Abstract
Antiestrogen therapy induces the unfolded protein response (UPR) in estrogen receptor-positive (ER+) breast cancer. X-box binding protein 1 (XBP1), which exists in the transcriptionally inactive unspliced form [XBP1(U)] and the spliced active form [XBP1(S)], is a key UPR component mediating antiestrogen resistance. We now show a direct link between the XBP1 and NF-κB survival pathways in driving the cell fate decisions in response to antiestrogens in ER+ breast cancer cells, both in vitro and in a xenograft mouse model. Using novel spliced and nonspliceable forms of XBP1, we show that XBP1(U) functions beyond being a dominant negative of XBP1(S). Both isoforms regulate NF-κB activity via ERα; XBP1(S) is more potent because it also directly regulates p65/RelA expression. These findings provide new insights into the fundamental signaling activities of spliced and unspliced XBP1 in breast cancer, establish NF-κB to be a mediator of these activities, and identify XBP1 and its splicing to be novel therapeutic targets.
INTRODUCTION
Approximately 70% of all newly diagnosed breast cancers express the estrogen receptor alpha (ERα) protein. Antiestrogens, such as tamoxifen (TAM) and fulvestrant (ICI 182,780 [ICI]; Faslodex), are widely used to treat these breast cancers, but resistance, either de novo or acquired, limits their curative potential (1). More patients die from ERα-positive (ERα+) breast cancer than from any other breast cancer subtype. Identifying the underlying molecular mechanisms of antiestrogen resistance remains a critical and immediate need. The unfolded protein response (UPR) that cells initiate to recover from endoplasmic reticulum (EnR) stress plays a central role in mediating antiestrogen resistance (2).
EnR stress is sensed by the EnR transmembrane proteins IRE1, PERK, and ATF6; these proteins then activate downstream signaling to enhance proper protein folding, decrease the rate of protein biosynthesis, and promote cell survival in an attempt to recover homeostasis (3). Some UPR functions are prosurvival, and their expression/activation is often altered in cancer cells. We have previously shown that a key UPR effector, X-box binding protein 1 (XBP1), is involved in antiestrogen resistance in breast cancer (4). XBP1 exists in two forms, an unspliced form [XBP1(U)] and a spliced form [XBP1(S)]. The unconventional cytosolic splicing of XBP1 mRNA by IRE1α removes a 26-bp intron, resulting in a translational frameshift and a protein product with a distinct C terminus that can now act as a transcription factor (5, 6). The transactivation domain within the C terminus of XBP1(S) confers its transcription factor activity, whereas the C terminus of XBP1(U) lacks the transactivation domain but contains a nuclear export signal and a protein degradation domain (7).
XBP1(S) is a central UPR effector, binding to EnR stress response elements (ERSEs) and unfolded protein response elements (UPREs) in the promoter of its target genes to induce the transcription of EnR chaperones and other genes involved in coping with EnR stress. In contrast, XBP1(U) may act as an endogenous inhibitor of XBP1(S) by binding and sequestering XBP1(S) outside the nucleus (7, 8). XBP1 expression and splicing are increased in several cancers, including colorectal cancer, hepatocellular carcinoma, and multiple myeloma (9–11). In breast cancer, expression of XBP1 correlates with ERα (12, 13) and nuclear factor kappa B (NF-κB) (14) expression. XBP1(S) expression is upregulated in antiestrogen-resistant cells (4, 15), and overexpression of XBP1(S) can induce both estrogen independence and resistance to TAM and fulvestrant (4). In breast cancer patients, XBP1 mRNA correlates with TAM responses (16). Nonetheless, how XBP1(S) mechanistically confers antiestrogen resistance is unknown. Both XBP1(U) and XBP1(S) bind to ERα and can modulate its transcriptional activity (17), but the relative mechanistic importance of these interactions is unknown. Moreover, the respective importance of the role(s) of XBP1(U) and XBP1(S) in antiestrogen resistance and in most other biological systems remains to be fully determined. This fundamental lack of knowledge is not surprising because of the endogenous splicing of XBP1 and the frequent presence of both XBP1(U) and XBP1(S) in many cells.
NF-κB is also an important signaling molecule in driving antiestrogen resistance (18). NF-κB homodimers or heterodimers are formed from among its five family members, and the most prevalent form in breast cancer is the p50/RelA heterodimer (19, 20). In resting cells, association with the inhibitor of kappa B (IκB) holds p50/RelA heterodimers in a latent form and prevents them from entering the nucleus. Extracellular stimuli, such as tumor necrosis factor alpha, activate IκB kinase (IKK), which then phosphorylates and degrades IκB bound to p50/RelA. The released p50/RelA translocates to the nucleus and regulates transcription of its target genes. NF-κB signaling is upregulated in antiestrogen-resistant breast cancer cells, possibly through increased expression of both RelA and IKKγ (18). Inhibition of NF-κB signaling sensitizes antiestrogen-resistant cells to TAM and fulvestrant (21). Parthenolide, a sesquiterpene lactone isolated from feverfew leaves, inhibits NF-κB signaling by blocking IKK activity and has successfully entered phase I/II clinical trials in cancer patients (22).
In this study, we created a novel and powerful series of molecular constructs to enable us to explore the relative actions of the proteins translated from the spliced and unspliced XBP1 templates. Thus, we created an unspliceable XBP1(U) cDNA where we eliminated the IRE1 splice sites by introducing silent mutations that do not change the amino acid sequence of the unspliced XBP1(U). We now show that inhibition of XBP1(S) increases basal apoptosis and resensitizes antiestrogen-resistant breast cancer cells to antiestrogens. We also show that both XBP1(U) and XBP1(S) positively regulate NF-κB activity via ERα signaling, whereas XBP1(S) also can independently regulate p65/RelA expression. Inhibition of NF-κB signaling reduces the antiestrogen resistance mediated by XBP1 overexpression, apparently by modulating the balance between apoptosis and autophagy. These findings provide new insights into the fundamental biological activities of both the spliced and unspliced XBP1 proteins in breast cancer, establish the importance of NF-κB as a mediator of these activities, and define redundancy in this signaling to regulate NF-κB. These data also firmly establish XBP1 and its splicing to be novel therapeutic targets and directly link two important cell survival signaling pathways (XBP1 and NF-κB) in driving the cell fate decisions in response to antiestrogens in ERα+ breast cancer cells.
MATERIALS AND METHODS
Cell culture.
MCF-7 (ERα+, estrogen dependent, and antiestrogen sensitive), MDA-MB-231 (ERα negative), and MDA-MB-231-ERα (ERα+) human breast cancer cells were maintained in improved minimal essential medium (IMEM) with 5% fetal bovine serum (FBS). MCF-7-derived LCC1 (ERα+, estrogen independent, and antiestrogen sensitive) (23), LCC9 (ERα+, estrogen independent, and TAM and fulvestrant resistant) (24), LY2 (ERα+, estrogen independent, and LY117018, TAM, and fulvestrant resistant) (25, 26), and MCF-7/RR (ERα+, estrogen independent, and TAM and fulvestrant resistant) (27) cells were maintained in IMEM without phenol red and supplemented with 5% charcoal-stripped calf serum (CCS). COS-1 cells were maintained in Dulbecco's modified Eagle medium (DMEM) with 10% FBS. Fulvestrant and benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) were obtained from Tocris Bioscience (Ellisville, MO). 4-Hydroxytamoxifen (4-OHT) and parthenolide were purchased from Sigma-Aldrich (St. Louis, MO).
siRNA transfection.
