Abstract
The growth factor progranulin is as an important regulator of transformation in several cellular systems. We have previously demonstrated that progranulin acts as an autocrine growth factor and stimulates motility, proliferation, and anchorage-independent growth of castration-resistant prostate cancer cells, supporting the hypothesis that progranulin may play a critical role in prostate cancer progression. However, the mechanisms regulating progranulin action in castration-resistant prostate cancer cells have not been characterized. Sortilin, a single-pass type I transmembrane protein of the vacuolar protein sorting 10 family, binds progranulin in neurons and negatively regulates progranulin signaling by mediating progranulin targeting for lysosomal degradation. However, whether sortilin is expressed in prostate cancer cells and plays any role in regulating progranulin action has not been established. Here, we show that sortilin is expressed at very low levels in castration-resistant PC3 and DU145 cells. Significantly, enhancing sortilin expression in PC3 and DU145 cells severely diminishes progranulin levels and inhibits motility, invasion, proliferation, and anchorage-independent growth. In addition, sortilin overexpression negatively modulates Akt (protein kinase B, PKB) stability. These results are recapitulated by depleting endogenous progranulin in PC3 and DU145 cells. On the contrary, targeting sortilin by short hairpin RNA approaches enhances progranulin levels and promotes motility, invasion, and anchorage-independent growth. We dissected the mechanisms of sortilin action and demonstrated that sortilin promotes progranulin endocytosis through a clathrin-dependent pathway, sorting into early endosomes and subsequent lysosomal degradation. Collectively, these results point out a critical role for sortilin in regulating progranulin action in castration-resistant prostate cancer cells, suggesting that sortilin loss may contribute to prostate cancer progression.
Prostate cancer is the most frequently diagnosed cancer in the United States. Approximately 233 000 new cases will be diagnosed, and an estimated 29 480 deaths will occur, in the current year (1). Despite extensive experimental studies, the pathogenesis of prostate cancer remains largely unknown. In addition, the molecular mechanisms responsible for the transition to the castration-resistant stage of prostate cancer are very poorly characterized.
Progranulin, also known as proepithelin, PC cell-derived growth factor, granulin-epithelin precursor or acrogranin, is an evolutionary conserved, secreted glycoprotein containing 7 granulin repeats, which plays an important role as a bona fide growth factor in cell proliferation, wound healing, and transformation in several cancer systems (2–4). In addition, progranulin regulates inflammation and neurodegeneration (5), as in fact, progranulin has been suggested as a causative gene for frontotemporal dementia.
We have previously established that progranulin is essential for bladder cancer progression (6, 7). Progranulin promotes motility and invasion of urothelial cancer cells through the activation of the Akt (protein kinase B, PKB) and MAPK pathways and MAPK-dependent activation of paxillin, which may regulate focal adhesion dynamics (6, 7).
We have more recently demonstrated that progranulin plays also a critical role in prostate cancer by promoting castration-resistant prostate cancer cell motility (6) and contributes as an autocrine growth factor to the transforming phenotype by regulating invasion and anchorage-independent growth (6). In addition, we analyzed progranulin expression in different available prostate cancer microarray studies using the Oncomine database (8, 9) and found a statistically significant increase of progranulin mRNA expression levels in prostate cancers compared with nonneoplastic controls (6). Accordingly, progranulin is overexpressed in prostate cancer tissues vis-à-vis nonneoplastic tissue controls (6). Collectively, these results suggest a possible role of progranulin in driving the transition to the castration-resistant stage of prostate cancer.
In spite of the growing body of evidence supporting the important role of progranulin in several pathologies (10), the mechanisms regulating progranulin's mode of action are still very poorly characterized. Furthermore, very few proteins that regulate early stages of progranulin signaling from the membrane have been so far characterized.
Sortilin, a single-pass type I transmembrane protein of the vacuolar protein sorting 10 family that is localized to the cell surface, secretory, and endocytic compartments of eukaryotic cells (11), has been recently identified as a novel progranulin binding partner in neurons where sortilin acts as negative regulator of extracellular progranulin levels by targeting progranulin for rapid endocytosis and lysosomal degradation (11, 12). Notably, progranulin levels are significantly decreased in sortilin knockout mice (11). Reduced progranulin levels are associated with frontotemporal dementia similarly to haploinsufficiency associated with progranulin gene mutations (13, 14), and targeted manipulation of the sortilin/progranulin axis rescues progranulin haploinsufficiency (15).
However, whether sortilin is expressed in castration-resistant prostate cancer cells and plays any role in regulating progranulin actions has not been demonstrated, as are the mechanisms and pathways regulating progranulin endocytosis.
Here, we report that sortilin is expressed at very low levels in castration-resistant PC3 and DU145 cells. Significantly, enhancing sortilin expression in PC3 and DU145 cells severely reduces progranulin levels and inhibits motility, invasion, proliferation, and anchorage-independent growth of these cells. These results are recapitulated by targeting endogenous progranulin in PC3 and DU145 cells. Accordingly, stable ablation of endogenous sortilin by shRNA approaches enhances progranulin levels in PC3 and DU145 cells and promotes motility, invasion, and anchorage-independent growth.
We have also characterized the mechanisms of sortilin action and demonstrated that sortilin mediates progranulin endocytosis and clearance from the medium through clathrin-dependent pathways, sorting into early endosomes followed by lysosomal degradation.
Collectively, these results indicate a critical role for sortilin in regulating progranulin action in castration-resistant cells, suggesting that sortilin loss may contribute to prostate cancer progression.
Materials and Methods
Cell lines
PC3, LNCaP, and DU145 human prostate cancer cells were obtained from ATCC. PC3 and LNCaP cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. DU145 cells were maintained in EARL-modified MEM supplemented with 10% fetal bovine serum, 1% nonessential amino acid, and 1% vitamins. Serum-free medium (SFM) is DMEM containing 0.1% BSA and 50 μg/mL of bovine transferrin (Sigma-Aldrich).
In addition to PC3 and DU145 cells, sortilin expression was assessed by immunoblot using anti-sortilin monoclonal antibodies (R&D Systems) in lysates of urothelial carcinoma-derived 5637, T24, and UMUC-3 cells (from ATCC) and T24T cells, a kind gift of Dr Dan Theodorescu (16–18). R− cells are mouse embryo fibroblasts derived from IGF-IR knockout mice (a kind gift from Dr Renato Baserga) (19). All prostate and bladder cancer cell lines were received from ATCC in 2007, expanded, and stocked in our laboratory cell bank. Once in culture, cells authentication is performed every 6 months according to United Kingdom Co-ordinating Committee on Cancer Research guidelines, including morphology assessment, growth curve analysis, and PCR mycoplasma test. R− cells from Dr Baserga's original stocks are routinely authenticated by confirming lack of IGF insulin receptor (IGF-IR) protein expression by immunoblot analysis.
Human recombinant progranulin
Human progranulin was purified from conditioned medium of 293-EBNA/progranulin cells. This cell line expresses a 6His-tagged human progranulin (20). Serum-free conditioned medium was concentrated with polyethylene glycol, dialyzed, and purified on nickel-nitrilotriacetic acid resin eluted with 250mM imidazole, as previously described (21).
