODIPY-C12 uptake. WAT from animals containing a homogeneous population of large adipocytes was nonmosaic, in that every adipocyte exhibited a similar level of BODIPY-C12 fluorescence, suggesting that the development of obesity is associated with the loss of heterogeneity in WAT. Hence, for the first time, we demonstrate an intrinsic heterogeneity in FFA and glucose transport activity in WAT.
Triglyceride (TAG) synthesis in white adipose tissue (WAT) relies on the insulin-dependent uptake and esterification of free fatty acids (FFAs) and the uptake of glucose, the main substrate for the glyceride-glycerol backbone of TAG. Newly synthesized TAGs are packaged into adipocyte organelles known as lipid droplets (LDs) (1–3). WAT is composed of unilocular adipocytes, containing a large central LD (cLD), occupying more than 95% of the total volume of the cell. The enlargement of adipocytes (and cLD) associated with obesity leads to the development of local WAT inflammation and insulin resistance, whereas weight loss associated with a reduction in adipocyte size improves insulin sensitivity (4–9).
WAT is compartmentalized into two major anatomical depots, ie, sc and visceral WAT, whose relative distribution and functional properties are affected by sex, age, and energy balance (10). Current concepts of adipose heterogeneity also include the distinctions between white, brown, and beige/brite adipocytes (11, 12). Classical brown adipocytes have the capacity to increase energy expenditure through uncoupling protein-1-driven uncoupling of oxidative metabolism from ATP production, known as thermogenesis, and possess unique morphological characteristics, including a multilocular appearance (multiple LDs) and high mitochondrial content. Recent studies suggest that WAT contains a novel brown-like adipocyte type known as beige/brite adipocytes derived from a common WAT precursor (13, 14). The functional significance of this adipocyte type in adult humans is currently under intense investigation.
Although interdepot heterogeneity in WAT function is a well-established concept, differences in the behavior of individual adipocytes residing within the same cell population is not well understood, and studies addressing this have been limited to in vitro-differentiated adipocytes and rodent models. For example, mouse 3T3-L1 adipocytes display a significant variability in adipogenic gene expression, insulin sensitivity, and LD size and number, suggesting the existence of different adipogenic states within the same population of cells (15–19). Whether these differences in cellular states represent the natural variability in gene expression or artifacts of in vitro culture is unknown. A recent study using in vitro-differentiated mouse primary adipocytes revealed two stable subpopulations of cells based on the differential incorporation of the neutral lipid probe BODIPY493/503 into LDs. High and low lipid uptake phenotypes persisted after cell redifferentiation and correlated with distinct patterns of gene expression (19). One of the best examples of in vivo intratissue heterogeneity is the fat-specific insulin receptor knockout mouse, whose WAT is polarized into small and large adipocytes, with each adipocyte subpopulation displaying a unique gene expression pattern. Interestingly, fat-specific insulin receptor knockout mice were protected against obesity and glucose intolerance (20, 21).
Heterogeneity in cellular responses may be caused by noise, resulting in stochastic and reversible changes in various cellular functions, or by genetic mutations or epigenetic programming in individual cells, resulting in stable cell subpopulations with distinct functional properties (22–25). In fully differentiated nondividing cells, such as adipocytes, differences in function may arise from differentiation from distinct adipose precursors or from differences in the microenvironment that affect cellular function. To explore potential WAT heterogeneity and its mechanisms in nonhuman primates, we used single-cell imaging of rhesus macaque WAT, using the metabolizable fluorescent FFA 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY-C12) and fluorescent glucose[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG). The present study for the first time uncovers the intrinsic mosaic behavior of individual adipocytes in intact WAT with respect to insulin response and suggests a novel link between cellular heterogeneity and obesity.
Materials and Methods
Animals
WAT samples used in this study were obtained from animals studied under protocols previously approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center. Animals were maintained on a regular chow diet consisted of two daily meals of Purina LabDiet fiber-balanced monkey chow (15% calories from fat, 27% from protein, and 59% from carbohydrates; number 5000; Purina Mills), supplemented with fruits and vegetables. Animals used in the present study were rhesus macaques, including lean, insulin-sensitive, 1-year-old juvenile females, 2-year-old juvenile females (Figure 1C), and 12- to 13-year-old adult males (see Figure 5). All other WAT samples used in this study were obtained as excess tissue from healthy 6- to 10-year-old control rhesus macaques studied under different protocols. Animals were euthanized according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. The night prior to necropsy, food was withheld. Before necropsy, animals were sedated with ketamine in the home cage, transported to the necropsy suite, treated with pentobarbital (25 mg/kg), and exsanguinated by severance of the descending aorta.
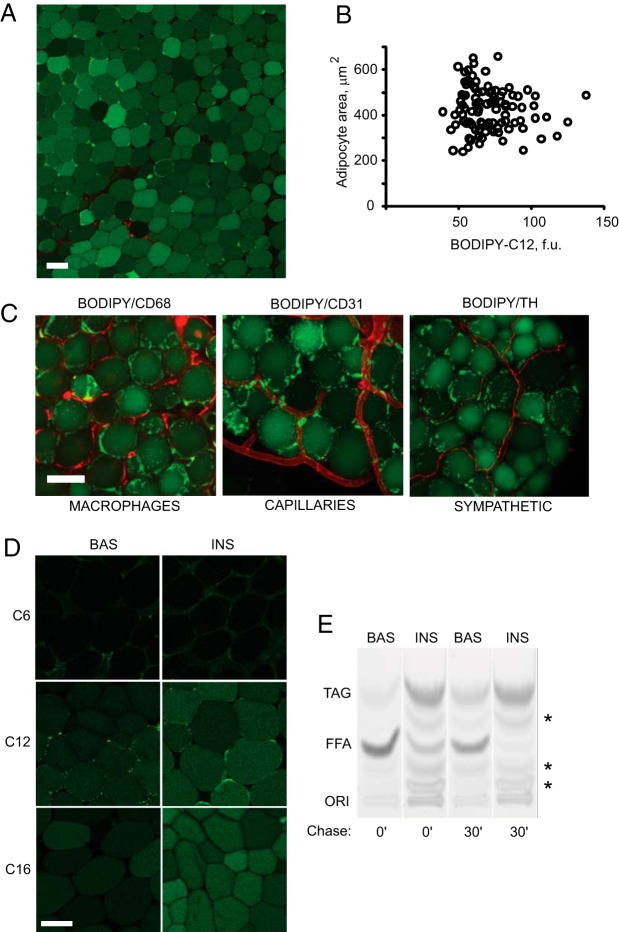
Figure 1. Identification of WAT mosaicism.