For short interfering RNA (siRNA) transfections, 2 × 105 cells were transfected with a final concentration of 25 nM siRNA (Qiagen) using the RNAiMax transfection reagent (Invitrogen, Carlsbad, CA). Sequences of the individual siRNAs are as follows: XBP1#1, GGAAGCCAUUAAUGAACUA; XBP1#2, AGCUAAUGUGGUAGUGAAATT; p65/RelA, GAUCUGCCGAGUGAACCGA; ERα, AAGUGGAAUUUGUGGAACAUC; and beclin 1 (BECN1), GGAAUGGAAUGAGAUUAAUGCUGCT. A nontargeting siRNA from Dharmacon RNA Technologies was used as a control.
qRT-PCR.
Total RNA was extracted from breast cancer cells using a Qiagen RNA extraction kit as described by the manufacturer. RNA (1 μg) was reverse transcribed with Bio-Rad iScript reverse transcription (RT) supermix for the quantitative RT-PCR (qRT-PCR) kit, and 1/50 of the resulting cDNA was used to detect mRNA abundance using an ABI 7500 system (Life Technologies). TaqMan assay primer/probe sets were purchased from Life Technologies (for 18S rRNA, primer/probe set Hs99999901; for XBP1, primer/probe set Hs00231936; for p65/RelA, primer/probe set Hs00153294). Relative mRNA levels were calculated using the comparative threshold cycle (CT) method (ΔΔCT) (28).
Western blot analysis.
The following primary antibodies were used: anti-p65/RelA was from BD Biosciences (San Jose, CA), anti-β-actin, p50, NEMO/IKKγ, and IκB antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), Flag M2 antibody was from Sigma-Aldrich, p52 was from Upstate Biotechnology (Charlottesville, VA), LC3II, cleaved caspase-7, cleaved procyclic acidic repetitive protein (PARP), Ki67, IRE1α, and p62 antibodies were from Cell Signaling Technology (Danvers, MA), anti-XBP1(S) antibody was obtained from BioLegend (San Diego, CA), anti-phospho-IRE1α antibody was obtained from Thermo Scientific, and the anti-BCL2 antibody was obtained from Stressgen (Plymouth Meeting, PA). Western blots were developed using chemiluminescent substrate (Denville Scientific, Metuchen, NJ) and quantified with Quantity One software (Bio-Rad, Hercules, CA).
XBP1-overexpressing cell lines.
XBP1(U) and XBP1(S) cDNA constructs (Origene, Rockville, MD) were cloned into the pENTR-D-TOPO vector (Life Technologies). We inserted a Flag tag sequence in the N terminus of XBP1. Mutations in the XBP1 cDNAs were introduced by site-directed mutagenesis using a QuikChange kit (Agilent); see Fig. 3 for the novel XBP1 cDNA constructs. The XBP1-ES48 mutant construct was also generated using a QuikChange kit (Agilent). After verification by DNA sequencing, the pENTR vectors were recombined with pLenti-puro/TO/DEST using an LR Clonase II kit (Life Technologies). The generated pLenti-puro/TO/Flag-XBP1 was packaged in Plat A cells from Cell Biolabs (San Diego, CA), and the resulting lentiviral supernatants were used to infect breast cancer cells. Infected cells were selected with 1 μg/ml puromycin for 3 days.
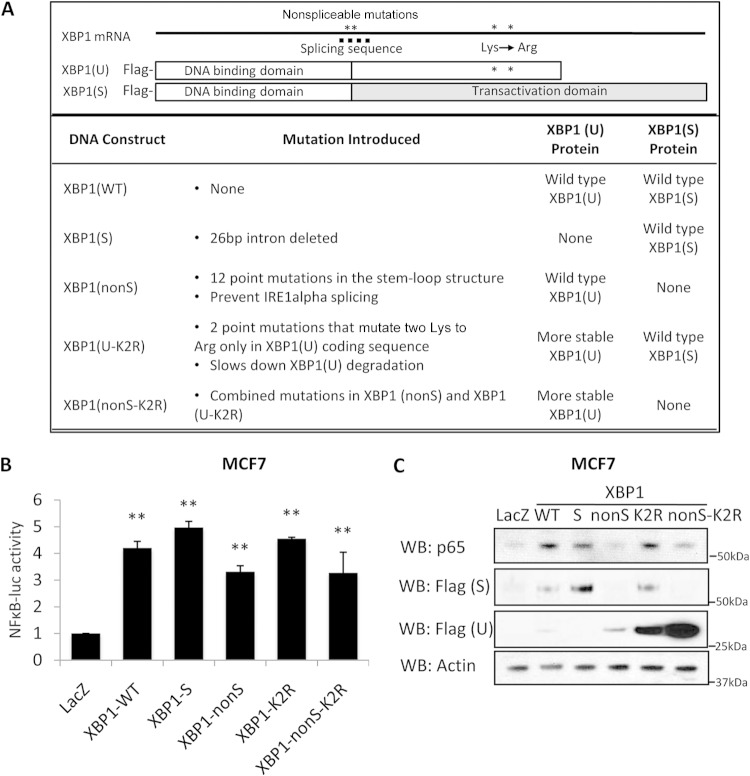
FIG 3.
XBP1(S) regulates the RelA protein expression level. (A) Representation of the XBP1 mutant constructs used in this study. (B) Antiestrogen-sensitive breast MCF-7 cells were transfected with various forms of XBP1 mutants together with pGL3-Basic or pGL3-NF-κB-luc luciferase constructs. At 24 h posttransfection, cells were lysed and luciferase activity was measured. (C) At 48 h after transfection, cell lysates were collected, and the levels of RelA and XBP1 (Flag tag) were examined by Western blot analysis; β-actin was used as the loading control. Data represent the means from at least three independent experiments. **, P < 0.01. For Western blots, representative images from three independent experiments are shown.
Luciferase assay.
The pGL3-NF-κB-luc, ERE-luc, or pGL3-Basic construct was transfected into breast cancer cells together with pRL-TK constructs as a control. At 48 h posttransfection, cells were lysed in passive lysis buffer for dual-luciferase assays according to the manufacturer's protocol (Promega Corp., Madison, WI).
Apoptosis, autophagy, and proliferation.
At 72 h after siRNA treatment, both the breast cancer cells and the culture medium were harvested and stained with annexin V-allophycocyanin-propidium iodide (PI) according to the manufacturer's instructions (BD Biosciences). After incubation, cells were diluted 1:4 in binding buffer and analyzed by flow cytometry. Autophagy was assessed by measuring the autophagy markers LC3II and p62 by Western blot analysis. Cell proliferation was measured by crystal violet staining (cell density). On the day before drug treatment, equal numbers of cells were seeded (2 × 104/well, 48-well plate). After drug treatment, cells were stained with 0.5% crystal violet. The optical density at 595 nm of the dye extract was measured with an automatic microtiter plate reader. Any significant increase at the end of the experiment is an estimate of the population's proliferation (sum of cell replication and cell death) (29).
Immunoprecipitation.
COS-1 cells were transfected with DNA constructs using Lipofectamine LTX and Plus reagents (Life Technology). At 24 h posttransfection, cells were lysed in radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors (Roche). After preclearance with agarose beads, cell lysates were incubated overnight with anti-Flag tag-agarose beads at 4°C (Sigma-Aldrich) and then washed twice with ice-cold phosphate-buffered saline and lysed directly in sample buffer, followed by Western blot analysis.
EMSAs.