Generation of sortilin-overexpressing PC3 and DU145 cells
PC3 and DU145 cells were transfected with human Sortilin in the pCMV6-XL5 vector (a kind gift from Dr Stephen M. Strittmatter, Yale University School of Medicine), using the TransIt-Prostate transfection reagent (Mirus Corp). Cells were selected in medium supplemented with 2 mg/mL of geneticin (G418) for PC3 and 1.5 mg/mL for DU145, pooled, and tested for sortilin expression in cell lysates by immunoblot using anti-sortilin monoclonal antibodies (R&D Systems).
Generation of progranulin and sortilin-depleted PC3 and DU145 cells
PC3 and DU145 cells stably depleted of either endogenous progranulin or sortilin were generated by transfecting the pRS-shRNA-control (scrambled shRNA) and pRS/shPGRN or pRS/shSORT plasmids (OriGene Technologies, Inc) using the TransIT-Prostate Transfection kit (Mirus). Cells were selected in medium supplemented with 2 μg/mL of puromycin. After selection, pools of progranulin and sortilin-depleted PC3 and DU145 cells were tested for progranulin expression levels in cell lysates and conditioned media by immunoblot using anti-progranulin polyclonal antibodies (US Biologicals) as previously described (6, 7). Sortilin expression was detected using anti-sortilin monoclonal antibodies (R&D Systems).
Migration assay
Transwells with 8.0-μm Polycarbonate Membrane inserts (Corning) were saturated with PBS-1% BSA for 2 hours at room temperature. PC3 and DU145 cells were serum starved for 24 hours. Cells (6 × 105 in 200 μL) were then seeded in upper chambers. Lower chambers contained 500 μL of SFM or SFM supplemented with recombinant progranulin (40nM). After 24 hours, cells in the upper chamber were removed, whereas the cells that migrated to the lower chamber were counted under the microscope after fixation and staining with Coomassie blue solution for 20 minutes.
Wound healing assay
PC3 and DU145 cells were seeded onto 35-mm plates in serum-containing medium until subconfluent and then transferred to SFM. After 24 hours, plates were scratched with a thin disposable tip to generate a wound (200 μm) in the cell's monolayer as previously described (6, 7). Cells were incubated for additional indicated time in SFM. Cells were analyzed and photographed with an Axiovert 200M cell live microscope (Zeiss) using the Metamorph Image Acquisition and Analysis software (Universal Imaging) at the Sidney Kimmel Cancer Center Bioimaging Core Facility.
Invasion assay
Cell invasion through a 3-dimensional extracellular matrix was assessed by a Matrigel invasion assay using BD Matrigel Invasion Chambers (BD Biocoat) with 8.0-μm filter membranes. Cells (5 × 104) in 500 μL of SFM were plated onto each filter, and 750 μL of SFM or SFM were supplemented with progranulin (40nM) in the lower chamber. After 24 hours, filters were washed, fixed, and stained with Coomassie brilliant blue. Cells on the upper surface of the filters were removed with cotton swabs. Cells that had invaded to the lower surface of the filter were counted under the microscope.
Cell growth assay
Cell proliferation was assessed as previously described (22). Briefly, PC3 and DU145 cells were plated in triplicate at a density of 5 × 104 cells/35-mm plate in serum-supplemented medium. After 24 hours, cells were washed 3 times in DMEM and transferred to SFM. Cells were counted after 48 hours with a hemocytometer.
Colony formation assay in soft-agar
Colony formation in soft-agar was performed as previously described (6). Cells were seeded in soft-agar at a density of 5 × 103 cells/35 mm for PC3 and 6 × 103 cells/35 mm for DU145 plate and counted after 4 weeks in culture. Colonies of more than 150 μm were scored as positive.
Confocal microscopy analysis
PC3 cells were plated onto cover glass (corning) and transfected with a green fluorescent protein-Sortilin plasmid (a kind gift from Dr Stephen M. Strittmatter). After transfection, cells were serum starved overnight and incubated with Alexa Fluor 594-labeled progranulin (40nM) at 37°C for indicated time points. To enrich progranulin detection in the lysosomal compartment, inhibitors of the lysosomal pathway (100μM leupeptin) were added at the time of incubation with progranulin. Cells were then washed with 1× PBS and fixed with 4% paraformaldehyde for 20 minutes at room temperature. Subsequently, slides were subjected to immunofluorescence and confocal microscopy analysis as previously described (6, 23–27). Primary antibodies were anti-clathrin and anti-caveolin monoclonal antibodies (BD Transduction Laboratories), anti-early endosome antigen 1 (EEA1) (BD Transduction Laboratories). Lysosomes were detected using LysoTracker deep red (life technologies). Secondary antibodies were goat antimouse IgG Alexa Fluor 647 and goat antirabbit IgG Alexa Fluor 647 antibodies (Invitrogen). Confocal analysis was carried out using a ×63, 1.3 oil-immersion objective of a Zeiss LSM-78 confocal laser-scanning microscope. All images were analyzed using ImageJ (National Institutes of Health [NIH]) and Adobe Photoshop CS6 (Adobe Systems).
For cell live experiments in the presence of lysosomal inhibitors, PC3/Control and PC3/Sortilin cells were plated onto 35-mm glass bottom culture dishes (MatTek Corp). Cells were serum starved for 24 hours, incubated on ice 10 minutes to attenuate endocytosis, and then incubated with Alexa Fluor 594-labeled recombinant progranulin (4 μg/mL) with or without 100μM leupeptin for 1 hour on ice. Cells were then washed 3 times with ice cold PBS and then transferred at 37°C and imaged every 12 seconds for 12 minutes by confocal microscopy (Zeiss LSM 510 UV META) with ×40 objective. The background-corrected Alexa Fluor 594 fluorescence intensity in 4 independent areas was obtained in each cell, which were plotted relative to time = 0 using the software Zeiss AIM 4.2 SP1. Images were analyzed and stacked into the movie using MetaMorph v7.6.5, ImageJ software (NIH Image).
Cycloheximide (CHX)-based Akt protein stability assay
Akt stability was assessed as described (28). Briefly, cells were seeded into 6-well plates in serum-containing medium. The next day, cells were treated with 100μM CHX in SFM and harvested at different time points. Proteins were extracted and analyzed by Western blotting to assess Akt protein stability. After membranes stripping, phosphatidylinositide 3-kinase (PI3K) was detected using anti-PI3K monoclonal antibodies (BD Transduction Laboratories). The intensities of protein bands were analyzed by densitometry using ImageJ software (NIH Image). Akt band intensities for each treatment condition were normalized to β-actin.
Statistical analysis
Results of multiple experiments are expressed as mean ± SD. All statistical analyses were carried out with SigmaStat for Windows version 3.10 (Systat Software, Inc). Results were compared using the two-sided Student's t test. Differences were considered statistically significant at P < .05.