Omental WAT explants were incubated for 2 hours in basal media (BAS) or with 10 nM insulin (INS), pulsed with BODIPY-labeled FFA for 15 minutes, and chased in BODIPY-free media for 0 or 30 minutes. A, Insulin-treated, BODIPY-C12-labeled WAT explants were stained with ethidium homodimer-1 dead cell marker (red), as described in Materials and Methods. B, Quantification of mean intracellular fluorescence as a function of cell area (n = 100). f.u., fluorescent units. C, Insulin-treated, BODIPY-C12-labeled WAT explants were fixed and subjected to indirect immunofluorescence analysis using antibodies to CD68 (macrophage marker), CD31 (endothelial marker), and tyrosine hydroxylase (sympathetic innervation marker). D, WAT was labeled with three different fluorescent FFAs, BODIPY-C6, BODIPY-C12, or BODIPY-C16, in basal or insulin-containing media. E, Cellular lipids were extracted and analyzed by thin-layer chromatography, as described in Materials and Methods. Chase time is indicated. Asterisks indicate BODIPY-labeled FFA β-oxidation products (56). ORI, origin. Scale bar, 50 μm.
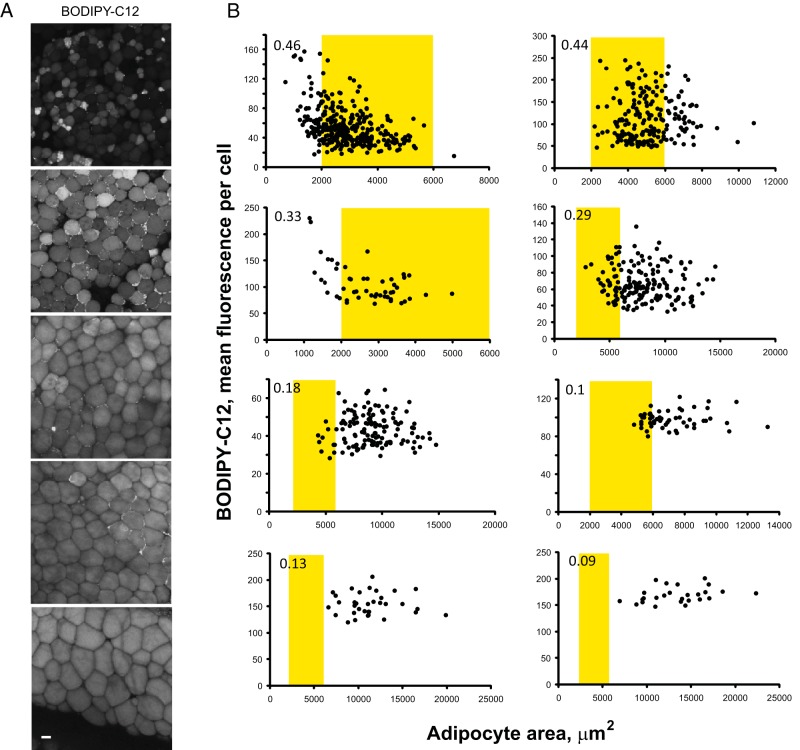
Figure 5. Adipocyte hypertrophy correlates with the loss of WAT mosaicism.
WAT biopsies collected from several control male rhesus were treated with insulin for 2 hours, labeled with BODIPY-C12 for 15 minutes, and chased in the BODIPY-free media for 30 minutes in the presence of insulin. A, Images show representative examples of adipocytes from animals containing small (top two panels), intermediate (middle panel), and large adipocytes (bottom two panels). Scale bar, 50 μm. B, Graphs depict the quantification of mean intracellular BODIPY-C12 fluorescence as a function of cell area for 8 animals (see Materials and Methods for details). Smaller adipocytes display a greater heterogeneity evident by a larger coefficient of variation (shown in the upper left corner of each graph). The areas shown in yellow indicate average adipocyte sizes found in lean animals (50 to 90 μm).
Thin-layer chromatography
Lipids were extracted and analyzed by thin-layer chromatography as previously described (26). Briefly, 100 mg of WAT were homogenized in chloroform-methanol [2:1 (vol/vol)], and lipid extracts corresponding to 5 mg of wet weight were dried under a stream of N2 and resuspended in equal volumes of chloroform-methanol [2:1 (vol/vol)] for loading onto thin-layer chromatography plates. The plates were developed in heptane/isopropyl ether/acetic acid (60/40/3) and fluorescently labeled lipids were visualized, using FluorChem M, ProteinSimple.