Electrophoretic mobility shift assay (EMSAs) were performed with a LightShift chemiluminescent kit from Thermo Scientific according to the manufacturer's protocol. Briefly, complementing biotinylated DNA from Integrated DNA Technologies (IDT) DNA was annealed to form double-stranded DNA. The DNA was incubated with hypoxia-inducible factor 1α (HIF1α) and/or XBP1(S) (Novus) for 20 min before it was loaded on a prerun 6% polyacrylamide gel at 100 V for 1 h. The gel was transferred to a nylon membrane in 0.5× Tris-borate-EDTA (TBE) buffer at 380 mA for 1 h and UV cross-linked at 120 mJ/cm2. The membrane was developed according to the manufacturer's instructions.
Xenograft model.
All animal experiments performed in this study were approved by the Georgetown University Institutional Animal Care and Use Committee (GU-IACUC). Five-week-old female ovariectomized NCR nu/nu mice were obtained from Taconic Farms, Inc. (Germantown, NY). Prior to the cell injection, a 60-day-release 0.36-mg 17β-estradiol pellet (Innovative Research of America, Sarasota, FL) was placed subcutaneously in the lower dorsal region of each nude mouse. Cells (2 × 106), resuspended in cell culture medium and an equal volume of Matrigel (BD Biosciences), were injected into each of the number 4 mammary fat pads. After the tumor size reached 6 mm in diameter, the mice were randomly grouped into four treatment groups: a control group (ethanol injection and regular diet) and groups treated with parthenolide, tamoxifen, and tamoxifen-parthenolide. Parthenolide was given at 1 mg/kg of body weight via intraperitoneal injection twice a week, and tamoxifen (260 mg/kg pure tamoxifen, which is from 400 mg/kg tamoxifen citrate; Voigt Global Distribution) was given in the diet. For all animals, tumor size was recorded twice each week until the end of the experiment, at which point the animals were euthanized.
Statistical analysis.
For each in vitro cell culture study, at least three independent experiments were performed. The collected quantified data were analyzed with one-way analysis of variance. For xenograft in vivo studies, tumor volumes were longitudinally measured. The expectation-conditional maximization (ECM) algorithm developed by Tan et al. was used to estimate any missing or truncated tumor volumes (30). Similar to the quasi-t test (see the work of Tan et al. [30]), we used the weighted F test for comparison between two groups with two tumor volumes (on a log10 scale), since the behaviors of two tumors growing in a single mouse are not considered to represent truly independent events. All computation was performed in the R environment.
Gene expression correlation analysis in public data sets.
Processed data sets were downloaded from the Gene Expression Omnibus (GEO) database, and samples with ERα+ status were selected. The interquartile range (IQR) was calculated from all corresponding probeset_ids for XBP1 and p65/RelA. The probeset_id (Affymetrix) with the larger IQR was used for further analysis of each gene. The Pearson correlation coefficient and the associated P value were calculated for each comparison.
RESULTS
XBP1 inhibition sensitizes antiestrogen-resistant breast cancer cells to antiestrogens.
We have previously shown that XBP1 is upregulated in antiestrogen-resistant LCC9 cells compared with its level of regulation in sensitive parental cells (15). To understand better the role of increased XBP1 in antiestrogen resistance, we knocked down endogenous XBP1 with two independent siRNAs in antiestrogen-resistant LCC9 cells and measured cell proliferation. Depletion of XBP1 strongly inhibited cell proliferation under basal conditions (Fig. 1A). Antiestrogen (fulvestrant) treatment further inhibited cell proliferation in XBP1-depleted cells. To understand further the mechanism responsible for the decreased proliferation seen with XBP1 depletion, we examined the effect on apoptosis/autophagy and cell cycle progression in cells transfected with XBP1 siRNA. XBP1 knockdown induced apoptosis in antiestrogen-resistant cells, as indicated by increased annexin V staining (Fig. 1B). Consistent with the cell proliferation data, antiestrogen treatment further increased the level of apoptosis induced by XBP1 depletion. Increased expression of cleaved caspase-7 and PARP was also observed in XBP1-knockdown cells (Fig. 1C). In contrast, XBP1 knockdown decreased the level of the prosurvival BCL2. Furthermore, pretreatment of cells with the caspase inhibitor z-VAD-fmk prevented apoptosis and reversed the effects on cell growth induced by XBP1 inhibition (see Fig. S1 in the supplemental material). Autophagy initiation was decreased by XBP1 knockdown, since LC3II levels decreased with both XBP1 siRNAs (Fig. 1C). However, XBP1 had no effect on cell cycle progression; the cell cycle profile remained unchanged between control and XBP1-depleted cells (Fig. 1D). In addition, expression of the proliferation marker Ki67 was unchanged in XBP1-depleted cells (see Fig. S2 in the supplemental material), also suggesting that cell cycling is unaltered by XBP1 inhibition. Thus, XBP1 inhibition sensitizes resistant breast cancer cells to antiestrogens by both inducing apoptosis and inhibiting prosurvival autophagy.
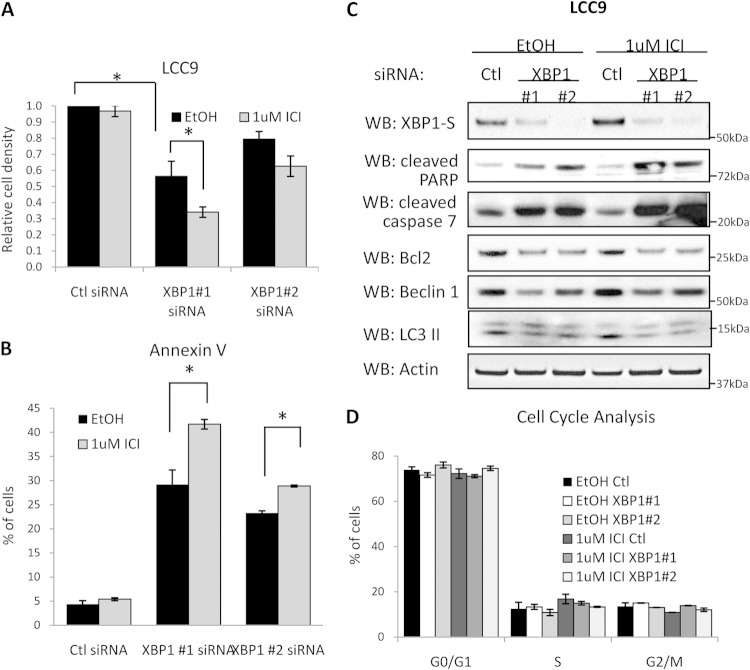
FIG 1.
XBP1 inhibition sensitizes antiestrogen-resistant breast cancer cells to antiestrogens. (A) LCC9 cells were treated with control (Ctl) siRNA or two different XBP1 siRNAs (XBP1#1 and XBP1#2 siRNAs). At 24 h posttransfection, cells were treated with 100 nM fulvestrant (ICI) or 0.01% ethanol (EtOH) as a vehicle control for an additional 7 days. Finally, cells were stained with crystal violet to measure cell density. (B) Transfected cells were treated with 100 nM fulvestrant for 48 h and then collected for annexin V staining followed by flow cytometry. (C) Cell lysates were subjected to Western blot (WB) hybridization for XBP1, cleaved caspase-7, cleaved PARP, p62, and LC3II protein expression; β-actin was measured as the loading control. (D) Transfected cells were treated with 100 nM fulvestrant for an additional 48 h. Cells were also collected and stained with propidium iodide (PI) for cell cycle analysis by flow cytometry. Data represent the means from at least three independent experiments. *, P < 0.05; **, P < 0.01. All Western blot images are representative images from at least three independent experiments.