Results
Sortilin expression in multiple cell lines
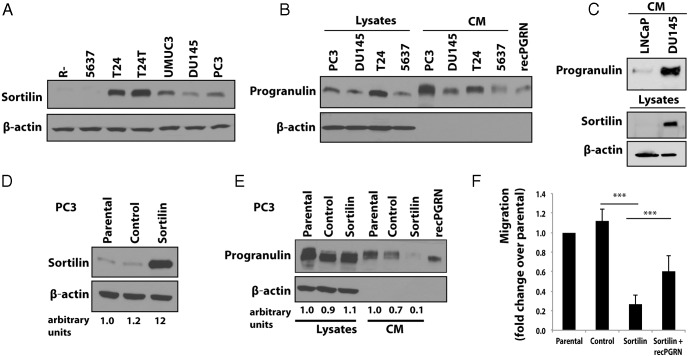
Sortilin has been recently shown to regulate progranulin endocytosis in neuronal cells (11). Because we previously demonstrated that castration-resistant PC3 and DU145 prostate cancer cells secrete progranulin, which acts as autocrine growth factor and promotes motility, proliferation, and invasion of these cells (6), we wanted to determine whether sortilin might play any role in regulating progranulin action in PC3 and DU145 cells. Thus, we first examined by immunoblot sortilin expression levels in PC3, DU145, and various progranulin-responsive cells, including urothelial carcinoma-derived cell lines (5637, T24, T24T, and UMUC-3) and mouse embryo fibroblasts (R−). Sortilin was expressed in all cell lines with the exception of mouse embryo fibroblasts and 5637 urothelial cells (Figure 1A). Significantly, PC3 and DU145 cells showed low levels of sortilin expression (Figure 1A) associated with progranulin expression and secretion in conditioned media (Figure 1B). 5637 and T24 cells were used as control for progranulin secretion (Figure 1B).
Figure 1. Sortilin is expressed at low levels in castration-resistant cells expressing progranulin and sortilin expression levels regulates motility.
A, A total of 40 μg of protein lysates from the indicated cell lines were analyzed for sortilin expression by immunoblot with anti-sortilin monoclonal antibodies. B and C, Progranulin expression in lysates and conditioned medium (CM) of various cell lines was detected by immunoblot as described (6, 7). Immunoblot for β-actin was used as loading control. Blots are representative of 2 independent experiments. D, PC3 cells were transfected with the human Sortilin vector and vector control as described in Materials and Methods. Sortilin expression was tested by immunoblot with anti-sortilin monoclonal antibodies. E, Progranulin expression in various PC3 cell lines was analyzed by immunoblot in lysates and CMs normalized over number of cells as previously described (6, 7). Blots are representative of 2 independent experiments. Densitometric analysis is expressed as arbitrary units. F, Migration was performed as described in Materials and Methods. Data are the average of 3 independent experiments run in triplicates ± SD. ***, P < .001.
We previously demonstrated that androgen-dependent LNCaP cells responded very poorly to progranulin, which barely increased motility and invasion of these cells (6). In addition, LNCaP cells, unlike castration-resistant DU145 and PC3 cells, showed very low migrating ability in serum-free medium, suggesting that LNCaP cells may produce very low levels of progranulin compared with castration-resistant cells (6). These results suggested that progranulin was not critical for biological responses in androgene-sensitive cells but played an important role in castration-resistant prostate cancer cells. However, in order to determine whether sortilin might play a role in regulating progranulin action in androgen-sensitive LNCaP cells, we tested by immunoblot progranulin and sortilin expression levels. Progranulin levels in media conditioned from LNCaP cells were 6-fold lower that DU145 cells (Figure 1C); more importantly, sortilin protein was not detectable in these cells, indicating that sortilin does not play a role in regulating progranulin levels in androgen-sensitive LNCaP cells.
Collectively, these results suggest that low sortilin expression levels might contribute to enhance progranulin action in PC3 and DU145 castration-resistant prostate cancer cells.
Sortilin overexpression regulates progranulin secretion level and biological responses in PC3 and DU145 cells
As a first approach to explore sortilin function in prostate cancer cells, we transfected PC3 and DU145 cells with a plasmid expressing human sortilin. After selection, we isolated 1 pool each of PC3 and DU145 cells expressing considerably higher levels of sortilin than parental and vector-transfected cells as assessed by immunoblot analysis. Compared with V-transfected cells, sortilin levels were up-regulated by 10-fold in PC3 cells (Figure 1D) and by 3-fold in DU145 cells (Supplemental Figure 1A) respectively. Significantly, sortilin overexpression determined a significant reduction of progranulin levels in media conditioned from both PC3 (Figure 1E) and DU145 cells (Supplemental Figure 1B). The decrease of progranulin levels in media of sortilin-overexpressing PC3 and DU145 cells determined a 4-fold inhibition (P < .001 vs control-transfected cells) of the ability of these cells to migrate in serum-withdrawal condition (Figure 1F and Supplemental Figure 1C). Importantly, migration of sortilin-overexpressing PC3 and DU145 cells was restored by incubating the cells with 40nM recombinant progranulin (Figure 1F and Supplemental Figure 1C), although the effect of progranulin stimulation of sortilin-overexpressing PC3 cells was only partial compared with sortilin-overexpressing DU145 cells. This difference is likely due to the higher level of sortilin expression in PC3 cells, which may more effectively counteract progranulin action in these cells.
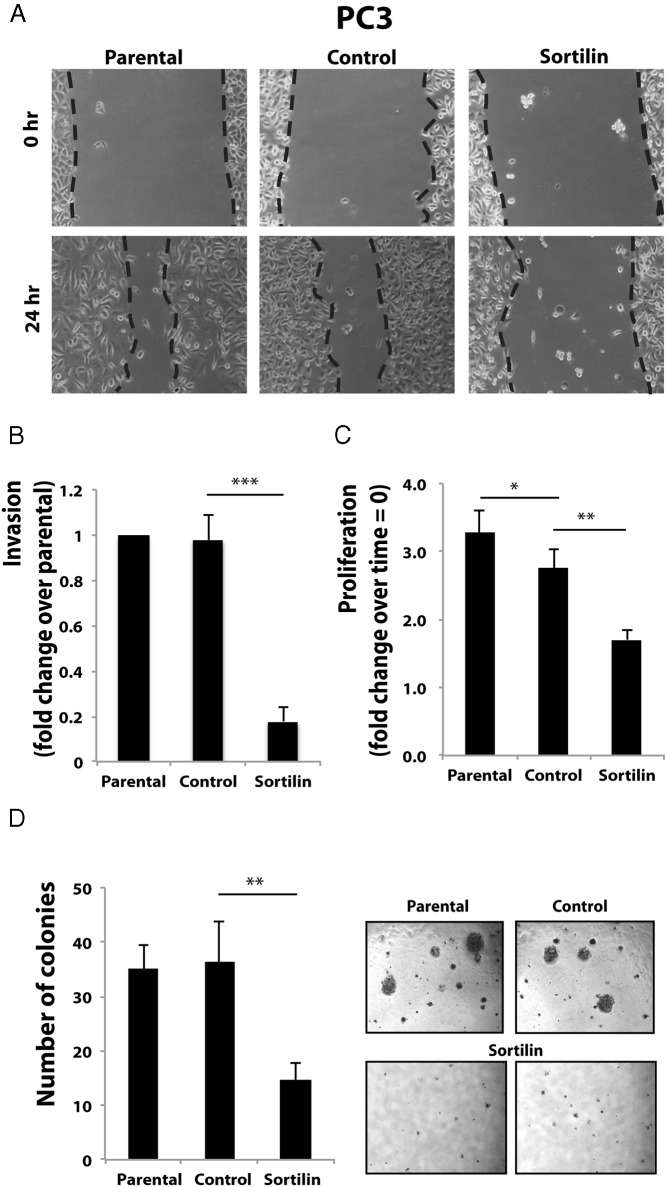
We further determined sortilin action in regulating motility of PC3 and DU145 prostate cancer cells using an in vitro “wound healing” assay (6, 7, 24). PC3 and DU145 cells were plated at high density in serum-containing medium. After 24 hours of starvation in SFM, confluent PC3 cells were wounded and incubated for additional 24 (PC3) or 48 (DU145) hours in SFM. In contrast to parental and control cells, sortilin overexpression determined a substantial inhibition of migration of PC3 (Figure 2A) and DU145 (Supplemental Figure 1D) cells into the denuded area.
