Abstract
Effective and safe antiobesity drugs are still needed in face of the obesity pandemic worldwide. Recent interventions in rodents revealed 3,5-diiodo-L-thyronine (3,5-T2) as a metabolically active iodothyronine affecting energy and lipid metabolism without thyromimetic side effects typically associated with T3 administration. Accordingly, 3,5-T2 has been proposed as a potential hypolipidemic agent for treatment of obesity and hepatic steatosis. In contrast to other observations, our experiments revealed dose-dependent thyromimetic effects of 3,5-T2 akin to those of T3 in diet-induced obese male C57BL/6J mice. 3,5-T2 treatment exerted a negative feedback regulation on the hypothalamus-pituitary-thyroid axis, similar to T3. This is demonstrated by decreased expression of genes responsive to thyroid hormones (TH) in pituitary resulting in a suppressed thyroid function with lower T4 and T3 concentrations in serum and liver of 3,5-T2-treated mice. Analyses of hepatic TH target genes involved in lipid metabolism revealed T3-like changes in gene expression and increased type I-deiodinase activity after application of 3,5-T2 (2.5 μg/g body weight). Reduced hepatic triglyceride and serum cholesterol concentrations reflected enhanced lipid metabolism. Desired increased metabolic rate and reduction of different fat depots were, however, compromised by increased food intake preventing significant body weight loss. Moreover, enlarged heart weights indicate potential cardiac side effects of 3,5-T2 beyond hepatic thyromimetic actions. Altogether, the observed thyromimetic effects of 3,5-T2 in several mouse TH target tissues raise concern about indiscriminate administration of 3,5-T2 as powerful natural hormone for the treatment of hyperlipidemia and pandemic obesity.
Thyroid hormones (THs) exert pronounced concentration-dependent actions on development, growth, body weight, temperature, oxygen consumption, and energy metabolism both during development but also in adult animals and humans (1). T3 is considered to be the main active TH, which modulates as specific endogenous ligand the activity of nuclear and mitochondrial TH receptors (TRs). Isoforms of the TRα and TRβ gene family have been identified and functionally characterized as ligand-regulated transcription factors with developmental and cell type-specific expression patterns (2–4). Currently, T4, the main secretion product of the thyroid gland, is considered a prohormone, which under physiological conditions might not reach the nuclear TR target. This might be due to limitations of intracellular nuclear ligand availability, which is controlled by transmembrane TH transporters, intracellular deiodinases (Dio) (Dio1, Dio2, and Dio3), and TH-metabolizing enzymes as well as by a cytosolic-nuclear gradient demonstrated for T3 (4, 5). However, also transcription-independent actions of TH mediated via membrane-bound receptors as well as nuclear receptors located in the cytosol have been demonstrated for T4 (6, 7).
Over the last decades, a further endogenous TH metabolite, 3,5-diiodo-L-thyronine (3,5-T2), present in significant subnanomolar concentrations in human serum (8), received marked attention. Several reports showed rapid 3,5-T2 effects on energy metabolism, lipid turnover, and oxidation in rat liver and skeletal muscle (6, 9). Many of these effects occurred without any interference of 3,5-T2 or its synthetic analog (10) with the hypothalamus-pituitary-thyroid (HPT) feedback axis or any adverse cardiac, bone, or central nervous system (CNS) effects typically observed after administration of T3 or liver-specific TR ligands, such as eprotirome (11, 12). Interestingly, administration of pharmacologically high concentrations of 3,5-T2 to hypothyroid rats on high-fat diet (HFD) resulted in antisteatotic action on liver and skeletal muscle without major changes in circulating TSH, T4, or T3 levels (9, 10, 13–16). An 8-day 3,5-T2 dose-escalation protocol in 2 healthy individuals resulted in increased resting metabolic rate and decreased body weight without any changes or adverse effects on serum TSH, TH, thyroid and cardiac function, blood pressure, or serum metabolic parameters, such as glycemia, total cholesterol, and triglycerides (17). In context of illegitimate use of 3,5-T2 in body builder communities and wellness scene (eg, SAN T2 Xtreme - Fat burner), a careful analysis of mechanism of action and safety of 3,5-T2 is needed. Considerable concern on ostensible solely beneficial effects of 3,5-T2 arises from several publications demonstrating considerable thyromimetic effects of 3,5-T2 by activating nuclear TR similar to T3 in various target tissues and from the recent development of a synthetic 3,5-T2 analog TRC150094, which might be used by humans before safety in long-term application is proven (10, 18–20). In addition, thyromimetic action and suppressive interference of 3,5-T2 with the HPT axis has been reported (21–24). So far, various experimental, subcellular, and cellular as well as different animal models have been exploited for better understanding of 3,5-T2 action in comparison with the classical TH T4 and T3. Whether endogenous 3,5-T2 directly originates from thyromimetic T3 via Dio3 action or whether it is directly formed on thyroglobulin during TH biosynthesis in the thyroid gland remains to be studied (8). So far, direct demonstration of enzymatic 3,5-T2 formation from T3 or T4 by Dio in vitro was not successful (5). In vivo administration of T3 resulted in rapid appearance of circulating 3,5-T2 (25), which might have a profile and mechanism of action distinct from that of T3.
Pandemic obesity, its metabolic sequel, and associated comorbidities represent an increasing challenge for medicine, health care systems, and societies worldwide, indicating the need for efficient, affordable, and safe modes of prevention and intervention. Under this perspective, the use of an endogenous hormone metabolite devoid of major side effects but exerting a beneficial profile on body weight and hepatic steatosis, such as 3,5-T2, might provide a promising route to tackle this challenge. Therefore, we tested the hypothesis whether 3,5-T2 exerts beneficial metabolic effects, independent and distinct from the classical thyromimetic T3. For this, we employed the mouse model of diet-induced obesity to investigate a putative therapeutic effect of 3,5-T2 treatment, because previously published studies mainly followed a “preventive” approach coadministering 3,5-T2 concomitant with high-fat feeding.
In this study, diet-induced obese male mice were treated with 3,5-T2 in comparison with T3 and measures of the HPT axis, expression of TH-regulated genes in classical target tissues of TH action, and hormone effects on parameters indicative of metabolic function, body weight control, and energy metabolism were analyzed. Our data demonstrate that 3,5-T2 indeed induces beneficial effects on body fat mass, serum leptin, and energy expenditure. However, in contrast to other reports, our data imply the risk of significant adverse interference with HPT axis as well as of cardiac and hepatic side effects with pharmacological use of 3,5-T2.
Materials and Methods
Animals and diets
All procedures were approved by the Animal Care and Use Committee at the State Ministry of Rural Development, Environment, and Consumer Protection of Brandenburg, Germany, in accordance with the German Animal Welfare Act. C57BL/6J breeding pairs, purchased from The Jackson Laboratory, received standard chow (ssniff) and were kept under standard conditions (22°C, 12-hour light, 12-hour dark cycle). After weaning, males were fed ad libitum a HFD (60 kJ% fat, 24.3 kJ/g, D12492; Research Diets, Inc).
At the age of 20 weeks, diet-induced obese mice were single housed and assigned to the experimental groups. Quantification of daily food intake was ensured by equipping cages with special food baskets to control for spillage.