XBP1 increases NF-κB signaling in antiestrogen-resistant breast cancer cells.
NF-κB is an important mediator of estrogen independence (31) and antiestrogen resistance (18, 21, 32) in breast cancer. To understand the possible interaction between NF-κB and XBP1 signaling and whether XBP1 regulates NF-κB activity, we knocked down XBP1 with siRNA in antiestrogen-resistant LCC9 cells. We measured the transcriptional activity of NF-κB using a pGL3-NF-κB-luc luciferase promoter-reporter construct containing three copies of the NF-κB response element. Depletion of XBP1 resulted in the downregulation of NF-κB luciferase activity (Fig. 2A). We also tested the effect of XBP1 on NF-κB activity in two additional antiestrogen-resistant breast cancer cell lines, MCF-7/RR and LY2, both of which showed enhanced dependence on NF-κB activity (21). A similar downregulation of NF-κB activity was observed in both cell lines following XBP1 knockdown (Fig. 2B and C). These data suggest that XBP1 regulates NF-κB signaling in antiestrogen-resistant breast cancer cells.
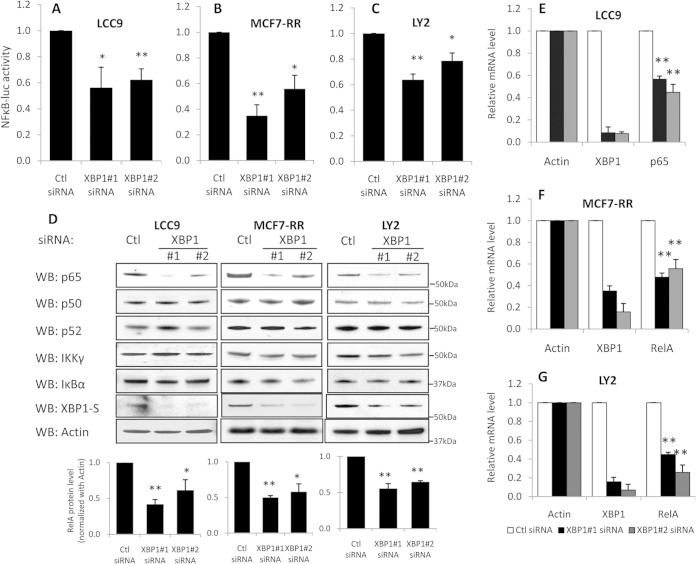
FIG 2.
XBP1 regulates NF-κB signaling by regulating p65/RelA transcription. Three antiestrogen-resistant breast cancer variants (LCC9, MCF-7/RR, LY2) were treated with control siRNA or two different XBP1 siRNAs for 72 h. (A to C) Cells were cotransfected with pGL3-NF-κB-luc or pGL3-Basic luciferase constructs. At 72 h after transfection, cells were lysed and the luciferase activities were measured. (D) Cell lysates were collected and subjected to Western blot hybridization for RelA, p50, IKKγ, and IκBα proteins; β-actin was used as the loading control. The quantification of p65/RelA expression from three independent experiments is shown in the graphs at the bottom. (E to G) Total RNA was extracted, and expression of the 18S, XBP1, and RelA mRNAs was measured by qRT-PCR. Data represent the means from at least three independent experiments. *, P < 0.05; **, P < 0.01. All Western blot images are representative images from at least three independent experiments.
XBP1 regulates p65/RelA expression.
In breast cancer cells, NF-κB signaling is activated in part by upregulation of both p65/RelA and IKKγ expression (18). To determine the mechanism by which XBP1 regulates NF-κB signaling, we knocked down XBP1 with siRNA in antiestrogen-resistant LCC9 cells and measured the expression of key NF-κB signaling components, including p65/RelA, p50, IκB, IKKγ, and p52. The protein level of p65/RelA was significantly lower in XBP1 siRNA-treated cells than control siRNA-treated cells (Fig. 2D). However, expression of IκB, IKKγ, p50, and p52 was not affected by XBP1 knockdown. Regulation of p65/RelA by XBP1 was not limited to LCC9 cells; a similar downregulation of p65/RelA was seen in two additional XBP1-depleted antiestrogen-resistant cell line models, MCF-7/RR and LY2 (Fig. 2D). Furthermore, qRT-PCR analysis showed that the RelA mRNA level was decreased in cells in which XBP1 was knocked down with siRNA, suggesting that XBP1 acts by regulating the rate of NF-κB transcription (Fig. 2E to G). IRE1α is a known mRNA stability regulator which could potentially mediate the downregulation of p65 mRNA in cells in which XBP1 was inhibited. We explored this possibility by determining the activation of IRE1α by measuring its phosphorylation and found that IRE1α is not activated by XBP1 inhibition (see Fig. S3 in the supplemental material).
Overexpression of XBP1 upregulates p65/RelA expression and NF-κB signaling in antiestrogen-sensitive breast cancer cells.
Next, we determined whether XBP1 overexpression is sufficient to regulate p65/RelA and NF-κB signaling in antiestrogen-sensitive breast cancer cells. XBP1 exists in two isoforms, a 37-kDa unspliced form [XBP1(U)] and a 50-kDa spliced form [XBP1(S)]. To determine which isoform is responsible for p65/RelA regulation, we overexpressed XBP1(U) and XBP1(S) in antiestrogen-sensitive MCF-7 breast cancer cells. The wild-type XBP1 [XBP1(WT)] construct encodes the full-length XBP1, which produces both XBP1(U) and XBP1(S) because an adequate expression of activated IRE1α is already present (4). The XBP1(S) construct that we used lacks the 26-bp splicing region, thereby producing only the XBP1(S) protein.
To obtain more definitive data for XBP1(U), we created a nonspliceable XBP1 mutant [XBP1(nonS)] that is resistant to IRE1α splicing and produces only the XBP1(U) protein. We mutated 12 nucleotides in the stem-loop structure that is recognized by IRE1α. The mutations introduced are no longer recognized by IRE1α for splicing, yet the original amino acid sequence of the XBP1(U) protein remains unchanged. Thus, the XBP1(nonS) mutant construct encodes only wild-type XBP1(U). In some cells, the XBP1(U) protein can be rapidly degraded in a proteasome-dependent manner (8). To ensure sufficient expression of XBP1(U), we also mutated the only two lysines (K278 and K286) that are unique to the XBP1(U) C terminus and required for its proteasomal degradation [this construct is referred to as XBP1(K2R)]. The two single nucleotide mutations that replaced lysine with arginine on XBP1(U) lie downstream of the 26-bp deletion and so do not affect the XBP1(S) protein sequence. Finally, we constructed the XBP1(nonS-K2R) mutant, which is both nonspliceable and more stable; this construct also produces only the XBP1(U) protein. The XBP1 constructs that we have created are shown in Fig. 3A.