Figure 2. Sortilin overexpression in PC3 cells affects progranulin levels and downstream biological responses.
Wound healing (A), invasion (B), proliferation (C), and anchorage-independent growth (D) in soft-agar were performed as described in Materials and Methods. In soft-agar assays, only colonies of more than 150 μm were counted. Data are the average of 3 independent experiments run in triplicates ± SD. *, P < .05; **, P < .01; ***, P < .001.
We then tested the ability of the various prostate cancer cells to invade a 3-dimensional extracellular matrix by performing invasion assays using Matrigel-coated transwells, as we previously described (6, 7, 24). As shown in Figure 2B, sortilin overexpression significantly blunted the ability of PC3 cells to invade (5-fold decrease, P < .001) through extracellular matrix compared with parental and V-transfected PC3 cells.
Because we previously reported that PC3 and DU145 cells express considerable levels of progranulin, which sustained the ability to proliferate in the absence of serum (6), we next determined whether sortilin expression by modulating progranulin levels might also affect the ability of PC3 cells to grow in the absence of serum. Although parental PC3 and V-transfected PC3 cells robustly grew in serum-free condition, sortilin overexpression determined a 2-fold reduction of mitogenesis in these cells (Figure 2C) compared with control-transfected cells (P < .01).
One critical parameter of cell transformation is also the ability of cancer cells to grow in anchorage-independent fashion (29). Thus, we wanted to determine whether sortilin overexpression in PC3 cells affected their capacity to form colonies in soft-agar. Parental and V-transfected PC3 cells formed several colonies bigger then 150 μm in soft agar plates (Figure 2D). On the contrary, sortilin-overexpressing PC3 cells had impaired (2-fold reduction, P < .01) colony formation ability (Figure 2D) as compared with cells expressing the control vector, indicating that enhanced sortilin expression affects anchorage-independent growth of castration-resistant prostate cancer cells by modulating progranulin levels.
Sortilin action in regulating invasion, proliferation, and anchorage-independent growth was confirmed in DU145 cells with results identical to the one obtained in PC3 cells (Supplemental Figure 1, E–G)
Collectively, our results indicate that sortilin may play a critical role in prostate cancer progression by reducing progranulin levels and progranulin-dependent migration, invasion, proliferation, and anchorage-independent growth of castration-resistant prostate cancer cells.
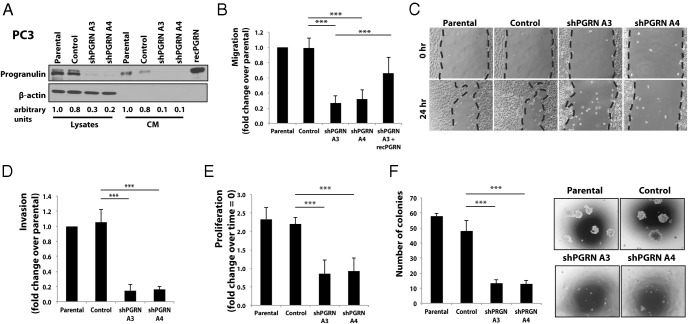
Progranulin depletion in PC3 cells recapitulates the biological responses of PC3/sortilin cells
In order to confirm that the impaired biological responses of sortilin-overexpressing PC3 cells were indeed due to decreased progranulin levels, we generated PC3 cells with a stable depletion of endogenous progranulin by transfecting a 29mer shRNA construct targeting progranulin. Mock-, scrambled-, and shRNA progranulin-transfected PC3 cells were selected in medium containing puromycin, and 2 resistant pools were isolated and tested for progranulin expression. Immunoblot analysis showed that shRNA progranulin cDNA transfection significantly reduced progranulin expression in PC3 cells in both cell lysates and conditioned media (Figure 3A), compared with parental and control-transfected PC3 cells. Progranulin depletion induced a significant reduction (P < .001) of migration (Figure 3B), wound-healing ability (Figure 3C), invasion (P < .001) (Figure 3D), and cell proliferation (P < .001) (Figure 3E) of PC3 cells compared with parental and scramble-control-transfected cells in serum-deprived condition. In addition, progranulin depletion severely reduced (P < .001) colony formation in soft-agar (Figure 3F). The capacity of progranulin-depleted PC3 cells to migrate was significantly restored (P < .001) by supplementing the SFM with 40nM recombinant progranulin (Figure 3B), confirming that the reduction in cell motility of PC3/shPGRN cells is due to reduced progranulin levels.
Figure 3. Progranulin depletion in PC3 cells inhibits motility, invasion, proliferation, and anchorage-independent growth.
A, PC3 cells, stably depleted of endogenous progranulin, were generated by transfecting the pRS-shRNA-control (scrambled shRNA) and pRS/shPGRN plasmids. Cells were selected in medium supplemented with 2 μg/mL of puromycin. After selection, pools of progranulin-depleted PC3 cells were tested for progranulin expression levels in cell lysates and conditioned media by immunoblot using anti-progranulin polyclonal antibodies as previously described (6, 7). Blots are representative of 2 independent experiments. Densitometric analysis is expressed as arbitrary units. Migration (B), wound healing (C), invasion (D), proliferation (E), and anchorage-independent growth (F) in soft-agar were performed as described in Materials and Methods. In soft-agar assays, only colonies of more than 150 μm were counted. Data are the average of 3 independent experiments run in triplicates ± SD. ***, P < .001.
Progranulin was additionally stably depleted in DU145 cells with consequent significant reduction (P < .001) of motility, invasion, proliferation, and anchorage-independent growth in serum-deprived condition (Supplemental Figure 2, A–F).
These results recapitulate the results obtained in sortilin-overexpressing cells strongly supporting the evidence that the impaired biological responses induced by sortilin overexpression are mediated by inhibiting progranulin levels in media of castration-resistant prostate cancer cells.
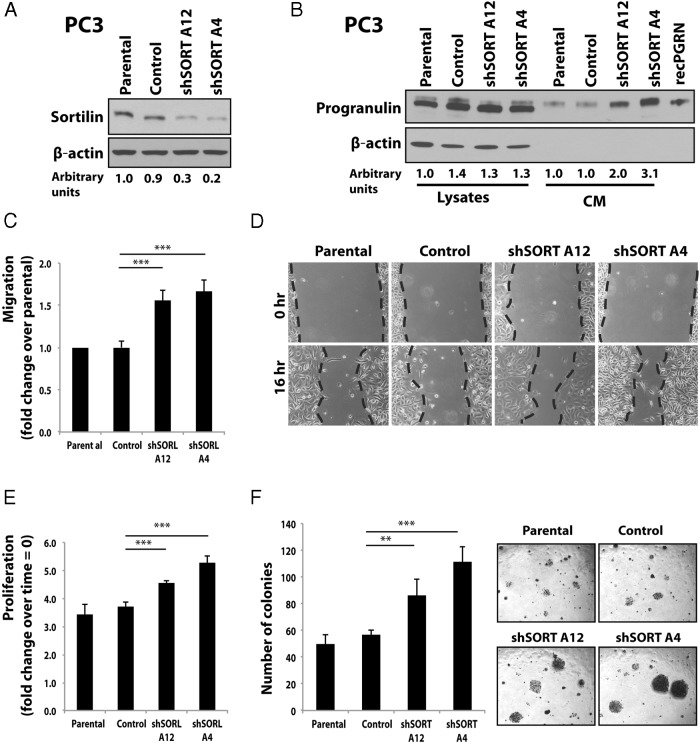
Sortilin depletion in PC3 cells increases progranulin levels and action
As complementary approach to establish sortilin function in regulating progranulin action in castration-resistant prostate cancer cells, we stably depleted endogenous sortilin in PC3 cells by transfecting an anti-sortilin shRNA-expressing plasmid as described for progranulin depletion. Decreased sortilin expression in 2 pools of shSORT-transfected cells (Figure 4A) led to 2-fold (shSORT A12) and 3-fold (shSORT A4) increased levels of extracellular progranulin in conditioned media from PC3 cells (Figure 4B), which significantly (P < .001) enhanced migration (Figure 4C), wound healing (Figure 4D), and proliferation (Figure 4E) in the absence of serum. Notably, sortilin-depleted PC3 cells showed significantly increased anchorage-independent growth as assessed by colony formation in soft-agar (Figure 4F).