At the end of the study, mice were restricted from food for 6 hours before killing (from 7 am to 1 pm). Under isoflurane anesthesia, blood was retrieved from vena cava to obtain serum. Subcutaneous (inguinal) and epididymal white fat pads, liver, and heart were sectioned quantitatively and immediately weighed on a balance. All tissue samples were immediately frozen in liquid nitrogen and stored at −80°C until further processing.
Study design
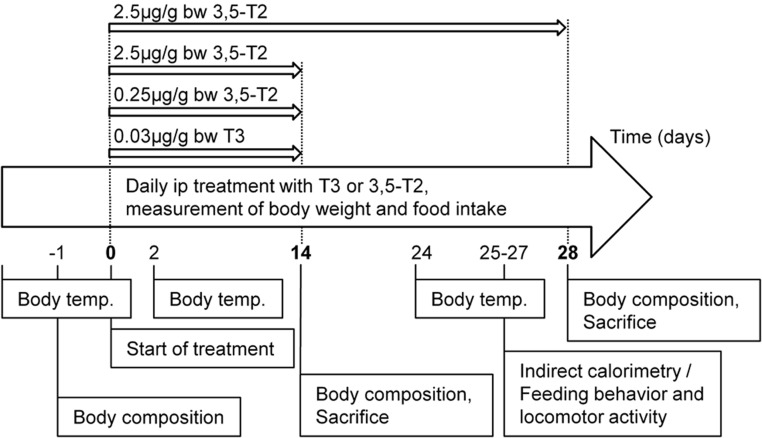
Single-caged obese mice were equally distributed to experimental groups. At time point 0 mean body weight of mice were as followed: controls, 50.8 ± 1.6 g; T3, 50.6 ± 1.4 g; 3,5-T2 (0.25 μg), 51.2 ± 1.2 g; and 3,5-T2 (2.5 μg), 52.3 ± 1.6 g, without showing significant difference between groups. Mice were injected (ip) with either T3 (0.03 μg/g body weight) or 3,5-T2 (0.25 or 2.5 μg/g body weight) (Sigma-Aldrich) once daily at onset of dark cycle for 14 days to validate effects of 3,5-T2 in comparison with T3 (n = 7–8). A second cohort of obese mice was treated with 2.5 μg/g body weight 3,5-T2 for 28 days to investigate the impact of 3,5-T2 on metabolic parameters. Therefore, separate groups of mice were used for measurement of energy expenditure (n = 6–8) or feeding behavior and locomotor activity (n = 9–11). Controls were injected daily with the same volume of solvent (0.15% NaOH in physiological saline). Body weight and food intake were recorded at time of treatment as described earlier. Body composition was determined at beginning and end of experiment by a nuclear magnetic resonance spectrometer as described (26). Body temperature was measured 2 days before first treatment and at different time points during the experiment with electronic thermometer (Almemo 2290–2/3, Ahlborn Mess- und Regelungstechnik), when animals were resting at onset of light cycle (Figure 1).
Figure 1. Study design.
Diet-induced obese C57BL/6J male mice were treated with either 3,5-T2 or T3 for 14 days to validate the thyromimetic potential of 3,5-T2 in relation to T3. Metabolic parameters were measured in a second cohort of mice after 3,5-T2 treatment for 28 days.
Feeding behavior and cage activity
Food intake was monitored with an automatic Drinking & Feeding Monitor System (TSE Systems) with food baskets connected to weight sensors. Mice were adapted to the setting for 2 days followed by a 3-day period of data collection. Cumulative food intake and locomotor activity (based on infrared beams, InfraMot-Activity System; TSE) were recorded. Mice had ad libitum access to food and water throughout the measurements.
Energy expenditure by indirect calorimetry
Total energy expenditure (TEE) and respiratory quotient were measured with an open circuitry calorimetry system equipped with the gas analyzing system Advance Optima from ABB AG (oxygen consumption [VO2] analyzer Magnos 16, carbon dioxide production [VCO2] analyzer Uras14) as described previously (26, 27). Mice were adapted to respiratory cages for 24 hours. Adaptation was followed by a 21-hour measurement period at 22°C. TEE was calculated with the equation TEE = 16.17 × VO2 + 5.03 × VCO2 − 5.98 × N (26, 27), where TEE is expressed in kJ per day, and VO2 and VCO2 are expressed in liters per day. N is excreted nitrogen and was assumed to be 0.1 g/d. Resting energy expenditure (REE) was defined as the mean of lowest 8 energy expenditure values during the 21-hour period (27).
Extraction and quantification of TH and 3,5-T2 from liver and serum
Extraction of TH and 3,5-T2 from liver was performed using a liquid-liquid extraction procedure (5). Liver tissue was homogenized followed by protein precipitation, acidification, and delipidation to remove potentially interfering substances. For the extraction, ethyl acetate served as organic solvent. For extraction of TH and 3,5-T2 from serum, a solid phase extraction procedure was used. Samples were subjected to protein precipitation followed by removal of phospholipids by transferring the supernatant to HybridSPE cartridges from Sigma-Aldrich.
The resulting serum eluate as well as the organic layer of liver extraction were evaporated to dryness and reconstituted for liquid chromatography/mass spectrometry (LC-MS/MS) analysis. Identification and quantification of TH and 3,5-T2 were performed using a binary pump HPLC system (Agilent 1200; Agilent) and a 5500 QTRAP triple-quadrupole tandem mass spectrometer (Applied Biosystems) equipped with TurboIonSpray interface (for LC-MS/MS parameters see Supplemental Table 1). The next standards were used as internal controls during the extraction procedure: 13C6-T3, 13C6-rT3, 2H5-T4 (Isoscience), and 15N-3,5-T2 (kindly donated by Thomas S. Scanlan, Oregon Health and Science University, Portland, Oregon). Data processing was performed using Bio Analyst version 1.5. Software (Applied Biosystems).
Determination of hepatic enzyme activities
Hepatic Dio1 activity was measured with a nonradioactive iodide release assay (28). Then 10 μg of pulverized protein were incubated with rT3 substrate (10μM rT3; Formula) for 2 hours at 37°C, and Dio1 activity was measured via an iodide-mediated redox reaction (29). Specific Dio1 activity was calculated as pmol iodide released per mg protein and min.
Measurement of hepatic citrate synthase (CS) activity was performed as described elsewhere (30). Shortly, frozen liver samples were homogenized in Tris-EDTA buffer (pH 7.4), and cleared supernatant after centrifugation was used for a spectrophotometric assay.
Hepatic triglyceride content
Quantification of liver triglyceride content was performed by enzymatic assay (Randox Laboratories Ltd) after homogenization buffer extraction according to manufacturer's instructions.
Quantitative real-time PCR of TH-responsive gene expression in the liver and pituitary
Total mRNA from liver was extracted with peqGOLD TriFast (Peqlab) according to manufacturer's instructions. Total mRNA from pituitaries was isolated using the Aurum kit (Bio-Rad) following manufacturer's guidelines. Purity and concentration were determined with a NanoDrop ND-1000 (Peqlab). Approximately 500 ng/μL of total RNA was required to synthesize cDNA using the iScript cDNA synthesis kit (Bio-Rad). Expression of mRNA was determined by quantitative real-time PCR on an iCycler detection system (Bio-Rad) using Absolute qPCR SYBR Green Fluorescein mix (ABgene). Primers were designed using the Primer-Blast tool of NCBI (Supplemental Table 2). Data were normalized using the 2−ΔΔCT method, and Hprt served as endogenous control (31).