BODIPY-C12 and 2-NBDG uptake in WAT explants
Five-millimeter WAT explants were incubated free-floating in plastic 24-well plates filled with 0.4 mL of medium 99 (M199)/0.1% FFA-free BSA (incubation media) supplemented with the indicated concentrations of insulin. Where indicated, the ER-tracker red (BODIPY-Glibenclamide; Life Technologies) was added to the media at a 1:1000 dilution. Green fluorescent BODIPY-500/510 C1, C12, or red fluorescent BODIPY-558/568 dodecanoic acid (Life Technologies) were prepared in advance by diluting a 2.5 mM methanol stock solution in incubation media to a final concentration of 10 μM. A ten-micromolar BODIPY-C12 solution was incubated for 15 minutes, protected from light, in a 37°C water bath. One hundred microliters of 10 μM BODIPY-C12 solution were added to each well and mixed by repeated pipetting, and the plates were incubated for an additional 15 minutes at 37°C. Medium was removed by aspiration, the tissue was washed three times with warm incubation media, and incubation continued for an additional 30 minutes in the presence of indicated reagents. WAT explants were fixed at room temperature with fresh 4% paraformaldehyde in PBS for 20–30 minutes, washed four times with PBS, and stored in PBS at 4°C, protected from light, for up to 48 hours before confocal microscopy analysis.
For dual-BODIPY-C12/2-NBDG uptake, WAT explants were incubated for 2 hours in basal media or with 10 nM insulin, labeled with red BODIPY-C12 for 15 minutes at 37°C, and chased in BODIPY-free media for 120 minutes in the absence or presence of insulin (this step is required for complete incorporation of red BODIPY-C12 into cLDs), followed by incubation with 0.5 mM 2-NBDG for 1 hour at 37°C. WAT explants were washed five times with ice-cold M199/0.1% FFA-free BSA, fixed with paraformaldehyde, and washed five times for 5–10 minutes in PBS supplemented with 100 mM CoCl2, a membrane-impermeable quencher of 2-NBDG fluorescence. A ten-millimolar 2-NBDG stock solution in PBS was stored at 4°C and used within a week. Ethidium homodimer-1 dead cell marker (Life Technologies) was added to WAT explants at a 1:200 during insulin preincubation.
Indirect immunofluorescence analysis
Paraformaldehyde-fixed BODIPY-C12-labeled WAT explants were placed into a 24-well plate containing 500 μL of blocking solution (5% fatty acid free BSA, 0.4% Triton X-100 in PBS) and incubated at room temperature for 1 hour. Primary antibodies to CD31 at a 1:10 dilution (R&D Systems), CD68 (Abcam), tyrosine hydroxylase (Millipore), or FFA transporter protein-1 (FATP-1) and CD36 (Santa Cruz Biotechnology) at 1:100 dilution were added and incubated on a rocker overnight at 4°C. The WAT explants were washed four times for 5–10 minutes in blocking solution without BSA, followed by a 1-hour incubation with secondary antibodies in blocking solution at a 1:1000 dilution. Samples were washed five times with BSA-free blocking solution, followed by two PBS washes, and immediately analyzed by confocal microscopy.
Image processing
Confocal stacks of images were collected at 1-μm intervals, opened with the LOCI plug-in data browser, and sum projections generated using Fiji. Regions of interest were manually selected for 20–30 cells per animal. Total BODIPY and NBDG fluorescence intensity were used as a measure of FFA and glucose uptake in the cell, respectively.
Statistical analysis
Statistical differences between groups were determined using the t test. A linear regression analysis was performed using GraphPad Prism-4.
In vitro differentiation of human adipocytes
Omental WAT (part of visceral depot) was obtained from obese female subjects undergoing laparoscopic bariatric surgery, and written informed consent was obtained with the Oregon Health and Science University Institutional Review Board approval as described (27, 28). Stromovascular fraction (SVF) isolation and adipocyte differentiation was previously described (27, 28).
Isolation of primary adipocytes
Twenty to thirty grams of omental WAT was washed with PBS and minced at room temperature into small fragments in 30 mL of PBS. Tissue fragments were transferred into 50-mL conical tubes and centrifuged for 10 minutes at 1000 rpm. Floating WAT fragments were transferred into fresh tubes and washed with PBS. WAT was transferred into 50-mL tubes containing 30 mL of M199 media supplemented with 0.1% FFA-free BSA and 20 mg (315 U/mg) collagenase, type II (EMD Millipore). Tissue fragments were incubated for 1 hour at 37°C with slow agitation. The digested cell suspension was centrifuged for 10 minutes at 1500 rpm. Additional washes with incubation media were performed on a bench, without centrifugation, based on a differential floatation of adipocytes. The top layer containing purified adipocytes was transferred into new tubes, and 100 μL of cells were embedded into a collagen matrix using the 3D Collagen cell culture kit (Millipore).
Results
We have previously described that single-cell responses to insulin in WAT can be assessed by monitoring uptake of the fluorescent FFA BODIPY-C12 and that fluorescent FFAs enter in vitro-differentiated adipocytes and WAT explants by an insulin-dependent mechanism (8, 29–31). We noted that insulin-treated omental WAT explants often displayed an unusual mosaic pattern of BODIPY-C12 fluorescence (Figure 1A), suggesting an intrinsic cellular heterogeneity in FFA incorporation in adipocytes. This heterogeneity was cell-size independent, in that adipocytes with similar surface area displayed a significant variability in fluorescence signals (Figure 1B). Adipocytes with different levels of fluorescence were viable (Figure 1A, nuclei of dead cells marked by red staining) and not differentially inflamed, based on the lack of crown-like structures or preferential microphage infiltration (Figure 1C, CD68). The capillary and tyrosine hydroxylase-positive fiber densities surrounding fluorescently dim and bright cells were similar, suggesting adequate tissue vascularization and the lack of preferential sympathetic innervation (Figure 1C, CD31 and TH). Thus, adipocyte heterogeneity is not clearly related to cell size, cell death, inflammation, or capillary density. FFA uptake in WAT and the mosaic pattern of fluorescence depended on the length of the aliphatic carbon chain attached to BODIPY, in that BODIPY-C12 and BODIPY-C16 entered the cLD in an insulin-dependent manner but displayed cellular heterogeneity in uptake, whereas BODIPY-C6 was not efficiently transported into adipocytes (Figure 1D). In the basal state, BODIPY-C12 and BODIPY-C16 uptake in WAT was also heterogeneous, although the amounts of intracellular fluorescence were lower compared with insulin-stimulated conditions (Figures 1D and 2B).