Figure 4. Sortilin depletion in PC3 cells enhances progranulin levels and downstream biological responses.
A, PC3 cells stably depleted of endogenous sortilin were generated by transfecting the pRS-shRNA-control (scrambled shRNA) or pRS/shSORT plasmids using the TransIT-Prostate Transfection kit. Cells were selected in medium supplemented with 2 μg/mL of puromycin. After selection, pools of sortilin-depleted PC3 cells were tested for sortilin expression by immunoblot using anti-sortilin monoclonal antibodies (R&D Systems). Densitometric analysis is expressed as arbitrary units. B, Progranulin expression levels in cell lysates and conditioned media as detected by immunoblot using anti-progranulin polyclonal antibodies as previously described (6, 7). Blots (A and B) are representative of 2 independent experiments. Migration (C), wound healing (D), proliferation (E), and anchorage-independent growth (F) in soft-agar were performed as described in Materials and Methods. In soft-agar assays, only colonies of more than 150 μm were counted. Data are the average of 3 independent experiments run in triplicates ± SD. **, P < .01; ***, P < .001.
These results were confirmed in DU145 cells, where sortilin depletion enhanced extracellular progranulin levels and biological responses (Supplemental Figure 3, A–F).
Collectively, these results further support the hypothesis that sortilin is an important regulator of progranulin action in castration-resistant prostate cancer cells.
Characterization of progranulin endocytosis and sorting in PC3 cells
In neurons, sortilin has been shown to promote rapid progranulin endocytosis and subsequent lysosomal degradation (11, 15). However, the pathways by which sortilin mediates progranulin clearance from the medium and internalization from the cell membrane have not been characterized.
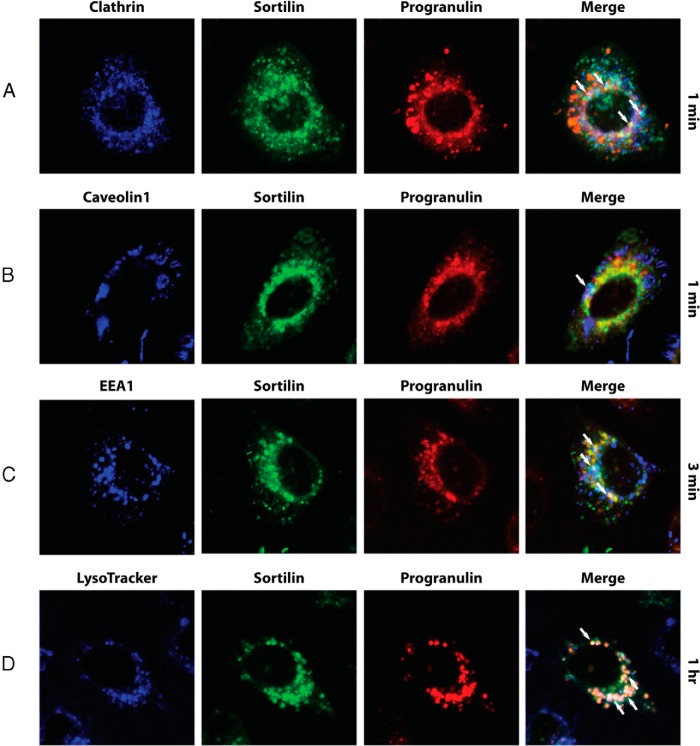
Because growth factor/receptor complexes are internalized by clathrin-dependent and independent pathways, as demonstrated for several tyrosine-kinase receptors, including the epidermal growth factor receptor (30, 31), the IGF-IR (32), and the IR (33), we determined by confocal microscopy analysis whether labeled recombinant progranulin after uptake from media and internalization may colocalize with clathrin or caveolin-1, 2 markers of clathrin-dependent and independent endocytosis pathways, respectively (30, 31, 34). The sortilin/progranulin complex significantly colocalized with clathrin-enriched vesicles (Figure 5A, arrows), whereas there was minimal colocalization between sortilin, progranulin, and caveolin-1 (Figure 5B, arrows) after 1 minute of labeled-progranulin uptake.
Figure 5. The sortilin/progranulin complex preferentially internalizes through clathrin-dependent endocytosis.
Sortilin and progranulin colocalization with clathrin (A), caveolin-1 (B), EEA1 (C), and lysosomes (D) was assessed by confocal microscopy at the Kimmel Cancer Center Bioimaging Core Facility as described in detail in Materials and Methods. All images were analyzed using ImageJ (NIH Image) and Adobe Photoshop CS6 (Adobe Systems). Pictures are representative of at least 10 independent fields from 3 independent experiments. An average of 300 cells was examined for each condition.
To further investigate sortilin-mediated trafficking of progranulin, we next tested whether progranulin colocalized with EEA1, a marker of early endosomes, or with LysoTracker, which specifically labels lysosomes (34). The sortilin/progranulin complex colocalized in EEA1-positive endosomes (Figure 5C, arrows) at 3 minutes, which was followed by progranulin and sortilin sorting into lysosomes, as indicated by a significant colocalization of the sortilin/progranulin complex with LysoTracker staining, colocalization which increased at 1 hour of progranulin uptake (Figure 5D, arrows).