Serum parameters
Serum leptin levels were measured by a murine ELISA kit (EIA-4564; DRG International, Inc). Serum lipids were measured with commercially available kits: cholesterol (Human), triglycerides (Sigma-Aldrich), and free fatty acids (Wako Chemicals).
Statistical analysis
Values are presented as mean ± SEM unless otherwise stated. Statistical differences of normally distributed data between 2 groups were determined by unpaired Student's t test or for more groups by one-way ANOVA (Bonferroni post hoc test), respectively. Data that did not meet these criteria were analyzed by nonparametric Mann-Whitney test or Kruskal-Wallis test (Dunn's post hoc test), respectively. Differences in overall time course between controls and 3,5-T2 treatment were evaluated by two-way ANOVA (Bonferroni post hoc test). Comparison of energy expenditure was carried out by analysis of covariance (ANCOVA) with lean mass as a covariate. Differences were considered significant when P < .05. Statistical analysis was performed by GraphPad Prism 5 (GraphPad Software, Inc) or SPSS Statistics 20 (IBM).
Results
Adaptability of HPT axis upon TH treatment as indicated by TH concentrations and gene expression profile
In order to evaluate the impact of 3,5-T2 on adiposity and the regulation of the HPT axis, 2 doses were tested in diet-induced obese C57BL/6J male mice in a 14-day study followed by a longer study for 28 days as indicated in Figure 1.
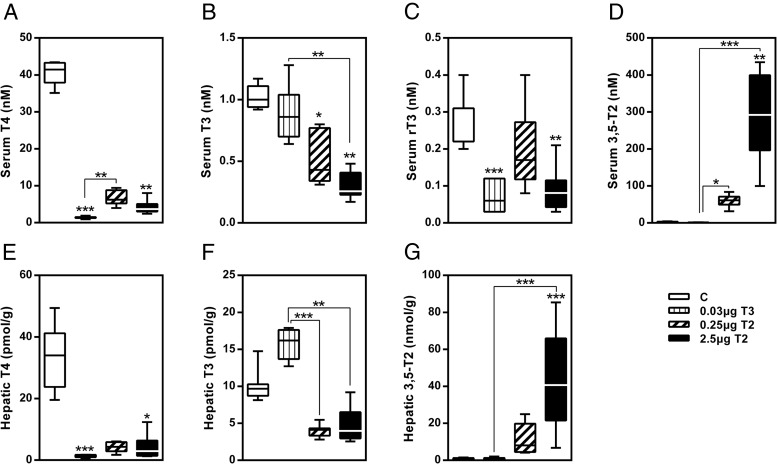
The application of TH for 14 days had substantial impact on HPT axis as shown by analysis of serum TH concentrations (Figure 2, A–D). T3 treatment efficiently decreased serum T4 and rT3 concentrations but maintained T3 serum levels in the euthyroid range. T3 administration did not alter serum 3,5-T2 concentrations. 3,5-T2 administration dose-dependently repressed serum T4 and T3. However, serum rT3 was only significantly suppressed by 2.5μg 3,5-T2. Reduction of T4 by both, T3 and 3,5-T2, indicated a physiological suppression of the HPT axis by negative feedback mechanisms (Figure 2, A–D). Additionally, we detected a dose-dependent increase of serum 3,5-T2 in 3,5-T2-treated mice with concentrations more than 25-fold (0.25 μg) or 120-fold (2.5 μg) higher compared with controls or T3-treated mice (Figure 2D). Alterations in hepatic TH levels after hormone treatment (Figure 2, E–G) reflected the serum profile with the exception of elevated hepatic T3 levels in the T3 group (Figure 2F). Furthermore, we observed a dose-dependent hepatic accumulation of 3,5-T2 in 3,5-T2-treated mice with levels being increased 18-fold (0.25 μg) and 70-fold (2.5 μg), respectively, similar to elevated serum concentrations (Figure 2G). The detection of TSH in serum could give a more direct indication for feedback effects of 3,5-T2. However, because no specific mouse immunoassay has been established so far, we do not have data on TSH concentration.
Figure 2. Profile of TH after a 14-day treatment as measured by LC-MS/MS.
Serum (A–D) and hepatic (E–G) TH concentrations. Data are shown as boxplots. *, P < .05; **, P < .01; ***, P < .001 vs control or as indicated (n = 6–8).
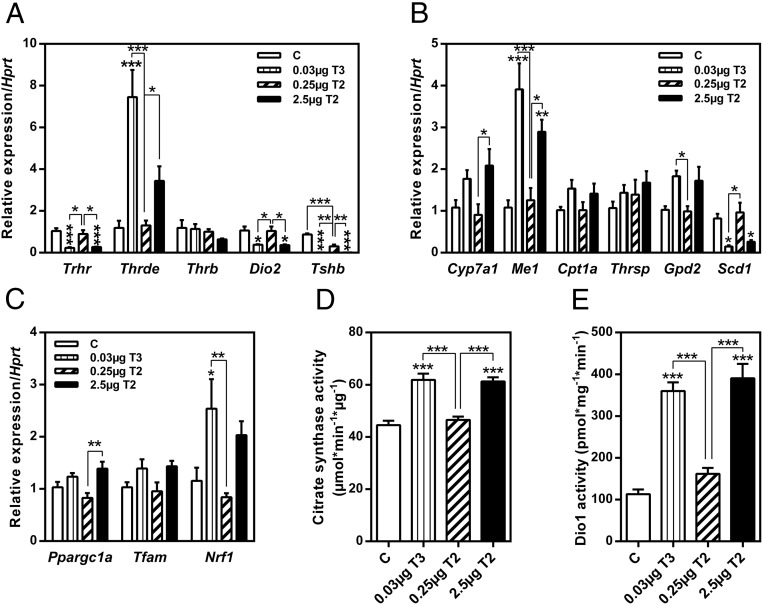
In addition to TH concentrations, expression of genes related to the regulation of the HPT axis in pituitary was evaluated (Figure 3A). A similar expression pattern was observed for TSH-releasing hormone receptor (Trhr), Dio2, and thyroid stimulating hormone β (Tshb), all of which were significantly down-regulated by T3 treatment. The higher dose of 3,5-T2 reduced the expression of the same genes. However, the lower 3,5-T2 dose (0.25 μg) had no impact on gene expression with the exception of significantly decreasing Tshb. Transcript levels of TRH-degrading enzyme (Thrde) were increased by T3 and 2.5μg 3,5-T2, whereas TRβ (Thrb) expression was not affected by either treatment. The expression of classical hepatic T3 target genes, such as cytochrome P450, family 7, subfamily a, polypeptide 1 (Cyp7a1), malic enzyme (Me1), carnitine palmitoyltransferase 1a (Cpt1a), and glycerol phosphate dehydrogenase 2 (Gpd2), were up-regulated by T3 and 3,5-T2 (2.5 μg), with the exception of the TH-responsive gene (Thrsp) (Figure 3B). Repeatedly, the lower 3,5-T2 dose was ineffective. Transcript levels of stearoyl-Coenzyme A desaturase 1 (Scd1), a major enzyme in hepatic lipogenesis converting saturated fatty acids into monounsaturated fatty acids, were decreased by both T3 and 3,5-T2 (2.5 μg). In contrast to our hypothesis of detecting 3,5-T2 actions distinct from T3, we observed similar thyromimetic actions in regard to TH profile as well as changes in pituitary and hepatic gene expression.