First, we measured the effect of overexpressing each XBP1 construct on NF-κB transcriptional activity using a promoter-reporter assay. All XBP1 mutants showed enhanced NF-κB luciferase activity, although XBP1(S)-overexpressing cells exhibited slightly higher activity (Fig. 3B). Hence, both XBP1(U) and XBP1(S) contribute to the upregulation of NF-κB activity. Next, we measured p65/RelA expression, which is downregulated by XBP1 knockdown in LCC9 cells. Among each of the mutated cDNA-transfected cells, expression of p65/RelA was upregulated only in the XBP1(WT)-, XBP1(S)-, and XBP1(K2R)-transfected MCF-7 cells. p65/RelA expression in XBP1(nonS)- and XBP1(nonS-K2R)-transfected cells was comparable to that in the control cells (Fig. 3C). These data from the promoter-reporter assay studies suggest that while both XBP1(U) and XBP1(S) promote NF-κB activity, only XBP1(S) directly regulates p65/RelA protein level in ERα+ breast cancer cells. Consistently, transcription from the p65 promoter was activated only in cells in which XBP1(S) was overexpressed and not in cells in which XBP1(U) was overexpressed (see Fig. S4A in the supplemental material). We further explored the mechanism by which XBP1(S) might regulate p65/RelA transcription. Due to the lack of an XBP1(S) conserved binding sequence in the p65/RelA promoter, we tested whether XBP1(S) regulates p65/RelA transcription via another transcription factor, such as HIF1α. HIF1α has been reported to bind with XBP1(S) to regulate transcription, and there is a highly conserved HIF1α binding site in the p65/RelA promoter (33). Analysis by electrophoretic mobility shift assay (EMSA) showed that HIF1α binds to the p65/RelA promoter sequence (see Fig. S4B in the supplemental material). However, we did not observe a further band shift with XBP1(S), suggesting that XBP1(S) does not regulate p65/RelA transcription via interactions with HIF1α. Currently, the transcription factor that is responsible for the XBP1(S) regulation of p65/RelA remains unknown and is under investigation.
XBP1(U) effects on NF-κB signaling require ERα.
XBP1 can regulate ERα transcriptional regulatory activity by directly binding to ERα (17). Moreover, increased ERα expression was observed in XBP1(S)-overexpressing MCF-7 cells (4). As ERα is a putative regulator of NF-κB, we explored the possibility that XBP1 regulates NF-κB activity through ERα signaling. ERE-luc activity was activated by all forms of XBP1 introduced (Fig. 4A). Immunoprecipitation experiments with the XBP1 mutants showed that both XBP1(U) and XBP1(S) can be coimmunoprecipitated with ERα (Fig. 4B). We then knocked down ERα with siRNA in MCF-7 cells overexpressing either LacZ (control), XBP1(S), or XBP1(nonS-K2R). Inhibition of NF-κB transcriptional activity was seen in all cases with ERα knockdown (Fig. 4C), suggesting that NF-κB activity is also regulated by ERα. Moreover, inhibition of NF-κB in cells expressing XBP1(nonS-K2R) (68%) was greater than that in cells expressing XBP1(S) (42%) and the LacZ control (24%). Thus, for the induction of NF-κB activity, XBP1(U) is more dependent upon the presence of ERα than is XBP1(S).
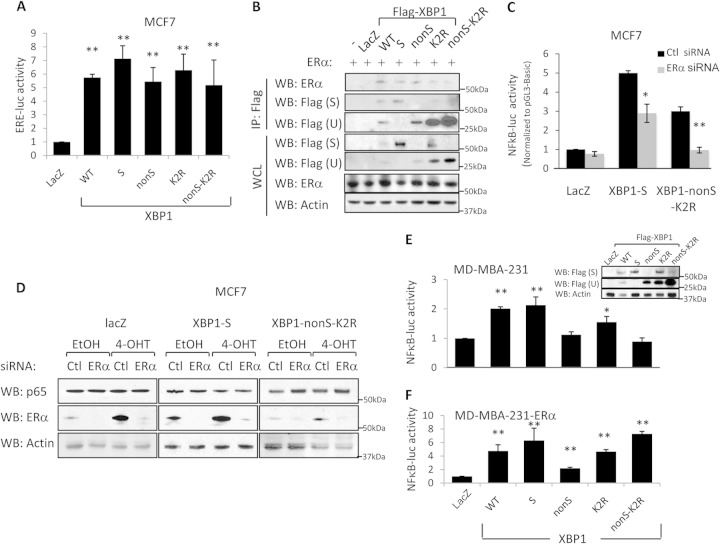
FIG 4.
XBP1 regulates NF-κB via ERα signaling. (A) MCF-7 cells were transfected with XBP1 mutants together with the pGL3-Basic or ERE-luc luciferase construct. At 24 h posttransfection, cells were lysed and luciferase activity was measured. (B) COS-1 cells were transfected with the respective XBP1 constructs together with an ERα-encoding construct. At 24 h posttransfection, cells were lysed, immunoprecipitated with the Flag tag antibody, and subjected to Western blot hybridization for ERα and Flag tag; β-actin was used as the loading control. WCL, whole-cell lysate; IP, immunoprecipitation. (C) XBP1(S)-, XBP1(nonS-K2R)-, and LacZ-overexpressing MCF-7 control cells were transfected with control or ERα siRNA together with pGL3-Basic or ERE-luc luciferase constructs. At 24 h posttransfection, cells were treated with 1 μM 4-OHT for an additional 48 h. Cells were lysed, and luciferase activity was measured. (D) XBP1(S)- and XBP1(nonS-K2R)-overexpressing MCF-7 cells were transfected with control or ERα siRNA. At 24 h posttransfection, cells were treated with 1 μM 4-OHT for an additional 48 h. Cell lysates were then collected, and the expression of RelA, ERα, and β-actin (the loading control) was examined by Western blot analysis. (E and F) Same as the assay described in the legend to Fig. 3B, except that MD-MBA-231 (E) and MD-MBA-231-ERα (F) cells were used. Data represent the means from at least three independent experiments. *, P < 0.05; **, P < 0.01. For Western blots, representative images from three independent experiments are shown.
We also transfected the XBP1 mutant constructs into MDA-MB-231 cells (a triple-negative breast cancer cell line lacking ER, progesterone receptor, and HER2 expression). NF-κB activity was elevated only in cells that overexpressed XBP1(S): XBP1(WT)-, XBP1(S)-, and XBP1(K2R)-transfected cells (Fig. 4E). However, when ERα was introduced into MDA-MB-231 cells by stably overexpressing the ERα cDNA, NF-κB activity was augmented by XBP1(U) overexpression (Fig. 4F). Thus, XBP1(U) and XBP1(S) can affect NF-κB signaling through their respective ability to bind ERα, whereas regulation by XBP1(S)-p65/RelA can also occur independently of ERα signaling. These observations are consistent with the lack of a transcriptional activation domain in XBP1(U), which may act through the ability of ERα-XBP1(U) heterodimers to regulate NF-κB transcription, whereas XBP1(S) can act both through its interactions with ERα [ERα-XBP1(S) heterodimers] and directly as a transcription factor.
NF-κB signaling is required for XBP1-mediated antiestrogen resistance.