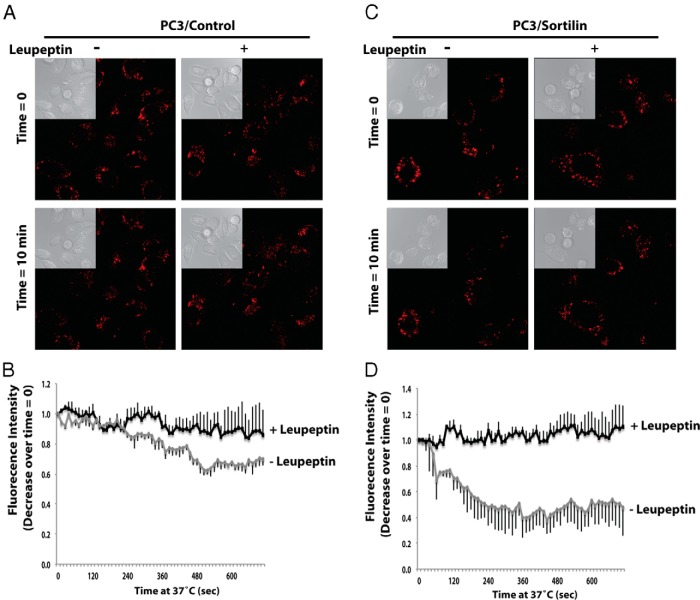
To confirm that progranulin is indeed degraded by the lysosomes, we monitored by cell live microscopy the levels of internalized Alexa Fluor 594-labeled recombinant progranulin in the presence or absence of leupeptin, a specific lysosomal inhibitor. Serum-starved PC3/Control and PC3/Sortilin cells were preincubated on ice to block internalization and then incubated on ice with progranulin for 1 hour. After transferring to 37°C, cells were analyzed by confocal microscopy in continuous for 12 minutes for labeled-progranulin levels. Blocking the lysosomal degradative pathway significantly reduced progranulin degradation in PC3/Control (Figure 6, A and B) and PC3 sortilin-overexpressing cells (Figure 6, C and D, and Supplemental Movies 1 and 2), confirming that sortilin-dependent progranulin degradation occurs in the lysosomes.
Figure 6. Progranulin levels are stabilized by lysosomal inhibition.
Serum starved PC3/Control (A and B) and PC3/Sortilin (C and D) cells were incubated with Alexa Fluor 594-labeled recombinant progranulin with or without leupeptin on ice for 1 hour. After washings, cells were shifted to 37°C and imaged at the indicated time points. These images were also acquired every 12 seconds for 12 minutes using confocal microscopy and Zeiss AIM (Supplemental Movies 1 and 2). A and C, Insets are light microscopy views of the same areas of fluorescence images. B and D, The background-corrected Alexa Fluor 594 fluorescence intensity relative to time 0 was quantified and plotted using the software Zeiss AIM 4.2 SP1. It is expressed as a function of time from experiments as in A and C, respectively. Bars are SD for n = 4.
Collectively, these results demonstrate that sortilin-mediated progranulin uptake from medium and endocytosis occurs preferentially through the clathrin-dependent pathway followed by sorting into early endosomes and subsequent targeting to the lysosomal compartment for degradation.
Sortilin and progranulin levels modulate Akt stability in PC3 cells
We have previously shown that progranulin action in DU145 cells required the activation of both the Akt and the MAPK pathways (6). However, PC3 cells, which lack phosphate and tensin homolog (35), show strong Akt activation in the absence of serum but no detectable MAPK activation as assessed by immunoblot with phospho-specific anti-ERK1/2 antibodies (our unpublished data). Thus, in order to investigate how sortilin and progranulin levels may affect progranulin-dependent downstream signaling, we analyzed Akt activation in parental, V-transfected, and sortilin-overexpressing PC3 cells by immunoblot using phospho-Akt-specific antibodies. Sortilin overexpression significantly reduced Akt activation after 24 hours of incubation in the absence of serum compared with parental and control cells (Figure 7A). Notably, sortilin overexpression was also associated with a 2-fold decrease of Akt protein levels (Figure 7A), suggesting that sortilin overexpression down-regulates Akt levels, thereby only indirectly affecting Akt phosphorylation. These results were confirmed in progranulin-depleted PC3 cells, which showed similar down-regulation of total Akt levels and phosphorylation compared with parental and control-transfected cells (Figure 7B). The levels of the PI3K in PC3 cells were not instead affected by either sortilin overexpression (Figure 7A) or progranulin depletion (Figure 7B), indicating that this effect is specific for Akt and not a more general perturbation of the PI3K/Akt pathway.
Figure 7. Sortilin and progranulin levels modulate Akt stability.
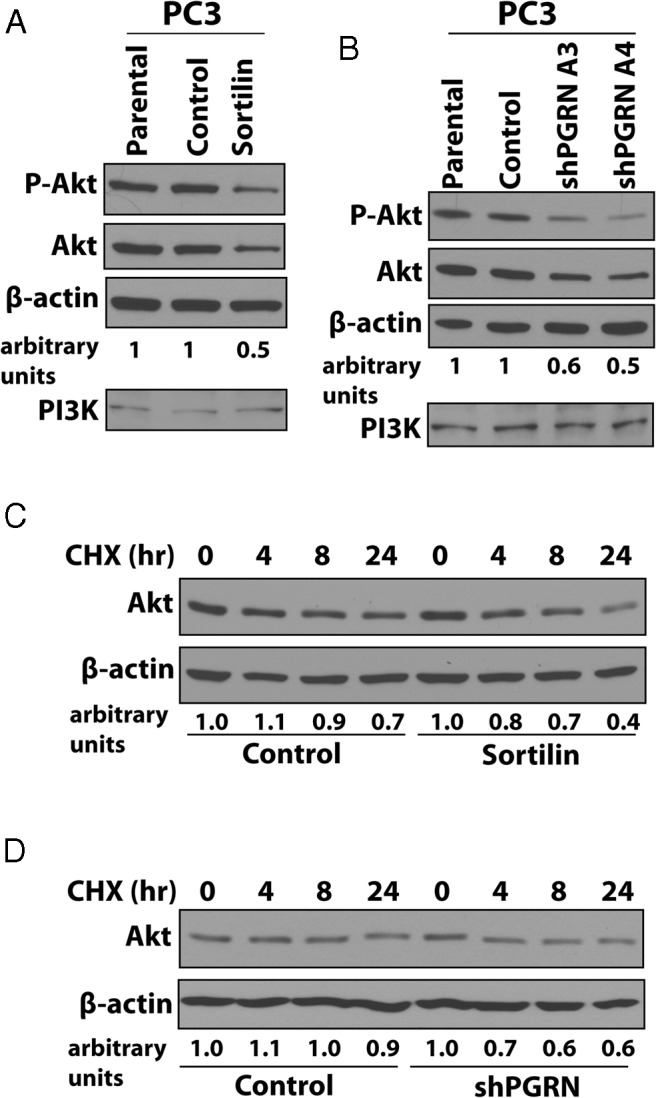
Sortilin-overexpressing (A) and progranulin-depleted (B) cells and controls were tested by immunoblot for phospho-Akt and total Akt levels. As control, the levels of the PI3K were also assessed. Blots are representative of 2 independent experiments. C and D, Akt stability in the presence of 100μM CHX in SFM was assessed by measuring Akt levels by immunoblot at different time points of incubation in SFM (h) as described (28) in (C) sortilin-overexpressing and (D) progranulin-depleted PC3 cells. Blots are representative of 2 independent experiments. The intensities of protein bands were analyzed by densitometry using ImageJ software (NIH Image). Densitometric analysis is expressed in arbitrary units.
Lastly, we determine Akt stability by measuring Akt levels at different time points of incubation in serum-free condition in the presence of 100μM CHX. In both sortilin-overexpressing (Figure 7C) and progranulin-depleted (Figure 7D) PC3 cells, Akt stability decreased by 30% and 32% compared with control-transfected cells, confirming that sortilin and progranulin levels are important in regulating Akt stability and not de novo synthesis of Akt proteins in PC3 cells.
Discussion
In this report, we provide several lines of evidence that underscore a key role for sortilin in the regulation of progranulin action in castration-resistant prostate cancer cells. First, sortilin is expressed at very low levels in castration-resistant PC3 and DU145 cells, which express high levels of progranulin. Second, sortilin expression in PC3 and DU145 cells severely diminishes progranulin levels in conditioned media and inhibits motility, invasion, proliferation, and anchorage-independent growth. Third, these results are recapitulated by stable depletion of endogenous progranulin in PC3 and DU145 cells by transfection of progranulin-specific shRNA plasmids. Fourth, targeting sortilin by shRNA approaches in PC3 and DU145 cells enhances extracellular progranulin levels and promotes motility, invasion, and anchorage-independent growth. Fifth, sortilin promotes progranulin clearance from medium by endocytosis through a clathrin-dependent pathway, sorting into early endosomes and subsequent lysosomal degradation.
Although cases of localized prostate cancer can be managed by surgery or radiation techniques, there are no effective treatments for disseminated prostate cancer. First line of therapy for metastatic prostate cancer is androgen-deprivation therapy, but tumors often recur and progress to the castration-resistant stage with limited therapeutic options (36–38). Moreover, there is a lack of reliable prognostic markers that would identify low- or intermediate-grade prostate cancers that are likely to progress to metastatic disease after local therapy.
We have previously demonstrated that the growth factor progranulin may play a significant role as an autocrine growth factor in prostate tumor progression by promoting proliferation, migration, and anchorage-independent growth of castration-resistant cells prostate cancer cells (6). In addition, we showed that progranulin is overexpressed in prostate cancer compared with normal prostate tissue controls. However, the mechanisms and/or proteins regulating progranulin function in prostate cancer have not been previously characterized. Thus, our results provide the first mechanistic evidence of sortilin function in prostate cancer.
Sortilin has been originally characterized as one of the neurotensin receptors (39). Two of them, neurotensin receptor (NTR)1 and NTR2, are G protein-coupled 7 transmembrane receptors, whereas NTR3 is a single-transmembrane protein identical to sortilin (39). Neurotensin is expressed in prostate cancer cells, where it acts as a growth factor (40) and may play an important role with its receptors in advanced prostate cancers when tumors become enriched with neuroendocrine cells (41). In primary prostate tumors cell cultures, NTR1 expression is up-regulated in cell with luminal phenotype, whereas NTR2 and NTR3 are overexpressed in cells with basal phenotype (42). Sortilin expression in normal and prostate cancer tissues has not been so far examined, and further studies are therefore warranted to clarify this issue.
Our results provide the first demonstration that sortilin modulates extracellular levels of the growth factor progranulin in castration-resistant cells providing an additional layer of complexity on sortilin biological function in prostate cancer, as in fact, our result would suggest that sortilin loss/decreased expression may contribute to prostate cancer progression by enhancing progranulin signaling. On the contrary, sortilin is not expressed in androgen-sensitive LNCaP prostate cancer cells, indicating a nonrelevant role of sortilin in androgen-responsive cells.
Previous work has elegantly demonstrated that in neuronal cells, sortilin affects progranulin levels by promoting endocytosis and clearance from media without affecting progranulin secretion (11, 12). We took into consideration the possibility that the mechanism of sortilin action may differ in prostate cancer cells. However, our experimental approaches strongly support the role of sortilin in regulating progranulin endocytosis in prostate cancer cells and not secretion. In fact, our confocal experiments, which showed progranulin endocytosis and colocalization with clathrin, EEA1, and lysosomal markers, were performed by monitoring solely Alexa Fluor 594-labeled recombinant progranulin, which was exogenously supplied to cells and uptaken from media after internalization. These results support the view that it is the progranulin internalized from the outside the one targeted by sortilin for lysosomal degradation and not endogenous progranulin along the secretory route.
We have characterized the internalization route of the sortilin/progranulin complex and demonstrated that progranulin clearance from media occurs preferentially through clathrin-dependent endocytosis, as in fact we did not detect significant colocalization of labeled-progranulin with caveolin-1, a marker of clathrin-independent/lipid raft endocytosis (30, 31, 34). From clathrin-coated vesicles, the progranulin/sortilin complex is sorted into early endosomes and subsequently routed into lysosomes for degradation. Progranulin sorting is reminiscent of tyrosine-kinases receptors trafficking, where endocytosis is a fine-tuning mechanism regulating the timing and intensity of membrane tyrosine-kinase receptor signaling, as we have discovered for the IGF-IR (32) and the IR (33).
We have previously demonstrated that the E3 ubiquitin ligase neural precursor cell expressed developmentally down-regulated protein 4 (Nedd4) mediates IGF-IR ubiquitination, which is critical for receptor endocytosis and sorting (23, 32). In contrast, ubiquitination is not essential for IR endocytosis and trafficking (33). Significantly, it has been recently shown that the pool of nonpalmitoylated sortilin is ubiquitinated by Nedd4 and targeted for lysosomal degradation (43). However, whether progranulin may induce sortilin ubiquitination in prostate cancer cells has not been established as is the role that ubiquitination may play in regulating progranulin turnover. Based on the above-mentioned data, we could speculate that sortilin by interacting with progranulin and Nedd4 might work as a bridge to bring the ligase and possibly regulate progranulin ubiquitination. Experiments are currently under way to explore this hypothesis.
Another major finding of this work is that sortilin and progranulin expression levels are important in the regulation of Akt stability in PC3 cells, because in fact sortilin overexpression or progranulin depletion severely reduced Akt stability in the absence of serum. Recent work has shown that NEDD4-1 is a novel E3 ligase that specifically regulates ubiquitin-dependent trafficking of P-Akt in IGF-I signaling. NEDD4-1 physically interacts with Akt and promotes homologous to the E6-AP carboxyl terminus domain-dependent ubiquitination of exogenous and endogenous Akt (44). Because sortilin binds Nedd4 (43), these results suggest a possible scenario where sortilin and progranulin may modulate Akt degradation by regulating Nedd4-dependent ubiquitination and proteosomal degradation of Akt.
Sortilin overexpression or progranulin depletion had a really dramatic effect in inhibiting growth of PC3 and DU145 cells in anchorage independency in the presence of serum, a condition that is closer to the cellular environment in vivo. It is, therefore, reasonable to speculate that targeting of progranulin may be very effective on metastases, which can be considered a model of anchorage independence, because they usually arise from single cells or small clusters of metastatic cells. These results strongly suggest that progranulin may be a very attractive target in prostate cancer cells. Further experiments are required to address the role that progranulin may play in regulating these processes in vivo.
In summary, our results indicate that the balance between sortilin and progranulin levels may be critical for prostate cancer progression. In addition sortilin and progranulin expression may constitute novel biomarkers for diagnosis and prognosis of prostate cancer.
Acknowledgments
We thank Dr Strittmatter for the kind gift of the sortilin expression plasmids. Dr Dan Theodorescu kindly provided T24T cells, and R− embryo fibroblast cells were a kind gift from Dr Renato Baserga.
This work was supported in part by Benjamin Perkins Bladder Cancer Fund and National Institutes of Health Grant RO1 CA164462 (to A.Morr. and R.V.I.) and by the Associazione Italiana per la Ricerca sul Cancro (AIRC) Grant 10625/12, AIRC Project Calabria 2014, Fondazione Cassa di Risparmio di Calabria e Lucania PON01_01078 (to A.B.), and the Italian Ministry of Research and Instruction (F.I.R.B. project number RBAP11884M_005) (to K.S.). A.Morc. was supported in part by Fondazione Diabete Ricerca. The Bioimaging Core Facility of the Sidney Kimmel Cancer Center is supported by the National Cancer Institute of the National Institutes of Health under Award P30CA056036.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Akt
- protein kinase B (PKB)
- CHX
- cycloheximide
- EEA1
- early endosome antigen 1
- IGF-IR
- IGF-insulin receptor
- Nedd4
- neural precursor cell expressed developmentally down-regulated protein 4
- NIH
- National Institutes of Health
- NTR
- neurotensin receptor
- PI3K
- phosphatidylinositide 3-kinase
- PRGN
- progranulin
- SORT
- sortilin
- SFM
- serum-free medium
- shRNA
- short hairpin RNA.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2. He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81(10):600–612. [DOI] [PubMed] [Google Scholar]
- 3. He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62(19):5590–5596. [PubMed] [Google Scholar]
- 4. He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9(2):225–229. [DOI] [PubMed] [Google Scholar]
- 5. Cenik B, Sephton CF, Kutluk Cenik B, Herz J, Yu G. Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J Biol Chem. 2012;287(39):32298–32306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Monami G, Emiliozzi V, Bitto A, et al. Proepithelin regulates prostate cancer cell biology by promoting cell growth, migration, and anchorage-independent growth. Am J Pathol. 2009;174(3):1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lovat F, Bitto A, Xu SQ, et al. Proepithelin is an autocrine growth factor for bladder cancer. Carcinogenesis. 2009;30(5):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhodes DR, Yu J, Shanker K, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA. 2004;101(25):9309–9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31(11):1245–1254. [DOI] [PubMed] [Google Scholar]
- 11. Hu F, Padukkavidana T, Vægter CB, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68(4):654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng Y, Brady OA, Meng PS, Mao Y, Hu F. C-terminus of progranulin interacts with the β-propeller region of sortilin to regulate progranulin trafficking. PLoS One. 2011;6(6):e21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;24(442):916–919. [DOI] [PubMed] [Google Scholar]
- 14. Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442(7105):920–924. [DOI] [PubMed] [Google Scholar]
- 15. Lee WC, Almeida S, Prudencio M, et al. Targeted manipulation of the sortilin-progranulin axis rescues progranulin haploinsufficiency. Hum Mol Genet. 2014;23(6):1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gildea JJ, Golden WL, Harding MA, Theodorescu D. Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer. Genes Chromosomes Canc. 2000;27(3):252–263. [DOI] [PubMed] [Google Scholar]
- 17. Gildea JJ, Harding MA, Seraj MJ, Gulding KM, Theodorescu D. The role of Ral A in epidermal growth factor receptor-regulated cell motility. Cancer Res. 2002;62(4):982–985. [PubMed] [Google Scholar]
- 18. Gildea JJ, Seraj MJ, Oxford G, et al. RhoGDI2 is an invasion and metastasis suppressor gene in human cancer. Cancer Res. 2002;62(22):6418–6423. [PubMed] [Google Scholar]
- 19. Sell C, Dumenil G, Deveaud C, Miura, et al. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14(6):3604–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. J Biol Chem. 2003;278(40):38113–38116. [DOI] [PubMed] [Google Scholar]
- 21. Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278(6):4238–4249. [DOI] [PubMed] [Google Scholar]
- 22. Morcavallo A, Buraschi S, Xu SQ, et al. Decorin differentially modulates the activity of insulin receptor isoform A ligands. Matrix Biol. 2014;35:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monami G, Emiliozzi V, Morrione A. Grb10/Nedd4-mediated multiubiquitination of the insulin-like growth factor receptor regulates receptor internalization. J Cell Physiol. 2008;216(2):426–437. [DOI] [PubMed] [Google Scholar]
- 24. Monami G, Gonzalez EM, Hellman M, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66(14):7103–7110. [DOI] [PubMed] [Google Scholar]
- 25. Iozzo RV, Buraschi S, Genua M, et al. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem. 2011;286(40):34712–34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and down-regulates β-catenin and Myc levels. J Biol Chem. 2010;285(53):42075–42085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genua M, Xu SQ, Buraschi S, et al. Proline-rich tyrosine kinase 2 (Pyk2) regulates IGF-I-induced cell motility and invasion of urothelial carcinoma cells. PLoS One. 2012;7(6):e40148. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Hong J, Katsha A, Lu P, Shyr Y, Belkhiri A, El-Rifai W. Regulation of ERBB2 receptor by t-DARPP mediates trastuzumab resistance in human esophageal adenocarcinoma. Cancer Res. 2012;72(17):4504–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyd DD, Levine AE, Brattain DE, McKnight MK, Brattain MG. Comparison of growth requirements of two human intratumoral colon carcinoma cell lines in monolayer and soft agarose. Cancer Res. 1988;48(9):2469–2474. [PubMed] [Google Scholar]
- 30. Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15(2):209–219. [DOI] [PubMed] [Google Scholar]
- 31. Sigismund S, Woelk T, Puri C, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102(8):2760–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vecchione A, Marchese A, Henry P, Rotin D, Morrione A. The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol. 2003;23(9):3363–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morcavallo A, Genua M, Palummo A, et al. Insulin and insulin-like growth factor II differentially regulate endocytic sorting and stability of insulin receptor isoform A. J Biol Chem. 2012;287(14):11422–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6(2):112–126. [DOI] [PubMed] [Google Scholar]
- 35. Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58(13):2720–2723. [PubMed] [Google Scholar]
- 36. Wong YN, Ferraldeschi R, Attard G, de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol. 2014;11(6):365–376. [DOI] [PubMed] [Google Scholar]
- 37. Gomella LG, Petrylak DP, Shayegan B. Current management of advanced and castration resistant prostate cancer. Can J Urol. 2014;21(2 suppl 1):1–6. [PubMed] [Google Scholar]
- 38. Schrecengost R, Knudsen KE. Molecular pathogenesis and progression of prostate cancer. Semin Oncol. 2013;40(3):244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dal Farra C, Sarret P, Navarro V, Botto JM, Mazella J, Vincent JP. Involvement of the neurotensin receptor subtype NTR3 in the growth effect of neurotensin on cancer cell lines. Int J Cancer. 2001;92(4):503–509. [DOI] [PubMed] [Google Scholar]
- 40. Seethalakshmi L, Mitra SP, Dobner PR, Menon M, Carraway RE. Neurotensin receptor expression in prostate cancer cell line and growth effect of NT at physiological concentrations. Prostate. 1997;31(3):183–192. [DOI] [PubMed] [Google Scholar]
- 41. Falkmer S, Askensten U, Grimelius L, Abrahamsson PA. Cytochemical markers and DNA content of neuroendocrine cells in carcinoma of the prostate gland during tumour progression. Acta Histochem Suppl. 1990;38:127–132. [PubMed] [Google Scholar]
- 42. Swift SL, Burns JE, Maitland NJ. Altered expression of neurotensin receptors is associated with the differentiation state of prostate cancer. Cancer Res. 2010;70(1):347–356. [DOI] [PubMed] [Google Scholar]
- 43. Dumaresq-Doiron K, Jules F, Lefrancois S. Sortilin turnover is mediated by ubiquitination. Biochem Biophys Res Commun. 2013;433(1):90–95. [DOI] [PubMed] [Google Scholar]
- 44. Fan CD, Lum MA, Xu C, Black JD, Wang X. Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4-1, in the insulin-like growth factor-1 response. J Biol Chem. 2013;288(3):1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]