Figure 3. Treatment of mice with 3,5-T2 or T3 affected regulation of HPT axis and its negative feedback mechanisms.
Pituitary gene expression (A), expression of hepatic target genes (B) and of mitochondrial biogenesis in liver tissue (C), hepatic CS activity (D), and hepatic Dio1 activity (E). Data are expressed as mean ± SEM. *, P < .05; **, P < .01; ***, P < .001 vs control or as indicated (n = 6–8).
To investigate whether T3 or 3,5-T2 affected mitochondrial biogenesis in liver tissue, expression of peroxisome proliferative-activated receptor-γ, coactivator 1α (Ppargc1a), nuclear respiratory factor 1 (Nrf1), and mitochondrial transcription factor A (Tfam) was measured. Overall, no significant changes of expression of these genes were detected by the treatments except for a trend towards slightly increased transcript levels in T3 and 3,5-T2 (2.5 μg)-treated mice in comparison with controls (Figure 3C). However, hepatic CS activity, a marker of mitochondrial content, was significantly increased by T3 and 3,5-T2 (2.5 μg) treatment (P < .001), indicative of enhanced mitochondrial oxidative capacity (Figure 3D).
Hyperthyroidism induced by TH supplementation is typically accompanied by counteracting compensatory mechanisms. One such reaction is an enhanced hepatic metabolism of active TH by increased hepatic Dio1 activity as seen after treatment with T3 (3.2-fold; P < .001) or 2.5μg 3,5-T2 (3.4-fold; P < .001) (Figure 3E). Hepatic hyperthyroid state is in accordance with a down-regulation of pituitary Dio2 expression (Figure 3A).
Overall, the alterations in serum and hepatic TH concentrations, observed changes of pituitary transcripts, and enhanced hepatic Dio1 activity are strong indicators for hyperthyroidism and enhanced negative feedback regulation of HPT axis.
3,5-T2 reduces fat mass and leptin concentration without affecting body weight
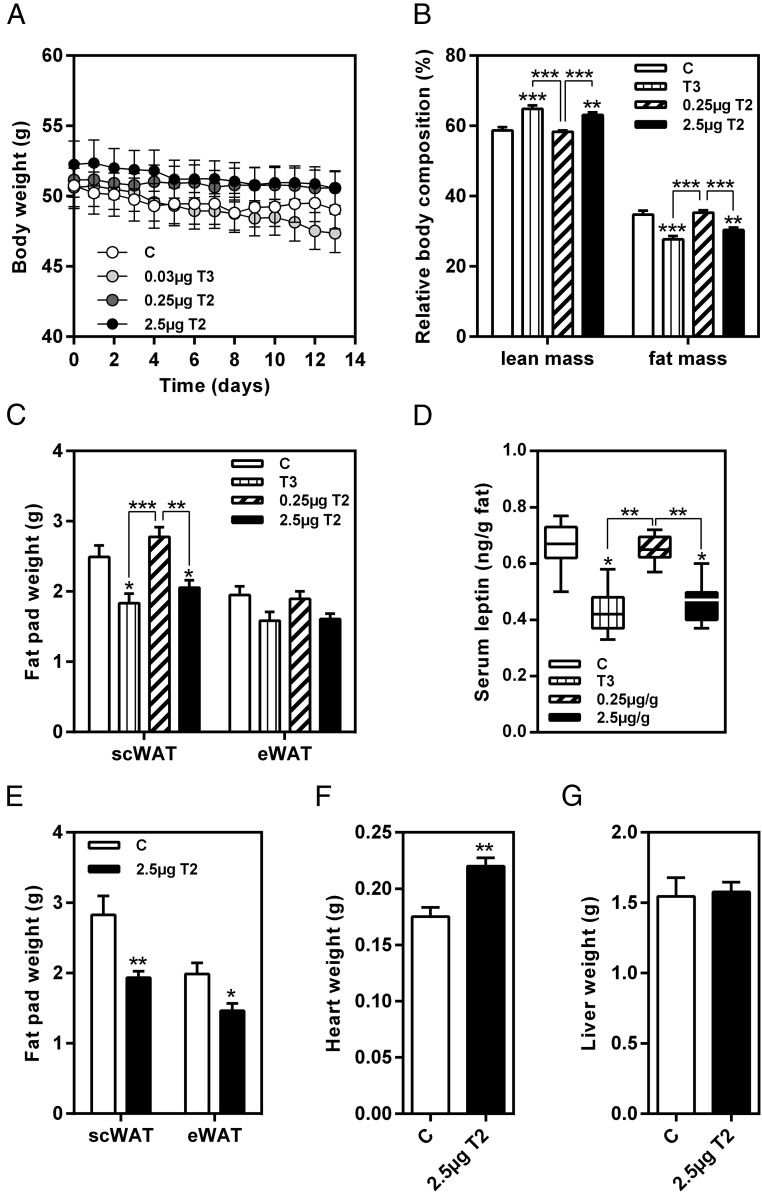
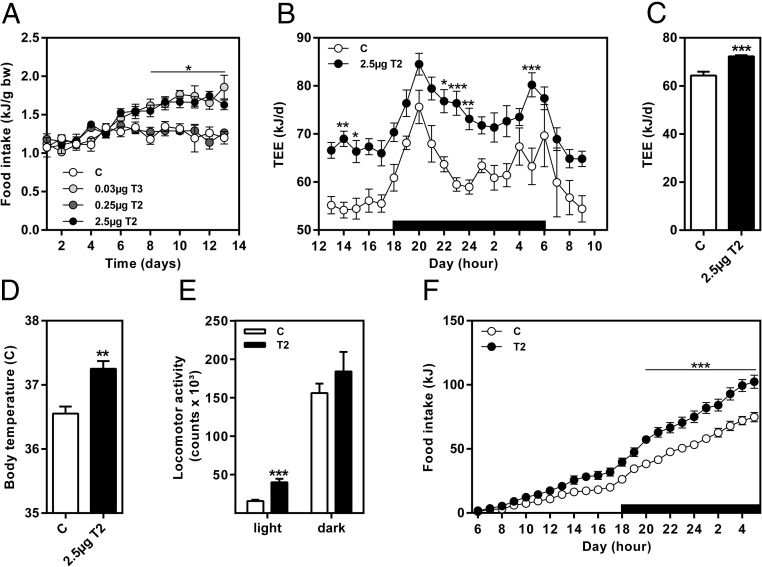
Recent publications showed a marked reduction of body weight and fat mass due to 3,5-T2 in rats (13, 24). In contrast, in our experiment, neither treatment with T3 or 3,5-T2 caused decrease of body weight after either 14 (Figure 4A) or 28 days. However, relative body composition of mice treated with 2.5 μg/g body weight 3,5-T2 was significantly changed in favor of higher fat-free mass (Figure 4B). This change was mainly attributable to a reduction of the sc fat depot (Figure 4C). The loss of fat mass was further increased after prolonged treatment with 3,5-T2. Weights of both, sc and epididymal, fat depots were significantly decreased after 28 days of intervention (Figure 4E). Positive effects of T3 and 3,5-T2 on body fat reduction were further emphasized by significantly reduced serum leptin concentrations in these mice (Figure 4D).