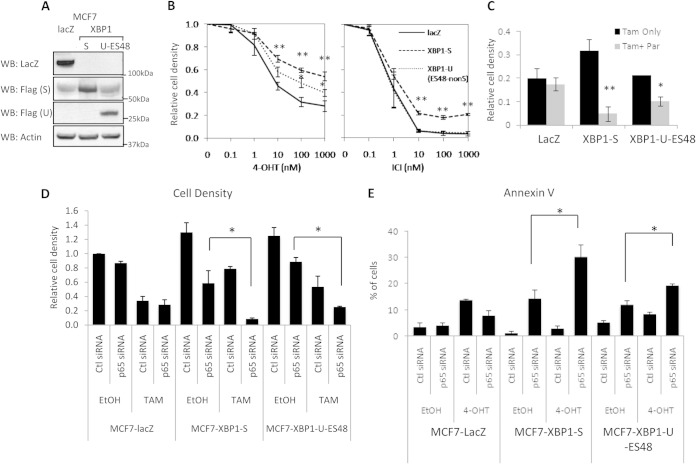
We next determined the role of upregulated NF-κB signaling in XBP1-mediated antiestrogen resistance. We generated MCF-7 cell lines that stably overexpress XBP1(S) and LacZ by lentiviral infection; however, we experienced difficulty in consistently maintaining stable XBP1(U) overexpression. Thus, we generated an XBP1(U)-ES48 construct that encodes a nonspliceable XBP1(U) that produces a truncated form of XBP1(U) lacking 48 amino acids at the C terminus that are essential for XBP1(U) proteasomal degradation. The resulting XBP1(U)-ES48 cells stably overexpress the truncated XBP1(U) (Fig. 5A). Using this novel construct, we first determined the basal responses to antiestrogens of XBP1-overexpressing cells. Both XBP1(S)- and XBP1(U)-ES48-overexpressing cells showed decreased sensitivity to tamoxifen compared with that of LacZ-overexpressing cells (Fig. 5B). Furthermore, XBP1(S) conferred resistance to fulvestrant, whereas XBP1(U)-ES48-overexpressing cells remained sensitive to this antiestrogen.
FIG 5.
NF-κB signaling is required for XBP1-mediated antiestrogen resistance. (A) Lysates from MCF-7 cells overexpressing LacZ, XBP1(S), and XBP1(U)-ES48 were subjected to Western blot hybridization with the antibodies indicated. (B) MCF-7 cells overexpressing XBP1 were treated with different doses of 4-OHT or fulvestrant for 7 days before crystal violet staining to measure changes in cell density. (C) MCF-7 cells overexpressing XBP1 mutants were treated with 4-OHT with or without parthenolide (Par) for 7 days. Cells were then stained with crystal violet. (D) XBP1(S)-overexpressing MCF-7 cells were transfected with control or p65/RelA siRNA. At 24 h posttransfection, cells were treated with 4-OHT or vehicle for an additional 48 h. Cells were stained with crystal violet to assess changes in cell density. (E) Same as the assay described in the legend to panel D, except that the cells were collected for annexin V staining followed by flow cytometry. Data represent the means from at least three independent experiments. *, P < 0.05; **, P < 0.01. For the Western blots, representative images from three independent experiments are shown.
Next, we treated XBP1- or LacZ-overexpressing MCF-7 cells with the NF-κB inhibitor parthenolide. As expected, LacZ-overexpressing MCF-7 cells were sensitive to 4-OHT treatment, and parthenolide had only limited effects when combined with 4-OHT (Fig. 5C). However, addition of parthenolide strongly sensitized XBP1-overexpressing cells to 4-OHT, suggesting that NF-κB signaling is required for XBP1-mediated antiestrogen resistance (Fig. 5C). To avoid possible off-target effects of parthenolide, we also used p65/RelA siRNA to inhibit NF-κB signaling in LacZ-, XBP1(S)-, and XBP1(U)-ES48-overexpressing MCF-7 cells (Fig. 5D). Similar to the results observed with parthenolide, p65/RelA siRNA had only minimal effects on LacZ-overexpressing MCF-7 control cells, either alone or when combined with 4-OHT treatment. In contrast, strong growth-inhibitory effects were observed in XBP1(S)-overexpressing MCF-7 cells and, to a lesser extent, in XBP1(U)-ES48-overexpressing MCF-7 cells, when treated with p65/RelA siRNA alone. In addition, p65/RelA-depleted XBP1-overexpressing MCF-7 cells became highly sensitive to 4-OHT, further establishing a role for p65/RelA in XBP1-mediated antiestrogen resistance.
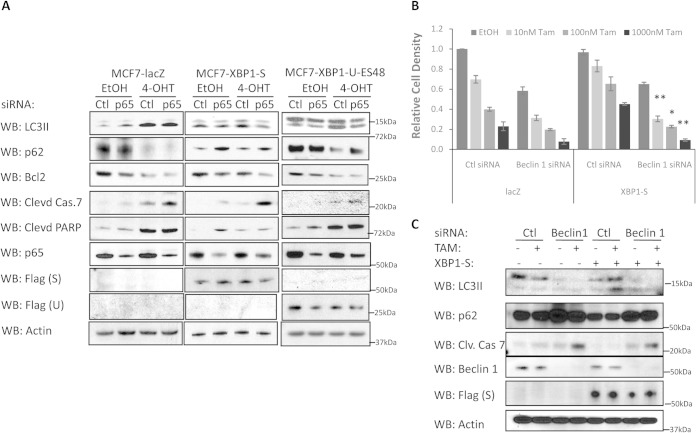
We measured apoptosis in p65/RelA-depleted LacZ-overexpressing or XBP1-overexpressing MCF-7 cells in the presence or absence of 4-OHT. Inhibition of NF-κB signaling strongly induced apoptosis in XBP1(S)-overexpressing MCF-7 cells, as measured by annexin V staining (Fig. 5E). Consistent with this observation, cleaved caspase-7 and PARP also were induced in p65/RelA-depleted XBP1-overexpressing MCF-7 cells, especially when cotreated with 4-OHT (Fig. 6A). Consistent with these observations, prosurvival BCL2 expression was downregulated by p65/RelA knockdown in these cells, and cotreatment with 4-OHT further decreased BCL2 expression. Thus, blocking apoptosis, likely through inhibiting activation of BCL2, appears to be a major mechanism by which XBP1–NF-κB signaling mediates antiestrogen resistance.
FIG 6.
p65/RelA inhibition leads to increased apoptosis in XBP1-overexpressing cells. (A) XBP1(S)-, XBP1(U)-ES48-, or LacZ control-overexpressing MCF-7 cells were transfected with control or p65/RelA siRNA for 24 h before being treated with 1 μM 4-OHT or an ethanol control for an additional 72 h. Cell lysates were obtained and subjected to Western blot hybridization for the antibodies indicated. Clevd, cleaved; Cas.7, caspase-7. (B) MCF7 cells were transfected with control or beclin 1 siRNA together with LacZ or XBP1(S) DNA constructs, followed by a 4-OHT or ethanol control treatment for 7 days before crystal violet staining. (C) Same as the assay described in the legend to panel B, except that cell lysates were collected 48 h after 4-OHT treatment and Western blot hybridization for the antibodies indicated. Clv. Cas 7, cleaved caspase-7. Data represent the means from at least three independent experiments; *, P < 0.05; **, P < 0.01. For the Western blots, representative images from three independent experiments are shown.
Autophagy can act as a survival mechanism and contribute to antiestrogen resistance in breast cancer. Antiestrogen-resistant breast cancer cells display higher levels of basal autophagy than sensitive cells (34). To evaluate the involvement of autophagy in XBP1–NF-κB signaling as it relates to antiestrogen resistance, we measured expression of the autophagy indicators LC3II and p62. As expected, LacZ-overexpressing MCF-7 cells had low levels of basal autophagy, which was strongly increased by 4-OHT treatment. Consistent with its lack of influence on antiestrogen responsiveness in these cells, depletion of p65/RelA had no effect on either LC3II or p62 levels with or without 4-OHT treatment. Similar to antiestrogen-resistant LCC9 cells, XBP1(S)-overexpressing MCF-7 cells displayed much higher basal levels of autophagy (increased basal LC3II levels and decreased p62 levels). The slight induction of LC3II suggests that 4-OHT increases autophagy in XBP1(S)-overexpressing MCF7 cells, but to a much lesser extent than in LacZ-overexpressing MCF7 cells.