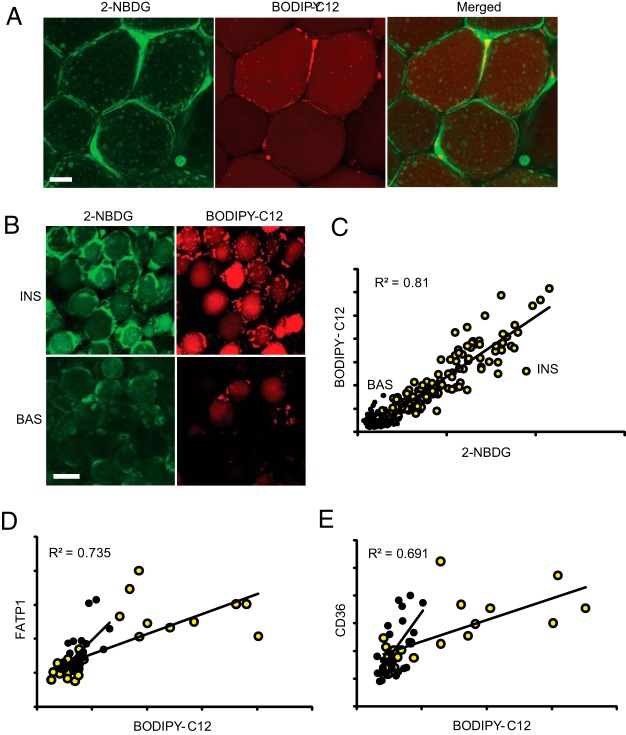
Figure 2. WAT mosaicism is related to heterogeneity in nutrient transporters.
Omental WAT explants were incubated for 2 hours in basal media (BAS) or with 10 nM insulin (INS), labeled with red BODIPY-C12 for 15 minutes, and chased in BODIPY-free media for 120 minutes in the absence or presence of insulin, followed by incubation with 2-NBDG for 1 hour, as described in Materials and Methods. A, Enlarged images show the sites of BODIPY-C12 and 2-NBDG couptake (see Results for details). B, Representative images show insulin stimulation of BODIPY-C12 and 2-NBDG uptake. Scale bar, 50 μm. C, Quantification of the total intracellular fluorescence for BODIPY-C12 and 2-NBDG. Yellow circles, insulin treated; black circles, basal. Red and green channels show significant correlation under insulin-stimulated conditions (P < .0001). D and E, Indirect immunofluorescence of BODIPY-C12-labeled WAT explants with antibodies to FATP-1 and CD36. Total BODIPY-C12, 2-NBDG, and immunofluorescent signals per cell are shown in arbitrary units.
Previous studies have indicated that fluorescent FFAs can be esterified to TAG in 3T3-L1 adipocytes (32–37). To verify that BODIPY-C12 enters the TAG pool in mature adipocytes, WAT explants were incubated with basal or insulin-containing medium, labeled for 15 minutes with BODIPY-C12, chased in label-free media, and lipid extracts were analyzed by thin-layer chromatography. Immediately after addition of BODIPY-C12 in basal medium, it migrated primarily as FFA and only a minor fraction of total fluorescence corresponded to BODIPY-TAG (Figure 1E, lane 1). After a 30-minute chase in basal medium, the proportion of BODIPY-TAG and BODIPY-labeled FFA β-oxidation products increased (Figure 1E, lane 3, and Ref. 56). Insulin treatment stimulated the synthesis of BODIPY-labeled neutral lipids, whereas an insulin chase accelerated the conversion of FFA into TAG (Figure 1E, lanes 2 and 4). We conclude that BODIPY-C12 is a readily metabolizable FFA analog that can be converted by WAT into TAG by an insulin-dependent mechanism.
Because both FFA and glucose transport in adipocytes is potentiated by an insulin-signaling pathway acting upstream of corresponding transporters, we assessed insulin response in individual adipocytes using two independent tracers: red fluorescent BODIPY-C12 and green fluorescent 2-NBDG. 2-NBDG has been previously used for monitoring glucose uptake in cell monolayers (38–40) but not in WAT explants. Two-color imaging revealed that both 2-NBDG and BODIPY-C12 were taken up by WAT adipocytes. BODIPY-C12 accumulated in micro-LDs (56) and cLDs (Figure 2A, red), whereas 2-NBDG was enriched in the cytoplasm surrounding the cLD (Figures 2A and Supplemental Figure 1 show organization of cytoplasmic architecture in adipocytes). Both tracers entered adipocytes in an insulin-dependent manner with apparent cellular heterogeneity (Figure 2B). There was a correlation between the levels of intracellular green and red fluorescence under insulin-stimulated conditions (Figure 2C), suggesting that a common regulatory mechanism acts upstream of both the glucose and FFA transport systems. Indirect immunofluorescence of WAT explants prelabeled with BODIPY-C12 showed that brighter adipocytes contained higher levels of FATP-1 (41–43) and the FFA scavenger receptor/fatty acid translocase/CD36 (44–45) (Figure 2, D and E). Thus, cellular mosaicism in FFA uptake is driven, at least in part, by differences in the levels of FFA transporters in adipocytes.
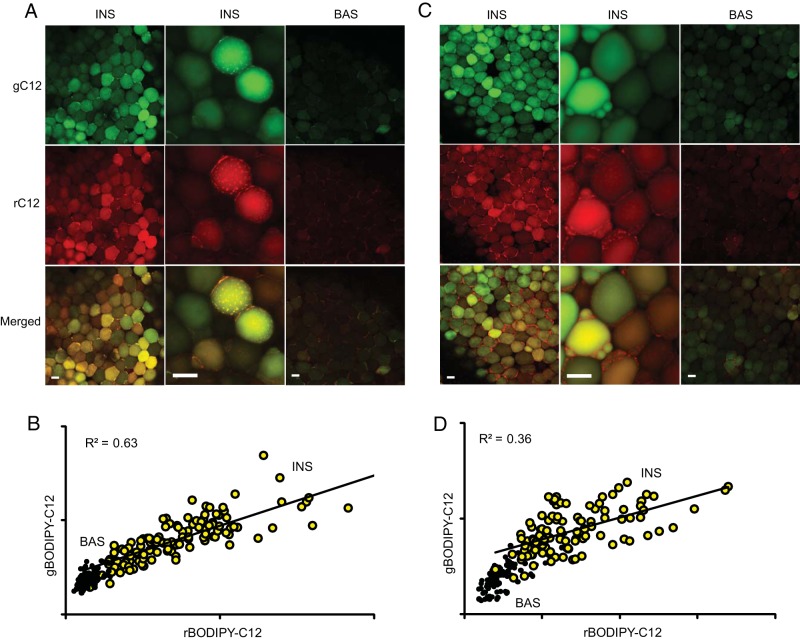
Cell-autonomous variability in the uptake of fluorescent tracers may represent simple noise due to the stochastic nature of the relevant transport processes. Alternatively, WAT may contain several stable subpopulations of cells with different functional properties. It is also possible that adipocytes switch their functional states in a cyclic manner. To distinguish between these possibilities, we tested whether the relative FFA uptake in individual adipocytes was conserved over time. WAT explants were preincubated in basal or insulin-containing media and colabeled with green and red fluorescent BODIPY-C12. In a parallel experiment, red fluorescent BODIPY-C12 was added 24 hours after the addition of green fluorescent BODIPY-C12. Simultaneous colabeling of WAT with red and green BODIPY-C12 showed that both channels correlated (R2 = 0.63) in that bright green cells were also bright red, whereas dim cells appeared dim in both channels (Figure 3, A and B). Delayed colabeling of WAT with red and green BODIPY-C12 showed a weaker linear correlation (R2 = 0.36) between the green and red channels in comparison with simultaneous colabeling (Figure 3, C and D). Thus, adipocytes may alter their intrinsic differential nutrient uptake over time.
Figure 3. Changes in individual adipocyte responses over time.
A and B, Omental WAT explants were incubated for 2 hours under basal conditions (BAS) or with 10 nM insulin (INS), labeled with a mixture of green and red fluorescent BODIPY-C12 for 15 minutes, and chased in BODIPY-free basal or insulin-containing media for 30 minutes, as described in Materials and Methods. C and D, The experiment was repeated as described in panel A, except red BODIPY-C12 (rC12) was added 24 hours after the removal of green BODIPY-C12 (gC12). Quantification of total intracellular BODIPY-C12 and 2-NBDG fluorescence for the experiments shown in panel A (B) and panel C (D). Yellow circles, insulin treated; black circles, basal. Red and green channels show significant correlation under insulin-stimulated conditions (P < .001). Scale bar, 50 μm. Total BODIPY-C12 and 2-NBDG fluorescence per cell and cell areas are shown in arbitrary units.
To rule out the effect of local tissue microenvironment on adipocyte function, human preadipocytes isolated from the SVF of omental WAT were differentiated into adipocytes. On day 14, cultures were treated with basal or insulin-containing media and labeled with BODIPY-C12. Adipocytes, but not preadipocytes, responded to insulin as evident by the accumulation of green fluorescence in LD-containing cells (Figure 4A). Interestingly, some adipocytes displayed a high fluorescent signal (high responders), whereas the others remained dim (low responders, Figure 4, A, D, and E, HR and LR). Both cell types were viable, based on positive staining with the cell viability marker calcein (Figure 4D). Calcein-negative and dead adipocytes did not display BODIPY-C12 transport activity (Figure 4, D and E, asterisks). Insulin treatment resulted in the increased fraction of responding adipocytes (Figure 4, B and C). These results suggest that in vitro-differentiated human adipocyte cultures also display a mosaic-like pattern of BODIPY-C12 uptake. Isolated primary adipocytes from WAT of nonhuman primates were also heterogeneous in respect to BODIPY-C12 uptake (Supplemental Figure 2), suggesting that the mosaic pattern seen in WAT explants is not due to uneven penetration of fluorescent dye into explants.
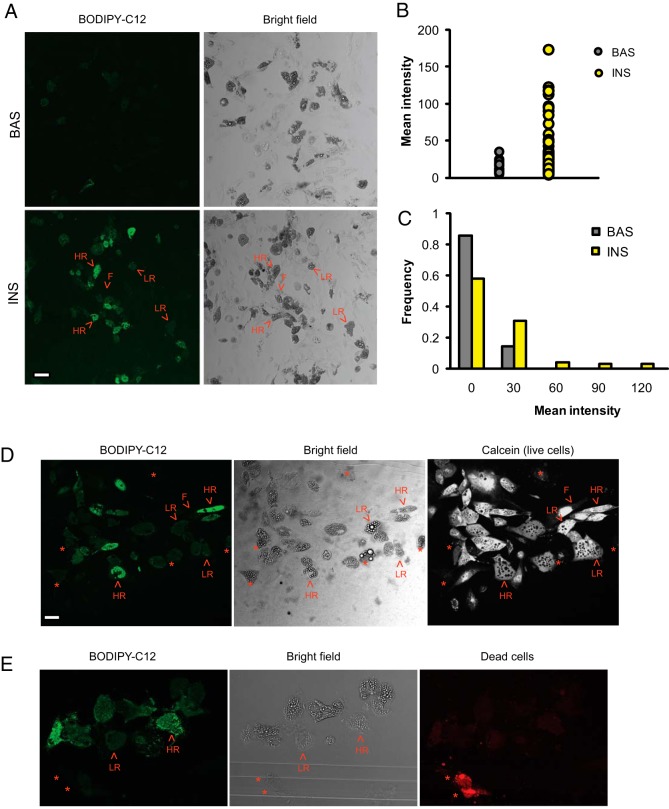
Figure 4. In vitro-differentiated human adipocytes exhibit heterogeneous FFA uptake.
SVF isolated from human omental WAT was differentiated into adipocytes as described in Materials and Methods. A, Insulin response in in vitro-differentiated adipocytes. Adipocytes were incubated for 2 hours in the basal media (Bas) or with 10 nM insulin (Ins), labeled with BODIPY-C12 for 15 minutes, and chased in BODIPY-free media for 30 minutes with or without insulin. A representative image shows the presence of high responders (HR), low responders (LR), and fibroblast-like cells (F). Quantification of mean intracellular fluorescence (B, shown in arbitrary units) and frequency plot (C) for WAT incubated under insulin-stimulated and basal conditions are shown. D, Cell viability test. Insulin-stimulated adipocytes were labeled with BODIPY-C12 and analyzed by confocal microscopy. After capturing images of BODIPY-labeled cells, calcein (cell viability marker) was added to the same field of living cells. After a 2-minute incubation, calcein incorporation was recorded using lower laser power. E, Costaining of BODIPY-C12 labeled adipocytes with the dead cell marker Ethidium homodimer-1 (red), which was added during the 30-minute chase as described in Materials and Methods. Scale bar, 25 μm.
Because the development of obesity is positively associated with adipocyte enlargement, we tested whether WAT hypertrophy affects the mosaic pattern of fluorescence. A group of animals receiving an identical diet exhibited a significant variability in omental fat cell size. The leanest animals had smaller adipocytes, and their WAT displayed the highest level of cellular heterogeneity with respect to BODIPY-C12 uptake (Figure 5A, top two panels). The progressive increase in adipocytes size correlated with lower levels of cellular heterogeneity in WAT (Figure 5A, bottom three panels). WAT enriched in adipocytes with average diameters of 50–90 μm2 had a higher coefficient of variation (0.46–0.33), whereas WAT containing larger adipocytes had a lower coefficient of variation (0.29–0.09; Figure 5B). WAT from three different depots of 1-year-old juvenile animals displayed a mosaic pattern of BODIPY fluorescence (Supplemental Figure 3), suggesting the development of adipocyte heterogeneity within 1 year after birth. In large adipocytes, basal and insulin-stimulated 2-NBDG uptake was low and the extend of mosaicism was difficult to assess. Thus, WAT hypertrophy correlates with the loss of cellular mosaicism.
Discussion
The present study demonstrates that WAT of the rhesus macaque is heterogeneous with respect to insulin-dependent uptake of FFAs and glucose by individual adipocytes residing within the same cell population. Both substrates are necessary and sufficient for TAG synthesis in adipocytes. Because BODIPY-C12 entered, in an insulin-dependent manner, the TAG pool and was concurrently detected in the cLD of individual adipocytes (Figure 1), it is likely that the mosaic pattern of fluorescence reflects differential rates of ongoing FFA esterification and TAG synthesis in WAT adipocytes. Consistent with this idea, our recent study demonstrated that the uptake of BODIPY-C12 in LDs correlates with the formation of BODIPY-TAG (56). Furthermore, the levels of FATP-1, the acyl-coenzyme A synthetase required for vectorial acylation of FFA in adipocytes (41–43), and the levels of fatty acid translocase/CD36 (44, 45) were higher in brighter adipocytes, suggesting that the biochemical machinery involved in FFA uptake and esterification is up-regulated in these cells.
We were not able to quantify glucose transporter-4 (46–48) levels in individual adipocytes due to the performance of the available antibodies; however, the concurrent activation of FFA and glucose uptake in adipocytes suggests that both FFA and glucose transport systems are regulated by a common mechanism. This mechanism may include the differential expression and/or activation of insulin signaling molecules such as the insulin receptor and insulin receptor substrate-1/2. Alternatively, the expression of key lipogenic enzymes and transporters may fluctuate in individual adipocytes. Consistent with this idea, recent studies in primary mouse adipocytes identified two subpopulations of adipocytes that accumulate low or high levels of neutral lipids. The latter had higher levels of mRNAs encoding essential lipogenic factors, such as glucose transporter-4 and fatty acid synthase (19).
We demonstrated that the relative responses of individual rhesus macaque adipocytes within the same population showed signs of desynchronisation within 24 hours of incubation (Figure 3). Hence, WAT adipocytes with a long life span (49) can switch their functional states over a period of weeks, months, or years. The presence of large cLDs in high- and low-responding cells suggests that the observed heterogeneity does not affect the steady-state levels of TAG in individual adipocytes, suggesting that less efficient adipocytes may represent a latent pool of cells. Alternatively, adipocytes may differ in the relative activities of the lipogenic pathway (de novo synthesis of FFA from glucose or other precursors), the levels of transcription factors involved in the regulation of lipogenesis (19, 50, 51), or lipolysis. As adipocyte size increased, there was a reduction in cellular heterogeneity. That larger adipocytes are more insulin resistant than smaller adipocytes has been previously documented (4–8). Thus, the loss of heterogeneity observed in the present study is likely to occur due to the inactivation of the insulin-signaling pathway in larger adipocytes.
Although the mechanisms responsible for the differences in insulin response in WAT remains to be investigated, the SVF cells isolated from human omental WAT can be differentiated in vitro into adipocytes with different functional propertied based on the differential uptake of BODIPY-C12 (Figure 4). SVF is a complex mixture of cell types, including preadipocytes, endothelial cells, adipose stem cells, and immune cells (52). Committed human aP2+CD68− preadipocytes have been shown to constitute up to 30% of SVF (53, 54). The present study suggests the existence of at least two subtypes of human adipocytes derived from in vitro differentiation of SVF cells. The first type displays high levels of FFA transport, whereas the second does not. Because in vitro differentiation of SVF cells is typically carried out in FFA-free media, TAG stores in these adipocytes are formed by the process of lipogenesis, with glucose serving as a fuel.
Although it is difficult to directly correlate the phenotype of human in vitro-differentiated adipocytes with authentic rhesus mature adipocytes, it is conceivable that the mosaic behavior seen in WAT explants arises from the differentiation from different adipocyte precursors. This hypothesis is supported by previous findings that differential adipocyte behavior has been observed in both males and females (30, 31), in different fat depots (8), and in WAT of juvenile and adult rhesus (present study). Alternatively, these differences may represent epigenetic reprogramming triggered by the local environment, as documented for tumor and adipocyte heterogeneity (19, 55).
The present study does have several limitations. Although we describe the mosaic behavior of rhesus adipocytes in an ex vivo organotypic system, in vivo, most FFA uptake in WAT occurs from FFAs generated locally by lipoprotein lipase. Hence, additional studies will be necessary to determine whether the latter is also differentially activated/expressed in WAT. It is difficult to extrapolate the data obtained in primary rhesus WAT to the data using in vitro-differentiated human adipocytes. Although both systems displayed cellular mosaicism in FFA uptake, the apparent heterogeneity may use distinct biochemical mechanisms. It is possible that the uneven delivery of nutrient may contribute to cellular heterogeneity seen in WAT explants. We argue against this possible artifact for the following reasons. First, resuspended primary adipocytes isolated from WAT display differential BODIPY-C12 uptake (Supplemental Figure 2). This rules out uneven dye penetration or preferential adsorption due to adipocyte polarization. Second, adjacent in vitro-differentiated human adipocytes display a mosaic behavior (Figure 4D), suggesting that intercellular architecture does not significantly contribute to differential FFA uptake. Third, our original studies demonstrated that BODIPY-C12 can rapidly penetrated several cell layers in WAT explants (8).
In conclusion, the identification of endogenous factors that control adipocyte heterogeneity in nutrient use may provide novel tools for designing new approaches for treatment of obesity and type 2 diabetes.
Acknowledgments
We thank Dr Robert O'Rourke (Department of Surgery, Oregon Health and Science University) for providing samples of human WAT.
This work was supported by National Institutes of Health Grants P50 HD071836 (to C.T.R.), R21 AG047543 (to O.V.), K99 DK100640 (to H.S.), and P51 OD011092 for the operation of the Oregon National Primate Research Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BODIPY
- 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
- cLD
- central LD
- FATP-1
- FFA transporter protein-1
- FFA
- free fatty acid
- LD
- lipid droplet
- M199
- medium 199
- 2-NBDG
- 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose
- SVF
- stromovascular fraction
- TAG
- triglyceride
- WAT
- white adipose tissue.
References
- 1. Brasaemle DL, Wolins NE. Packaging of fat: an evolving model of lipid droplet assembly and expansion. J Biol Chem. 2012;287(4):2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khor VK, Shen WJ, Kraemer FB. Lipid droplet metabolism. Curr Opin Clin Nutr Metab Care. 2013;16(6):632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thiam AR, Farese RV, Jr, Walther TC. The biophysics and cell biology of lipid droplets. Nat Rev Mol Cell Biol. 2013;14(12):775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith U. Effect of cell size on lipid synthesis by human adipose tissue in vitro. J Lipid Res. 1971;12(1):65–70. [PubMed] [Google Scholar]
- 5. Salans LB, Dougherty JW. The effect of insulin upon glucose metabolism by adipose cells of different size. Influence of cell lipid and protein content, age, and nutritional state. J Clin Invest. 1971;50(7):1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobsson B, Smith U. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res. 1972;13(5):651–656. [PubMed] [Google Scholar]
- 7. Olefsky JM. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976;57(4):842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varlamov O, Somwar R, Cornea A, Kievit P, Grove KL, Roberts CT., Jr Single-cell analysis of insulin-regulated fatty acid uptake in adipocytes. Am J Physiol Endocrinol Metab. 2010;299(3):E486–E496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27(3):234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. [DOI] [PubMed] [Google Scholar]
- 13. Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shigematsu S, Miller SL, Pessin JE. Differentiated 3T3L1 adipocytes are composed of heterogenous cell populations with distinct receptor tyrosine kinase signaling properties. J Biol Chem. 2001;276(18):15292–15297. [DOI] [PubMed] [Google Scholar]
- 16. Nagayama M, Uchida T, Gohara K. Temporal and spatial variations of lipid droplets during adipocyte division and differentiation. J Lipid Res. 2007;48(1):9–18. [DOI] [PubMed] [Google Scholar]
- 17. Loo LH, Lin HJ, Singh DK, Lyons KM, Altschuler SJ, Wu LF. Heterogeneity in the physiological states and pharmacological responses of differentiating 3T3-L1 preadipocytes. J Cell Biol. 2009;187(3):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le TT, Cheng JX. Single-cell profiling reveals the origin of phenotypic variability in adipogenesis. PLoS One. 2009;4(4):e5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katz LS, Geras-Raaka E, Gershengorn MC. Heritability of fat accumulation in white adipocytes. Am J Physiol Endocrinol Metab. 2014;307(3):E335–E344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bluher M, Michael MD, Peroni OD, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3(1):25–38. [DOI] [PubMed] [Google Scholar]
- 21. Bluher M, Patti ME, Gesta S, Kahn BB, Kahn CR. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem. 2004;279(30):31891–31901. [DOI] [PubMed] [Google Scholar]
- 22. Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297(5584):1183–1186. [DOI] [PubMed] [Google Scholar]
- 23. Raser JM, O'Shea EK. Noise in gene expression: origins, consequences, and control. Science. 2005;309(5743):2010–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bar-Even A, Paulsson J, Maheshri N, et al. Noise in protein expression scales with natural protein abundance. Nat Genet. 2006;38(6):636–643. [DOI] [PubMed] [Google Scholar]
- 25. Blainey PC, Quake SR. Dissecting genomic diversity, one cell at a time. Nat Methods. 2014;11(1):19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sampath H, Flowers MT, Liu X, et al. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem. 2009;284(30):19961–19973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Rourke RW, White AE, Metcalf MD, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54(6):1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Rourke RW, Meyer KA, Gaston G, White AE, Lumeng CN, Marks DL. Hexosamine biosynthesis is a possible mechanism underlying hypoxia's effects on lipid metabolism in human adipocytes. PLoS One. 2013;8(8):e71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Somwar R, Roberts CT, Jr, Varlamov O. Live-cell imaging demonstrates rapid cargo exchange between lipid droplets in adipocytes. FEBS Lett. 2011;585(12):1946–1950. [DOI] [PubMed] [Google Scholar]
- 30. Varlamov O, White AE, Carroll JM, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153(7):3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varlamov O, Chu MP, McGee WK, et al. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154(11):4126–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liao J, Sportsman R, Harris J, Stahl A. Real-time quantification of fatty acid uptake using a novel fluorescence assay. J Lipid Res. 2005;46(3):597–602. [DOI] [PubMed] [Google Scholar]
- 33. Li H, Black PN, DiRusso CC. A live-cell high-throughput screening assay for identification of fatty acid uptake inhibitors. Anal Biochem. 2005;336(1):11–19. [DOI] [PubMed] [Google Scholar]
- 34. Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol. 2006;26(9):3455–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Q, Kazantzis M, Doege H, et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes. 2006;55(12):3229–3237. [DOI] [PubMed] [Google Scholar]
- 36. Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 2008;9(3):338–352. [DOI] [PubMed] [Google Scholar]
- 37. Sun Z, Gong J, Wu H, et al. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat Commun. 2013;4:1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamada K, Nakata M, Horimoto N, Saito M, Matsuoka H, Inagaki N. Measurement of glucose uptake and intracellular calcium concentration in single, living pancreatic β-cells. J Biol Chem. 2000;275(29):22278–22283. [DOI] [PubMed] [Google Scholar]
- 39. Osorio-Fuentealba C, Contreras-Ferrat AE, Altamirano F, et al. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes. 2013;62(5):1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alonso-Castro AJ, Zapata-Bustos R, Gomez-Espinoza G, Salazar-Olivo LA. Isoorientin reverts TNF-α-induced insulin resistance in adipocytes activating the insulin signaling pathway. Endocrinology. 2012;153(11):5222–5230. [DOI] [PubMed] [Google Scholar]
- 41. Schaffer JE, Lodish HF. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 1994;79(3):427–436. [DOI] [PubMed] [Google Scholar]
- 42. Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J Biol Chem. 1999;274(51):36300–36304. [DOI] [PubMed] [Google Scholar]
- 43. Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell. 2002;2(4):477–488. [DOI] [PubMed] [Google Scholar]
- 44. Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda). 2006;21:259–268. [DOI] [PubMed] [Google Scholar]
- 46. Nishimura H, Saltis J, Habberfield AD, et al. Phosphorylation state of the GLUT4 isoform of the glucose transporter in subfractions of the rat adipose cell: effects of insulin, adenosine, and isoproterenol. Proc Natl Acad Sci USA. 1991;88(24):11500–11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ezaki O, Flores-Riveros JR, Kaestner KH, Gearhart J, Lane MD. Regulated expression of an insulin-responsive glucose transporter (GLUT4) minigene in 3T3-L1 adipocytes and transgenic mice. Proc Natl Acad Sci USA. 1993;90(8):3348–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25(2):177–204. [DOI] [PubMed] [Google Scholar]
- 49. Arner P, Bernard S, Salehpour M, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478(7367):110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herman MA, Peroni OD, Villoria J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484(7394):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marcelino H, Veyrat-Durebex C, Summermatter S, et al. A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth: a Randle cycle favoring fat storage. Diabetes. 2013;62(2):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53(2):227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tchoukalova YD, Sarr MG, Jensen MD. Measuring committed preadipocytes in human adipose tissue from severely obese patients by using adipocyte fatty acid binding protein. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1132–R1140. [DOI] [PubMed] [Google Scholar]
- 54. Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50(1):151–157. [DOI] [PubMed] [Google Scholar]
- 55. Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339(6127):1567–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chu M, Cahana DY, Kahl C, et al. Spatiotemporal dynamics of triglyceride storage in unilocular adipocytes [published online October 8, 2014]. Mol Biol Cell. doi:10.1091/mbc.E14-06-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]