Figure 4. 3,5-T2 treatment reduced fat mass in obese mice without affecting body weight.
Effect of 3,5-T2 treatment on (A) body weight, (B) relative body composition, (C) sc fat pad (scWAT) and epididymal fat pad (eWAT) weight, and (D) serum leptin concentrations after 14 days in comparison with T3 (n = 6–8); 28 days of 3,5-T2 application (E) reduced weight of scWAT and eWAT, (F) increased heart weight, and (G) did not affect liver weight (n = 7–8). Data are expressed as mean ± SEM. *, P < .05; **, P < .01; ***, P < .001 vs control or as indicated.
Changes in TH concentrations (Figure 2) and TH-related gene expression (Figure 3, A and B) were indicative for hyperthyroidism after 3,5-T2 treatment. To determine whether prolonged 3,5-T2 treatment induced symptoms of thyrotoxic state, heart weight of mice was evaluated after 28 days of intervention. Indeed, the observed increased heart weight of 3,5-T2-treated mice (P < .01) might point to such side effects due to the hormone treatment (Figure 4F). Liver weight was not affected by 3,5-T2 application (Figure 4G).
Energy expenditure and food intake are increased in 3,5-T2-treated obese mice
Body mass of 3,5-T2-treated mice did not change (Figure 4A). However, daily food intake was significantly increased during the second week of treatment in animals treated with 3,5-T2 (2.5 μg) and was similar to T3-treated mice (Figure 5A). This directed us to closer investigate the impact of 3,5-T2 (2.5 μg) on energy metabolism. The metabolic measurements at the end of the treatment period (d 25–27) (Figure 5, B and C) revealed elevated TEE throughout the 21-hour measurement (Figure 5B). Further adjustment of TEE (control, 64.31 ± 1.61 kJ/d and 3,5-T2, 72.29 ± 0.48 kJ/d; P < .01) (Figure 5C) and REE (control, 48.93 ± 1.15 kJ/d and 3,5-T2, 55.57 ± 1.55 kJ/d; P < .05) for lean mass by ANCOVA (common lean mass 28.4 g) showed significant increase of both metabolic parameters due to the 3,5-T2 treatment by 12% and 13%, respectively. Furthermore, body temperature was significantly elevated after 3,5-T2 application (d 24: control, 36.6 ± 0.1°C and 3,5-T2, 37.3 ± 0.1°C; P < .001) (Figure 5D). Increased locomotor activity during the light phase contributed to increased energy expenditure as well (Figure 5D). Elevated 3,5-T2-mediated energy expenditure did not reduce body weight (Figure 4A), because 3,5-T2 treatment increased 24-hour cumulative food intake (control, 74.6 ± 3.7 kJ; T2, 102.4 ± 5.1 kJ; P < .01) (Figure 5F), as already observed earlier during the 14-day intervention (Figure 5A).
Figure 5. Energy expenditure and food intake were increased by a 28-day treatment with 3,5-T2.
A, Daily food intake during a 14-day intervention. B, Energy expenditure during a 21-hour measurement from days 26 to 27 of intervention and (C) adjustment of TEE by ANCOVA with lean mass as covariate, (D) body temperature at day 24 of intervention, (E) 24-hour locomotor activity measurement, and (F) 24-hour cumulative food intake as measured at days 25–26 of intervention. Adjusted TEE was presented at a common lean mass of 28.4 g. Data are expressed as mean ± SEM. *, P < .05; **, P < .01; ***, P < .001 vs control or as indicated (n = 6–11).
Improvement of serum cholesterol and hepatic triglyceride content
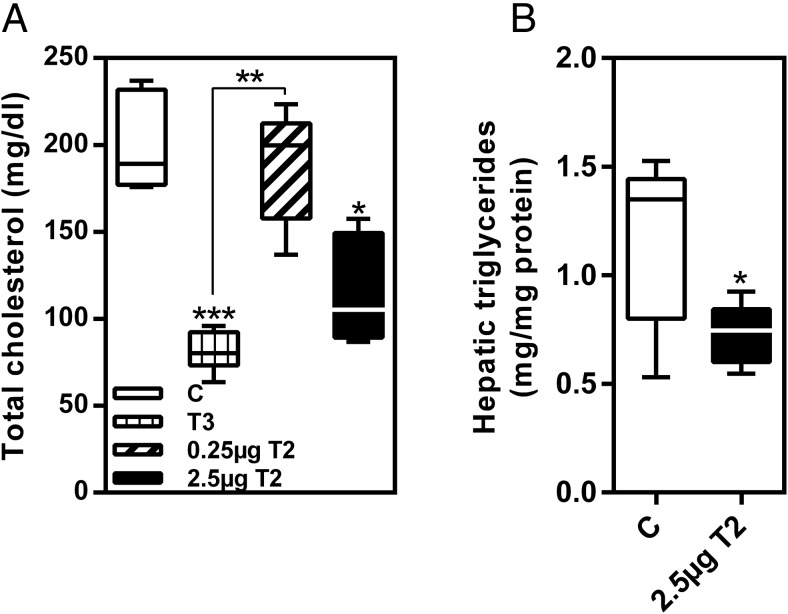
According to increased expression of Cyp7a1 (Figure 3B), serum cholesterol levels in T3 and 3,5-T2 (2.5 μg)-treated mice were decreased (Figure 6A and Supplemental Table 3). Serum triglycerides and free fatty acids were not affected. Furthermore, because 3,5-T2 was reported to affect hepatic triglyceride content in rats (13), we assessed triglycerides in livers of mice that were significantly reduced after 28 days of 3,5-T2 treatment (P < .05) (Figure 6B).
Figure 6. 3,5-T2 treatment caused (A) decrease of total serum cholesterol after 14 days and (B) reduction of hepatic triglyceride content after 28 days of application.
Data are shown as boxplots. *, P < .05; **, P < .01; ***, P < .001 vs control or as indicated (n = 6–8).
Discussion
The endogenous TH metabolite 3,5-T2 has been proposed as a powerful, natural hormone exerting rapid beneficial effects on mitochondrial function, lipid mobilization, and fatty acid oxidation in multiple organs of hypothyroid or euthyroid rats and in humans (9, 17). A series of recent reports indicated that the rapid metabolic action of 3,5-T2 and its synthetic analog TRC150094 are not mediated by classical TH action via nuclear TR (10, 14), thus avoiding suppressive interference with the HPT axis and adverse cardiac or bone action.
In contrast to previous studies, which mostly employed a preventive setting with focus on body weight gain upon HFD feeding in combination with 3,5-T2 treatment, we tested the effects of 3,5-T2 in comparison with T3 in a “therapeutic” approach as a potential antiobesity drug. Administration of 3,5-T2 for 14 or 28 days in diet-induced obese mice had positive effects on body composition characterized by reduction of different fat depots and increased metabolic rate. However, these positive effects were partially compromised by hyperphagia, which prevented any weight loss in diet-induced obese mice. Elevated food intake might be a consequence of reduced serum leptin concentrations after 3,5-T2 treatment. Furthermore, we found a major suppressive interference of the metabolically active dose of 3,5-T2 comparable with T3 on the HPT axis associated with tissue hyperthyroidism in the pituitary and liver, which was already evident after a 14-day treatment period. Moreover, heart weight was increased after 28 days of 3,5-T2 application. Apparent thyrotoxic effects of 3,5-T2 as indicated by enlarged hearts have previously been reported after 3,5-T2 treatment of both wild-type and Ldlr−/− mice (32) and are in line with our own findings, suggesting cardiac action of 3,5-T2 independent of TH-related alterations of lipid status and metabolism. Padron et al (24) observed no change in heart rate but also reported an increased relative heart weight in 3,5-T2-treated rats.
In agreement with data obtained in mice, rats, and humans (13, 15, 17, 32), we also observed a reduction in serum total cholesterol and hepatic triglycerides, whereas serum triglyceride and free fatty acid concentrations were not altered. These effects were already detectable after 14 days of treatment and similar to treatment with T3. Analysis of hepatic gene expression provided evidence for induction of Cyp7a1, a TH-regulated enzyme that converts cholesterol to bile acids (33), which may explain improvement of serum cholesterol status by 3,5-T2. Goldberg et al (32), who administered T3 in similar doses (0.0075–0.75 μg/g body weight) for 1 week and 3,5-T2 (1.25 and 12.5 μg/g) in higher dose than in our experiment to control and Ldlr−/− mice fed HFD, observed a drastic decrease in low density lipoprotein (LDL)-cholesterol indicative of a LDL receptor-independent regulation.
Similar to our findings, Goldberg et al (32) reported increased hepatic expression of genes involved in lipogenesis such as Me1, suggesting a change in hepatic lipid handling. Furthermore, 3,5-T2 is claimed to act antisteatotically in rats as shown by preventing the development of hepatosteatosis during concomitant feeding of HFD and 3,5-T2 treatment (13). In addition, Mollica et al (16) showed a reduction of preexisting hepatic fat accumulation by 3,5-T2 treatments of rats. In agreement with their data, we observed reduced hepatic triglyceride content in obese mice, which was obvious after 2 weeks of 3,5-T2 treatment and reached significance after 28 days of intervention. Data from rats suggested increased hepatic mitochondrial capacity due to 3,5-T2 treatment (13). Based on our findings, the elevated transcript levels of mitochondrial transcription factors and the elevated hepatic CS activity reflect enhanced mitochondrial capacity. Even though this translated into increased TEE and REE with concurrent elevated body temperature, none of the treatments led to a reduction of body weight.
This observation might be explained by increased food intake after T3 and 3,5-T2 treatment, which might have compromised loss of total body weight. Mechanisms underlying increased food intake and motor activity can be mediated via reduction of leptin concentration or by hypothalamic effects of T3 and 3,5-T2. Indeed, elevated lean body mass is compatible with anabolic effects of physiological TH concentrations, which might involve altered ghrelin secretion and/or vagal input (34). TH-induced increase in food intake and energy expenditure has recently been shown to depend on hypothalamic activation of the mammalian target of rapamycin signaling pathway leading to increased orexigenic agouti-related peptide, neuropeptide Y, and uncoupling protein 2 transcripts and decreased mRNA levels of anorexigenic proopiomelanocortin in the arcuate nucleus of the hypothalamus (35, 36) representing a strong orexigenic drive. In addition, the combination of TH administration and HFD might cooperate at the hypothalamic level via activation of JNK signaling in TRH-secreting neurons, resulting in decreased TRH, TSH, and subsequently TH secretion by the thyroid (37).
The intriguing concept of 3,5-T2 as a TH that might exhibit positive effects on metabolism without adverse side effects is being challenged by recent publications and our findings. Padron et al (24) reported on a dose-dependent suppression of serum TSH, T4, and T3 after 3,5-T2 treatment of adult male Wistar rats associated with increased hypothalamic and pituitary Dio2 activity, as well as increased hepatic and renal Dio1 activity, indicating a hyperthyroid state. In addition, the authors demonstrated altered thyroid function during 3,5-T2 treatment indicated by decreased iodide uptake, sodium-iodide symporter expression, thyroperoxidase expression and activity as well as Dio1 expression and activity. Furthermore, the expression levels of the TSH receptor and dual oxidase were increased, reflecting decreased stimulation of the thyroid gland by suppressed TSH. These findings indicate, in contrast to several previous reports in rats, that 3,5-T2 indeed exerts marked thyromimetic T3-like effects also in nonobese young rats, similar to our observations in diet-induced obese mice. This study did not report on cardiac side effects of 3,5-T2, which were independently observed by Goldberg et al (32) and ourselves in mice.
Our experiment for the first time revealed elevated serum 3,5-T2 concentration and unexpectedly high 3,5-T2 liver content after 3,5-T2 injections. The latter observation indicates 1) significant hepatic 3,5-T2 uptake by still unidentified transporter(s) followed by intrahepatic accumulation of 3,5-T2, and 2) inefficient elimination of 3,5-T2 from liver. This constellation resembles features of the high uptake and accumulation of the TRβ-selective ligand eprotirome, which exhibited remarkable hepatic tissue selectivity and strong antihyperlipidemic effects in human volunteers (11, 12). However, the human trial was prematurely discontinued due to indication of adverse effects of eprotirome in dogs questioning the further human application (12). Accumulation of 3,5-T2 in liver might also provide an explanation for the observation by Ball et al (38) in rats, who reported that 3,5-T2 was more effective in inducing hepatic Me1 gene expression than suppressing circulating TSH, indicating that tissue- and gene-selective effects of 3,5-T2 are not only related to differences in binding of this thyromimetic ligand to various TR isoforms but also to distinct local cellular ligand availability.
Effects of 3,5-T2 observed in our study and others are comparable with reported action of the synthetic 3,5-T2 analog TRC150094. TRC150094 at comparable dosage acted similar to high doses of 3,5-T2 in rat models, having positive effects on mitochondrial activity and lipid metabolism and preventing hepatosteatosis (10, 18, 19) without exerting unfavorable cardiac and CNS side effects typical for high-dose administration of T4 and T3 or leading to adverse bone effects as reported for preclinical trials in rabbits with the lipid lowering drug eprotirome, which preferentially targets the liver (39, 40). TRC150094 does not undergo metabolic modification to T3-antagonistic thyronamines like 3-iodothyronamine or its thyroacetic acid oxidation products generated by ubiquitous amine oxidases and exerting rapid T3-independent actions (41, 42). The above observation, therefore, clearly indicates specific targets and actions linked to endogenous formation of 3,5-T2. Unfortunately, these positive observations on 3,5-T2 action in animal models were not confirmed in a recently published human trial on subjects with impaired hepatic and peripheral insulin sensitivity (20).