Interestingly, 4-OHT-induced autophagy was prevented by p65/RelA siRNA knockdown in XBP1(S)-overexpressing cells, suggesting a role of NF-κB signaling in 4-OHT-induced autophagy in these cells. Unlike XBP1(S)-overexpressing MCF7 cells, the basal autophagy level in XBP1(U)-ES48-overexpressing MCF7 cells remained at a level similar to that in LacZ-overexpressing MCF7 cells. 4-OHT treatment strongly induced autophagy in XBP1(U)-ES48-overexpressing MCF7 cells. Similar to XBP1(S)-overexpressing cells, autophagy induction was weakened by inhibition of p65/RelA with siRNA also in the XBP1(U)-ES48-overexpressing MCF7 cells. To understand further whether the autophagy induced by XBP1(S) is prosurvival, we used siRNA to knock down the autophagy regulator beclin 1 (BECN1; ATG6) in XBP1(S)-overexpressing cells. XBP1(S)-overexpressing cells became sensitive to tamoxifen when autophagy was blocked by beclin 1 knockdown (Fig. 6B and C), demonstrating that the autophagy induced by XBP1(S) overexpression protects the cells against apoptosis. Taken together, these data suggest that prosurvival autophagy is activated in XBP1(S)-mediated antiestrogen resistance and NF-κB signaling contributes to the induction of autophagy.
XBP1 overexpression promotes antiestrogen resistance in a xenograft model in an NF-κB signaling-dependent manner.
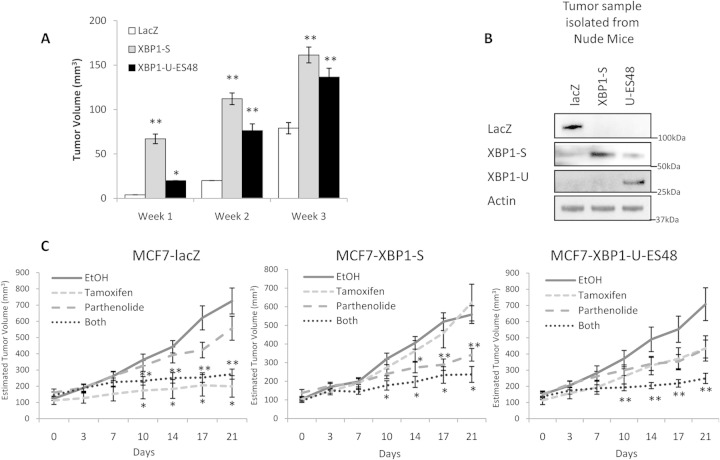
To confirm the role of XBP1 in antiestrogen resistance in vivo, we inoculated the mammary fat pads of athymic nude mice with LacZ-, XBP1(S)-, and XBP1(U)-ES48-overexpressing MCF7 cells. We did not observe any difference in the tumor take rate among the three different cell lines. However, the initial growth rate of XBP1-overexpressing tumors was notably higher than that of the LacZ-overexpressing control tumors (Fig. 7A). By week 3, the volumes of XBP1(S)- and XBP1(U)-ES48-overexpressing tumors were approximately 2-fold and 1.7-fold the volume of the LacZ-overexpressing tumors (controls). XBP1(S) and XBP1(U)-ES48 expression was maintained in the xenografts, as determined by Western blot analysis on materials collected at necropsy (Fig. 7B). To evaluate the effects of drug treatment, mice were randomly assigned to each experimental group (a control group and groups treated with tamoxifen, parthenolide, or tamoxifen-parthenolide) once the tumors had reached 6 mm in their longest diameter. Throughout the experiments, we did not observe significant differences in body weight gains between the different groups of treated animals (see Fig. S5 in the supplemental material). As expected, LacZ-expressing tumors were sensitive to tamoxifen treatment; tumors stopped growing shortly after the initiation of tamoxifen treatment (Fig. 7C). However, LacZ-expressing tumors did not respond to NF-κB inhibition alone, and the combination of tamoxifen and parthenolide did not provide any further reduction in tumor volume beyond that seen with tamoxifen alone. Xenografts from XBP1(S)- and XBP1(U)-overexpressing cells were less sensitive to tamoxifen treatment; tumor volumes did not differ significantly between the ethanol-treated and tamoxifen groups [P > 0.104 for XBP1(S), P > 0.070 for XBP1(U)]. Reflecting our in vitro data, XBP1(S)-overexpressing tumors were more sensitive to NF-κB inhibition. The average tumor volumes in the ethanol-treated group were significantly greater than those in the parthenolide-treated group (day 14, P = 0.021; day 17, P = 0.003; day 21, P = 0.001). However, tamoxifen-parthenolide treatment did not produce any further reduction in XBP1(S) tumor size from that achieved with parthenolide treatment alone, possibly because the suppression achieved with the dose of parthenolide given had reached the maximum suppression that can be achieved in our xenograft model. Consistently, XBP1(U)-overexpressing tumors, which were less sensitive to parthenolide alone, achieved a more significant repression with parthenolide-tamoxifen treatment than parthenolide treatment alone (Fig. 7C).
FIG 7.
XBP1-overexpressing tumors are resistant to tamoxifen in human breast cancer cell xenografts. (A) XBP1(S)-, XBP1(U)-ES48-, or LacZ control-overexpressing MCF-7 cells (2 × 106) were injected into the mammary fat pad of athymic nude mice, and tumors were measured weekly. The graph shows the average tumor volumes at weeks 1 to 3 after tumor injection. (B) Xenografts were harvested at necropsy, and tumor lysates were obtained and subjected to Western blot hybridization with the antibodies indicated. (C) After any tumor in an animal reached 6 mm in diameter, that animal was randomly assigned to a group and treated with either the tamoxifen diet or parthenolide injection, or both; animals receiving a diet without tamoxifen or administered ethanol injections served as controls. After randomization, each group contained 4 to 8 animals; two tumor inoculation sites were used for each animal. Day 0 was taken as the day that drug treatment started. Once treatments started, tumor sizes were measured twice weekly. Data represent the mean tumor volumes in each group. *, P < 0.05; **, P < 0.01. For the Western blots, representative images from three independent experiments are shown.
XBP1 expression correlates with p65/RelA expression in multiple ERα+ breast cancer data sets.
XBP1 and p65/RelA are both highly expressed in antiestrogen-resistant breast cancer. We performed further analysis of the pattern of XBP1 and p65/RelA expression in published clinical ERα+ breast cancer microarray data sets (35–37). Since the patient characteristics, treatments, and other clinical parameters may not have the same distributions across studies and the tissue collection, processing, and other technical issues may also be different, we might not expect all studies to exhibit the same correlations. Nonetheless, in the three independent data sets studied, we found a highly significant correlation between the expression values for XBP1 and p65/RelA mRNA (Table 1). Two of these data sets showed a very strong correlation (>0.70), consistent with our data from the in vitro and in vivo experimental models. This positive correlation is broadly consistent with our finding that XBP1 regulates the expression of p65/RelA, with our earlier study reporting that the proteins are correlated in human breast tumors (14), and with the correlation of XBP1(S) mRNA with a poor clinical response to tamoxifen (16).
TABLE 1.
Pearson correlations of public ERα+ breast cancer data setsa
| Data set name (reference) | Correlation coefficient | P value |
|---|---|---|
| Loi_133A (35) | 0.78 | <2.2e−16 |
| Sotiriou (36) | 0.73 | <2.2e−16 |
| Symmans (37) | 0.28 | <1.06e−06 |
Data from each study were downloaded from the GEO database, and samples with known ERα+ status were selected for analysis. The probeset_id (Affymetrix) with the larger IQR was used for each gene. The Pearson correlation coefficient (which is for XBP1 versus p65/RelA) and the associated P value were calculated for each comparison.
DISCUSSION
Splicing of XBP1(U) by IRE1 enables XBP1(S) to act as a transcription factor, and this has generally been thought to represent XBP1's primary mechanism of action. Previously, studying the role of XBP1(U) has been challenging because many cells express activated IRE1 and both the XBP1(S) and XBP1(U) isoforms are often concurrently present. Unspliced XBP1 has also been reported to act as a dominant negative of XBP1(S), further complicating data analysis/interpretation. To address this critical question, we created an unspliceable XBP1(U) cDNA where we eliminated the splice sites using site-directed mutagenesis. Importantly, these mutations do not change the amino acid sequence of the unspliced XBP1(U). Additional mutations in the ubiquitination sequence allowed us to increase the stability of the translated XBP1(U). Thus, for the first time, we were able to study the relative contributions of XBP1(U) and XBP1(S) in affecting cell survival.
We chose to study the respective roles of XBP1(U) and XBP1(S) in the context of antiestrogen resistance in breast cancer. Previously, we reported that XBP1(S) is overexpressed in antiestrogen-resistant breast cancer cells (15) and that overexpression of XBP1(S) can confer estrogen independence and resistance to both tamoxifen and fulvestrant (4). Moreover, upregulation of XBP1(S) in breast tumors is associated with a poor clinical outcome of tamoxifen treatment (16). Our results show that highly expressed XBP1(S) not only contributes to antiestrogen resistance but also is crucial to the survival of antiestrogen-resistant breast cancer cells. XBP1(S) depletion by siRNA strongly induced apoptosis and inhibited prosurvival autophagy.
Expression of the NF-κB components p65/RelA and IKKγ is upregulated in antiestrogen-resistant cells and may to contribute to antiestrogen resistance (18, 21). In XBP1(S)-depleted antiestrogen-resistant cells, we observed decreased expression of p65/RelA and a downregulation of NF-κB signaling. Conversely, XBP1(S)-overexpressing cells exhibited higher NF-κB activity; inhibition of NF-κB by either the small-molecule inhibitor parthenolide or p65/RelA siRNA sensitized XBP1(S)-overexpressing (antiestrogen-resistant) cells to antiestrogens. Our in vivo xenograft studies confirmed this finding by showing that XBP1(S)-overexpressing tumors become sensitive to NF-κB inhibition. Inhibition of NF-κB can also resensitize resistant XBP1(S)-overexpressing tumors to tamoxifen. These results strongly suggest that XBP1(S)-mediated antiestrogen resistance is NF-κB dependent. We further show that both apoptosis and prosurvival autophagy are regulated by NF-κB and contribute to the antiestrogen resistance mediated by XBP1(S).
While XBP1(S) is known primarily for its function in the UPR stress signaling pathway, functions outside UPR signaling or even independent of its transcriptional regulatory activities have also been reported. For example, in addition to interacting with p85α and FoxO1 (34, 38, 39), XBP1 may interact with ERα to increase ERα transcriptional activity (17). Previously, we have reported that XBP1(S) overexpression increases the level of ERα expression (4). Our results presented here, showing that ERE-luc activity is increased by XBP1(S) overexpression, likely explain these earlier findings. ERα signaling is also involved in the regulation of NF-κB activity by XBP1(S). For example, ERα knockdown by siRNA decreases the induction of NF-κB–luc produced by XBP1(S) overexpression. Upregulation of p65/RelA expression appears to be dependent on XBP1(S) and independent of ERα. However, the mechanism by which XBP1(S) regulates the transcription of p65/RelA needs further investigation.
Unexpectedly, overexpression of the unspliced form, XBP1(U), also resulted in antiestrogen resistance in vitro and in vivo, although to an extent less than that seen with XBP1(S) overexpression. Since XBP1(U) contains the same DNA binding domain as XBP1(S) but lacks a functional transactivation domain, it has generally been believed that XBP1(U) functions primarily as a dominant negative of XBP1(S) (8, 40, 41). However, XBP1(U) promotes cell survival and antiestrogen resistance, suggesting that XBP1(U) has additional mechanistically relevant functions. We show that XBP1(U), similar to prior reports for XBP1(S), directly binds to and activates ERα. ERα likely interacts with either XBP1(U) or XBP1(S) via the N terminus that is shared by both forms of XBP1. Activated ERα induces NF-κB signaling, which is higher in XBP1(U)- and XBP1(S)-overexpressing cells than control cells. However, the potency of XBP1(S) overexpression in inducing NF-κB activity and antiestrogen resistance is greater than that of XBP1(U) overexpression. This observation is consistent with our findings that XBP1(S), but not XBP1(U), can increase the expression of p65/RelA independently of ERα.
The novel XBP1 constructs created in this study have enabled us to determine directly, for the first time, specific functions of XBP1(U). We have demonstrated that XBP1(U) is more than simply a dominant negative for XBP1(S); for example, it can bind to ERα and activate downstream NF-κB signaling. However, we show that XBP1(S) is more potent in activating NF-κB signaling due to its ability to also upregulate p65/RelA expression independently of ERα. Thus, both the spliced and unspliced isoforms can influence cell fate decisions by affecting the balance between prosurvival autophagy and apoptosis in breast cancer. Importantly, this balance is partly mediated through their respective abilities to regulate NF-κB. Our findings establish a regulatory link between XBP1 and NF-κB survival signaling, and data from the in vivo studies strongly suggest that interfering with the XBP1–NF-κB pathway is a potentially novel therapeutic approach for the treatment of some antiestrogen-resistant breast cancers.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Public Health Service awards NIH R01-CA131465 and U54-CA149147 (to R.C.) from the National Institutes of Health and Breast Cancer Research Program postdoctoral fellowship BC100073 (to R.H.) from the U.S. Department of Defense.
We thank Kerrie B. Bouker, Ayesha N. Shajahan, and Katherine L. Cook for their comments on the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00847-14.
REFERENCES
- 1.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O'Brien K, Wang Y, Hilakivi-Clarke LA. 2003. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene 22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 2.Clarke R, Cook KL, Hu R, Facey CO, Tavassoly I, Schwartz JL, Baumann WT, Tyson JJ, Xuan J, Wang Y, Warri A, Shajahan AN. 2012. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res 72:1321–1331. doi: 10.1158/1538-7445.AM2012-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook KL, Shajahan AN, Clarke R. 2011. Autophagy and endocrine resistance in breast cancer. Expert Rev Anticancer Ther 11:1283–1294. doi: 10.1586/era.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC, Zhu Y, Zwart A, Wang M, Clarke R. 2007. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J 21:4013–4027. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- 5.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Oku M, Suzuki M, Mori K. 2006. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol 172:565–575. doi: 10.1083/jcb.200508145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee AH, Iwakoshi NN, Glimcher LH. 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto T, Yoshimatsu K, Watanabe K, Yokomizo H, Otani T, Matsumoto A, Osawa G, Onda M, Ogawa K. 2007. Overexpression of human X-box binding protein 1 (XBP-1) in colorectal adenomas and adenocarcinomas. Anticancer Res 27:127–131. [PubMed] [Google Scholar]
- 10.Munshi NC, Hideshima T, