Single Nucleotide Polymorphisms (SNPs) in Pigmentation-Related Genes Is Linked to Changes in Serum 25-Hydroxyvitamin D (25(OH)D)
In this issue of Endocrinology, Saternus et al (1) present genetic evidence that the activity of pigmentation-related genes can affect serum levels of 25(OH)D in the cohort of 2790 Caucasian patients hospitalized for coronary angiography at the Ludwigshafen General Hospital in Germany. Specifically, variants of 11 from 29 investigated genes that included exocyst complex component 2 (EXOC2), tyrosinase (TYR), TYR-related protein type 1 (TYRP1) affected most serum 25(OH)D levels. Furthermore, the presence of 46 SNPs located in 16 pigmentary genes was associated with either increased or decreased levels of 25(OH)D calculated for total cohort of vitamin D-deficient/insufficient patients (mean, 17.3 ng/mL and median, 15.5 ng/mL) (1). Twelve of these SNPs located at the endothelin 1 (n = 3), EXOC2 (n = 1), microphtalmia-associated transcription factor (MITF) (n = 2), TYR (n = 4), TYRP1 (n = 1), and cAMP-dependent protein kinase catalytic subunit-γ (n = 1) genes reached the significance level after correction for multiple comparisons. Unfortunately, lack of detailed information on the pigmentary phenotype of the patients and on the correlation between analyzed SNPs and functional activity of the proteins prevents a conclusive analysis of the relationship between pigmentary or associated activities and serum levels of 25(OH)D, leaving significant room for speculations and further research.
Melanin Is Securing the Skin Place Under the Sun
Human skin, during the day time, is exposed to electromagnetic wavelengths of solar radiation, including visible light (λ > 400 nm), UVA (λ = 320–400 nm), and UVB (λ = 280–320 nm), which imposes a considerable stress on this organ (2). Depending on the dose, UVA and highly energetic UVB in particular are cytotoxic, mutagenic, and induce multiple damage at the subcellular, cellular, and tissue levels, leading to a variety of skin pathologies, including, but not limited to, skin cancers (3). UVR (ultraviolet) also induces local and systemic immunosuppression effects (4).
In order to protect and/or restore local homeostasis against the UVR-damaging effects, the epidermis is armed with melanin-producing capability (5, 6). In general, melanin pigment protects epidermal cells from UVR through multiple mechanisms (5, 6). In addition, due to its biophysical and chemical properties, during noxious insults, melanin can maintain cellular and epidermal homeostasis (7), whereas intermediates of melanogenesis can modulate local metabolic and immune activities (8). These properties support the hypothesis that melanocytes can act as sensory and regulatory cells of the epidermis (9).
It must be emphasized that protective effects of melanin are assigned to eumelanin (black pigment), whereas pheomelanin (yellow-red pigment) can have mutagenic and carcinogenic influences depending on the context (5, 7, 10, 11). Of note, dark skin individuals predominantly produce eumelanin, whereas Caucasians of Northern or Central European extraction can produce pheomelanin (red-hair phenotype) or mixed melanin. The type of pigment and/or amount of eumelanin determines the susceptibility for development of skin cancer, including melanoma. Red-hair phenotype characterized by pheomelanogenesis is in particular associated with higher risk of developing skin cancer, including melanomas. This raises a question, why in humans an evolutionary pressure has not eliminated a pheomelanogeneic phenotype (it facilitates skin cancerogenesis), but in contrast, it allowed expansion of this population (a population used in this study) among Caucasians in Central or Northern Europe. This raises a challenging question: is pheomelanogenesis linked to some beneficial effects at higher latitude?
TYR as a Main and Obligatory Regulator of Melanin Pigmentation
TYR is a crucial enzyme of melanogenesis. It catalyzes 3 distinct reactions: hydroxylation of monophenol (L-tyrosine), dehydrogenation of catechol dihydroxyphenylalanine (L-DOPA), and dehydrogenation of dihydroxyindole (DHI), of which tyrosine hydroxylation represents an obligatory step of melanogenesis with a rate limiting step represented by DOPA oxidation (5, 6, 8). Lack of TYR function prevents melanin production, leading to the albino phenotype with high and moderate TYR activity favoring eumelanogenesis. Decreased TYR activity may lead to pheomelanin production as a result of conjugation of DOPAquinone with either cysteine or glutathione (5, 6, 8).
TYR is a product of TYR gene (C-locus) that can generate several alternatively spliced messages, of which one containing all 5 exons translates into the TYR enzyme (5, 6, 12). Other alternatively spliced forms may have nonenzymatic bioregulatory functions that remain to be established (5). The structure of TYR protein is highly conserved among different species and shows high homology with TYRPs, including TYRP1 and TYRP2/DCT (dopachrome tautomerase). The TYR and TYRP1/2 proteins are the subject of complex posttranslational processing and intracellular trafficking, with melanosomes being a main target (5, 6, 12). Fully processed TYR with catalytic site containing copper is ready to initiate melanogenesis once L-tyrosine is available and the proper chemical environment is formed in this compartment (5, 6, 12).
Murine Tyrp1 possesses dihydroxyindole carboxylic acid oxidase activity, but TYRP1 enzymatic function in human melanocytes is less clear, except for its role in stabilizing TYR protein, modulating its catalytic activity, and maintaining the structure of the melanosomes (6, 12). TYRP2/DCT acts as dopachrome tautomerase (12). Both enzymes also play important role in maintaining melanocyte viability (12).
TYR, TYRP1, TYRP2, and oculocutaneous albinism 2 belong to the family of melanogenesis-related proteins (MRPs), of which expression and activity is regulated by multiple factors acting through membrane bound receptors initiating signaling cascades involving protein kinase A, C, and G and MAPKs (5, 6, 12). The main master regulator of transcriptional activity of MRP is MITF, on which the above signaling systems merge to coordinate pigmentary activity of the melanocyte (5, 13).
Majority of Systemic Vitamin D Comes From the Skin
The vast majority of systemic vitamin D derives from the skin with a minority coming from dietary supplementation (14). Vitamin D formation in skin is a result of photochemical reactions initiated by the absorption of UVB energy by 7-dehydrocholesterol in the epidermis, of which final outcome depends on the UVB dose, temperature, and lipid environment (15). The vitamin D formed in the keratinocytes of the epidermis or provided by diet is transported to the liver, where it undergoes hydroxylation to 25(OH)D that serves as a prohormone that is either activated in the kidney or peripheral tissues expressing CYP27B1 (14). Although the transport of vitamin D from the gastrointestinal tract is reasonably well established (14), its transport from epidermis through basement membrane, large space acellular papillary dermis filled with extracellular matrix with final entry into the dermal vascular bed, represents an enigma. It is believed that as vitamin D is being generated in the plasma membrane, it is ejected into the extracellular space and is driven into the derma capillary bed by the concentration gradient that is due to the D binding protein in the circulation (14–16). Most vitamin D absorbed through the gastrointestinal tract passes through the liver, where it is hydroxylated at C25 (14). In contrast, cutaneous vitamin D can potentially access many organs besides the liver during initial passage through the circulation (14). The latter may allow its hydroxylation at positions other than C25 by CYP11A1 in steroidogenic organs as suggested by a recent report (17). Importantly, the epidermis has the capability to effect sequential hydroxylation of vitamin D: D→25(OH)D→1,25(OH)2D (16), and it is unclear whether cutaneous 25(OH)D can enter the circulation (Figure 1).
Figure 1.
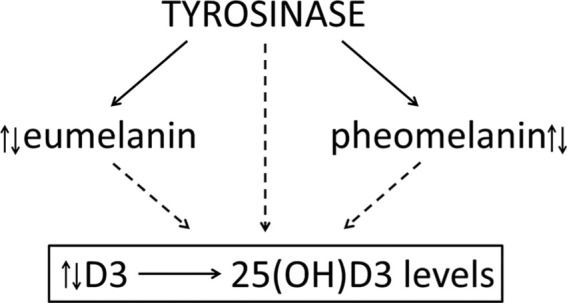
Hypothetical interaction between TYR and TYR-regulated melanin pigment and vitamin D3 producing and activating systems.
When Pigmentary System Meets Vitamin D
The majority of the experts in the field of vitamin D accept the assumption on the reverse correlation between the activity of the cutaneous pigmentary system and vitamin D production (1, 15, 18). We agree with this opinion, because the capability of melanin pigment to absorb UVB energy must attenuate the final dose of UVB energy reaching 7-dehydrocholesterol in basal and suprabasal layers of epidermis (14, 15). However, some authors have challenged this theory proposing that there is no correlation between skin pigmentation and cutaneous melanin pigment (see papers cited in Refs. 1, 18). The present study is in line with the majority's opinion that the melanin pigmentary system is affecting vitamin D signaling by showing genetic evidence for a linkage between circulating 25(OH)D and melanogenic machinery in the skin of Caucasian individuals (1).
However, lack of information on the level on skin pigmentation and types of melanin produced weakens a conclusion on a direct correlation between melanin pigment and vitamin D, because these patients, while being hospitalized, must have limited or none exposure to UVB, per the described protocol (1). Furthermore, mechanisms regulating melanin pigmentation are complex and nonlinear in nature (5), and melanocytes have several regulatory activities that are independent of melanin (19). In addition, although SNPs in the EXOC2 gene can affect hair pigmentation (20), the predominant role of its protein product is to interact with the actin cytoskeletal remodeling and vesicle transport machinery but not to regulate melanin pigmentation.
Nevertheless, SNPs in TYR clearly indicate a direct correlation with melanin pigmentation, because its protein product, TYR, is an obligatory enzyme of melanogenesis, and changes in its structure would have a profound effect on skin melanin pigmentation (5, 8). Other MRPs, such as TYRP1 and TYRP2/DCT, only modify melanogenesis, with the exception of OCA2 that may have a more profound effect (5, 6, 8), whereas MITF, endothelin 1, cAMP-dependent protein kinase catalytic subunit-γ, and other gene products, listed by Saternus et al (1), regulate expression and activity or TYR and other MRPs (5, 8).
Conclusion and Future Directions
Therefore, based on the information listed above and data presented by Saternus et al (1), one can safely conclude that there is a genetic correlation between the melanin-producing system and circulating levels 25(OH)D3 in the Caucasian population of predominantly German ancestry. However, establishment of a phenotypic correlation would require complementary biochemical experiments. Based on the crucial role of TYR in initiation and regulation of melanin production, the most definitive approach would involve testing whether listed SNPs have an effect on TYR activity, level, and type of melanin produced (Figure 1). The future challenge is to establish whether the type of melanin pigment (eumelanin vs pheomelanin) or its mixture and concentration levels can affect vitamin D production, its transport to circulation, and initial hydroxylation.
Acknowledgments
We thank Dr Holick for critical comments and advises during writing this manuscript.
This work was supported in part by NIH Grants 2R01AR052190, R21 AR066505-01A1, and 1R01AR056666-01A2 (to A.S.) and the Department of Veterans Affairs Grant 1IP1BX001607 (to A.E.P.).
Disclosure Summary: The authors have nothing to disclose.
For article see page 39
- EXOC2
- exocyst complex component 2
- 25(OH)D
- 25-hydroxyvitamin D
- MITF
- microphtalmia-associated transcription factor
- MRP
- melanogenesis-related protein
- SNP
- single nucleotide polymorphism
- TYR
- tyrosinase
- TYRP1
- TYR-related protein type 1
- EXOC2
- exocyst complex component 2
- 25(OH)D
- 25-hydroxyvitamin D
- MITF
- microphtalmia-associated transcription factor
- MRP
- melanogenesis-related protein
- SNP
- single nucleotide polymorphism
- TYR
- tyrosinase
- TYRP1
- TYR-related protein type 1.
References
- 1. Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1 and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39–47. [DOI] [PubMed] [Google Scholar]
- 2. Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii,, 1–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desotelle JA, Wilking MJ, Ahmad N. The circadian control of skin and cutaneous photodamage. Photochem Photobiol. 2012;88(5):1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kripke ML. Ultraviolet radiation and immunology: something new under the sun–presidential address. Cancer Res. 1994;54(23):6102–6105. [PubMed] [Google Scholar]
- 5. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. [DOI] [PubMed] [Google Scholar]
- 6. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21(4):976–994. [DOI] [PubMed] [Google Scholar]
- 7. Wood JM, Jimbow K, Boissy RE, S, et al. What's the use of generating melanin? Exp Dermatol. 1999;8(2):153–164. [DOI] [PubMed] [Google Scholar]
- 8. Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25(1):14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164(1):103–120. [DOI] [PubMed] [Google Scholar]
- 10. Takeuchi S, Zhang W, Wakamatsu K, et al. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc Natl Acad Sci USA. 2004;101(42):15076–15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitra D, Luo X, Morgan A, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491(7424):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olivares C, Solano F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009;22(6):750–760. [DOI] [PubMed] [Google Scholar]
- 13. Liu JJ, Fisher DE. Lighting a path to pigmentation: mechanisms of MITF induction by UV. Pigment Cell Melanoma Res. 2010;23(6):741–745. [DOI] [PubMed] [Google Scholar]
- 14. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 15. Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88(2):296–307. [DOI] [PubMed] [Google Scholar]
- 16. Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347(1–2):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slominski AT, Kim TK, Shehabi HZ, et al. In vivo evidence for a novel pathway of vitamin D metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26(9):3901–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elias PM, Menon G, Wetzel BK, Williams JJ. Evidence that stress to the epidermal barrier influenced the development of pigmentation in humans. Pigment Cell Melanoma Res. 2009;22(4):420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slominski A. Neuroendocrine activity of the melanocyte. Exp Dermatol. 2009;18(9):760–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han J, Kraft P, Nan H, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4(5):e1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]