A major question is whether 3,5-T2 acts only via TR or whether it induces additional signals to mediate changes on gene expression, energy metabolism, or mitochondrial function. Similar effects of the high 3,5-T2 dose and T3 indicate that 3,5-T2 acts like T3 via nuclear pathways regulating the expression of genes containing thyroid hormone response elements in their regulatory regions. However, it still need to be clarified whether 3,5-T2-specific pathways exist, which will be studied in the future, eg, by analyzing the liver transcriptome of 3,5-T2- and T3-treated mice. Based on several published reports and our data in regard to gene expression, we assume that 3,5-T2 acts at least partly via binding to TR. However, 3,5-T2 is known to bind with lower affinity to TR in comparison with the classical ligand T3 (38, 43). Recently, García-G et al (44) suggested differences in T3 and 3,5-T2 binding and activation of TR isoforms in killifish and their direct influence on the expression of TH-dependent genes through an interaction with thyroid hormone response elements in promoter regions of these genes. In their model, 2 TRβ isoforms with selective ligand binding properties have been identified. According to their findings, 3,5-T2 might be a relevant TRβ-specific ligand during tilapia development and also for human TRβ as indicated by recent observations (45, 46). Therefore, 3,5-T2 might act as potent endogenous ligand for some of the TR forms under physiological and pathophysiological conditions, albeit at much higher concentrations than T3.
Further experiments are needed to clarify differences observed with respect to cardiac side effects, suppression of HPT axis, and tissue hyperthyroidism after 3,5-T2 administration in our mouse model, as well as in several other reports in mice (32) and rats (21, 23, 24, 38). These observations contrast the impressive series of publications over the last 2 decades on solely beneficial effects of 3,5-T2 on hepatic, skeletal muscle, and systemic lipid profile, body fat, energy metabolism, and mitochondrial action devoid of reported adverse impact on cardiac, CNS, and HPT function (9, 10, 14). Mode of administration (oral, ip, sc), duration and dose of 3,5-T2, possible contamination by T3, age and strain differences of rodent models used as well as distinct composition of diets offered might affect different outcome of these experiments. However, several of these reports also present clear evidence for TR-mediated action of 3,5-T2 on expression of T3-responsive genes in several tissues in addition to rapid plasma membrane, cytosolic signal, and mitochondrial effects previously described. Therefore, a clinical long-term use of the endogenous TH metabolite 3,5-T2 as antihyperlipidemic drug similar to TRβ-selective synthetic ligands without risk of producing T3-like adverse effects might not be realistic yet. So far, there is only 1 publication by Antonelli et al (17) who applied 3,5-T2 to humans in a dosage by orders of magnitude lower than our effective one. However, in the body building scene and wellness internet market, multiple doses of 3,5-T2 tablets are used each containing up to 50 mg. Such intake might lead to comparable ranges of 3,5-T2 exposure in humans as used in our mouse model.
Taken together, our data indicate metabolic changes induced by 3,5-T2 in diet-induced obese male mice, which resulted in decrease of body fat mass and hepatic lipid content. However, 3,5-T2 was not potent enough to reduce body weight, but it favorably altered body composition. Moreover, we detected suppression of the HPT axis with concurrent hepatic accumulation of 3,5-T2. The obvious cardiac side effects of 3,5-T2 observed here in the obese mouse model were unexpected, and an independent study with appropriate cardiac readouts needs to clarify whether they lead to adverse cardiac function or just represent an adaptation to increased metabolic rate. In addition, it needs to be clarified whether effects of 3,5-T2 on cardiac function are either directly 3,5-T2 dependent, TRα or TRβ mediated, or indirect via activation of TH-sensitive adrenergic signaling pathways modulating cardiac function.
Based on our findings, which suggest clear thyromimetic activity of 3,5-T2, the uncritical use of this TH metabolite as a therapeutic antiobesity drug cannot be recommended.
Acknowledgments
We thank Christine Gumz, Carola Gehrmann and Anja Fischbach for skillful technical assistance. We also thank Hannelore Daniel for generously providing us with the opportunity to measure thyroid hormones by LC-MS/MS.
This work was supported by German Research Foundation Grants KFO 218/1, KFO 218/2 and GK 1208, by the German Ministry of Education and Research (DZD) Grant 01GI0922, and by the state of Brandenburg.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 5
- ANCOVA
- analysis of covariance
- CNS
- central nervous system
- CS
- citrate synthase
- Cyp7a1
- cytochrome P450, family 7, subfamily a, polypeptide 1
- Dio
- deiodinase
- HFD
- high-fat diet
- HPT
- hypothalamus-pituitary-thyroid
- LC-MS/MS
- liquid chromatography/mass spectrometry
- LDL
- low density lipoprotein
- Me1
- malic enzyme
- REE
- resting energy expenditure
- 3,5-T2
- 3,5-diiodo-L-thyronine
- TEE
- total energy expenditure
- TH
- thyroid hormone
- TR
- TH receptor
- VCO2
- carbon dioxide production
- VO2
- oxygen consumption.
References
- 1. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flamant F, Gauthier K. Thyroid hormone receptors: the challenge of elucidating isotype-specific functions and cell-specific response. Biochim Biophys Acta. 2013;1830(7):3900–3907. [DOI] [PubMed] [Google Scholar]
- 4. Oppenheimer JH. Thyroid hormone action at the nuclear level. Ann Intern Med. 1985;102(3):374–384. [DOI] [PubMed] [Google Scholar]
- 5. Piehl S, Heberer T, Balizs G, Scanlan TS, Köhrle J. Development of a validated liquid chromatography/tandem mass spectrometry method for the distinction of thyronine and thyronamine constitutional isomers and for the identification of new deiodinase substrates. Rapid Commun Mass Spectrom. 2008;22(20):3286–3296. [DOI] [PubMed] [Google Scholar]
- 6. Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol. 2008;29(2):211–218. [DOI] [PubMed] [Google Scholar]
- 7. Kalyanaraman H, Schwappacher R, Joshua J, et al. Nongenomic thyroid hormone signaling occurs through a plasma membrane-localized receptor. Sci Signal. 2014;7(326):ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lehmphul I, Brabant G, Wallaschofski H, et al. Detection of 3,5-diiodothyronine in sera of patients with altered thyroid status using a new monoclonal antibody based chemiluminescence immunoassay. Thyroid. 2014;24(9):1350–1360. [DOI] [PubMed] [Google Scholar]
- 9. Goglia F. Biological effects of 3,5-diiodothyronine (T(2)). Biochemistry (Mosc). 2005;70(2):164–172. [DOI] [PubMed] [Google Scholar]
- 10. Cioffi F, Zambad SP, Chhipa L, et al. TRC150094, a novel functional analog of iodothyronines, reduces adiposity by increasing energy expenditure and fatty acid oxidation in rats receiving a high-fat diet. FASEB J. 2010;24(9):3451–3461. [DOI] [PubMed] [Google Scholar]
- 11. Angelin B, Kristensen JD, Eriksson M, et al. Reductions in serum levels of LDL cholesterol, apolipoprotein B, triglycerides and lipoprotein(a) in hypercholesterolaemic patients treated with the liver-selective thyroid hormone receptor agonist eprotirome. J Intern Med. 2014. doi:10.1111/joim.12261. [DOI] [PubMed] [Google Scholar]
- 12. Sjouke B, Langslet G, Ceska R, et al. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): a randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol. 2014;2(6):455–463. [DOI] [PubMed] [Google Scholar]
- 13. Lanni A, Moreno M, Lombardi A. 3,5-diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 2005;19(11):1552–1554. [DOI] [PubMed] [Google Scholar]
- 14. Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18(2):239–253. [DOI] [PubMed] [Google Scholar]
- 15. de Lange P, Cioffi F, Senese R, et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes. 2011;60(11):2730–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mollica MP, Lionetti L, Moreno M, et al. 3,5-diiodo-l-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J Hepatol. 2009;51(2):363–370. [DOI] [PubMed] [Google Scholar]
- 17. Antonelli A, Fallahi P, Ferrari SM, et al. 3,5-diiodo-L-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J Biol Regul Homeost Agents. 2011;25(4):655–660. [PubMed] [Google Scholar]
- 18. Silvestri E, Glinni D, Cioffi F, et al. Metabolic effects of the iodothyronine functional analogue TRC150094 on the liver and skeletal muscle of high-fat diet fed overweight rats: an integrated proteomic study. Mol Biosyst. 2012;8(7):1987–2000. [DOI] [PubMed] [Google Scholar]
- 19. Zambad SP, Munshi S, Dubey A, et al. TRC150094 attenuates progression of nontraditional cardiovascular risk factors associated with obesity and type 2 diabetes in obese ZSF1 rats. Diabetes Metab Syndr Obes. 2011;4:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van der Valk F, Hassing C, Visser M, et al. The effect of a diiodothyronine mimetic on insulin sensitivity in male cardiometabolic patients: a double-blind randomized controlled trial. PLoS One. 2014;9(2):e86890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baur A, Bauer K, Jarry H, Köhrle J. 3,5-diiodo-L-thyronine stimulates type 1 5′deiodinase activity in rat anterior pituitaries in vivo and in reaggregate cultures and GH3 cells in vitro. Endocrinology. 1997;138(8):3242–3248. [DOI] [PubMed] [Google Scholar]
- 22. Chin WW. Molecular mechanisms of thyroid hormone action. Thyroid. 1994;4(3):389–393. [DOI] [PubMed] [Google Scholar]
- 23. Horst C, Rokos H, Seitz HJ. Rapid stimulation of hepatic oxygen consumption by 3,5-di-iodo-L-thyronine. Biochem J. 1989;261(3):945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padron AS, Neto RA, Pantaleão TU, et al. Administration of 3,5-diiodothyronine (3,5-T2) causes central hypothyroidism and stimulates thyroid-sensitive tissues. J Endocrinol. 2014;221(3):415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faber J, Heaf J, Kirkegaard C, et al. Simultaneous turnover studies of thyroxine, 3,5,3′ and 3,3′,5′-triiodothyronine, 3,5-, 3,3′-, and 3′,5′- diiodothyronine, and 3′-monoiodothyronine in chronic renal failure. J Clin Endocrinol Metab. 1983;56(2):211–217. [DOI] [PubMed] [Google Scholar]
- 26. Vogel H, Scherneck S, Kanzleiter T, et al. Loss of function of Ifi202b by a microdeletion on chromosome 1 of C57BL/6J mice suppresses 11β-hydroxysteroid dehydrogenase type 1 expression and development of obesity. Hum Mol Genet. 2012;21(17):3845–3857. [DOI] [PubMed] [Google Scholar]
- 27. Klaus S, Rudolph B, Dohrmann C, Wehr R. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol Genomics. 2005;21(2):193–200. [DOI] [PubMed] [Google Scholar]
- 28. Renko K, Hoefig CS, Hiller F, Schomburg L, Köhrle J. Identification of iopanoic acid as substrate of type 1 deiodinase by a novel nonradioactive iodide-release assay. Endocrinology. 2012;153(5):2506–2513. [DOI] [PubMed] [Google Scholar]
- 29. Sandell E, Kolthoff I. Micro determination of iodine by a catalytic method. Microchim Acta. 1937;1(1):9–25. [Google Scholar]
- 30. Kanzleiter T, Rath M, Penkov D, et al. Pknox1/Prep1 regulates mitochondrial oxidative phosphorylation components in skeletal muscle. Mol Cell Biol. 2014;34(2):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 32. Goldberg IJ, Huang LS, Huggins LA, et al. Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology. 2012;153(11):5143–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin DJ, Plateroti M, Samarut J, Osborne TF. Two uniquely arranged thyroid hormone response elements in the far upstream 5′ flanking region confer direct thyroid hormone regulation to the murine cholesterol 7α hydroxylase gene. Nucleic Acids Res. 2006;34(14):3853–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel K, Joharapurkar A, Dhanesha N, et al. Thyroid hormone modulates food intake and glycemia via ghrelin secretion in Zucker fatty rats. Drug Res (Stuttg). 2014;64(10):523–529. [DOI] [PubMed] [Google Scholar]
- 35. Coppola A, Liu ZW, Andrews ZB, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Varela L, Martínez-Sánchez N, Gallego R, et al. Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J Pathol. 2012;227(2):209–222. [DOI] [PubMed] [Google Scholar]
- 37. Sabio G, Cavanagh-Kyros J, Barrett T, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24(3):256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ball SG, Sokolov J, Chin WW. 3,5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J Mol Endocrinol. 1997;19(2):137–147. [DOI] [PubMed] [Google Scholar]
- 39. Williams GR. Thyroid hormone actions in cartilage and bone. Eur Thyroid J. 2013;2(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ladenson PW, Kristensen JD, Ridgway EC, et al. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362(10):906–916. [DOI] [PubMed] [Google Scholar]
- 41. Piehl S, Hoefig CS, Scanlan TS, Köhrle J. Thyronamines–past, present, and future. Endocr Rev. 2011;32(1):64–80. [DOI] [PubMed] [Google Scholar]
- 42. Hackenmueller SA, Scanlan TS. Identification and quantification of 3-iodothyronamine metabolites in mouse serum using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1256:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng SY, Ransom SC, McPhie P, Bhat MK, Mixson AJ, Wintraub BD. Analysis of the binding of 3,3′,5-triiodo-L-thyronine and its analogues to mutant human β 1 thyroid hormone receptors: a model of the hormone binding site. Biochemistry. 1994;33(14):4319–4326. [DOI] [PubMed] [Google Scholar]
- 44. García-G C, López-Bojorquez L, Nuñez J, Valverde-R C, Orozco A. 3,5-Diiodothyronine in vivo maintains euthyroidal expression of type 2 iodothyronine deiodinase, growth hormone, and thyroid hormone receptor β1 in the killifish. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R877—R883. [DOI] [PubMed] [Google Scholar]
- 45. Mendoza A, Navarrete-Ramírez P, Hernández-Puga G, et al. 3,5-T2 is an alternative ligand for the thyroid hormone receptor β1. Endocrinology. 2013;154(8):2948–2958. [DOI] [PubMed] [Google Scholar]
- 46. Navarrete-Ramírez P, Luna M, Valverde-R C, Orozco A. 3,5-di-iodothyronine stimulates tilapia growth through an alternate isoform of thyroid hormone receptor β1. J Mol Endocrinol. 2014;52(1):1–9. [DOI] [PubMed] [Google Scholar]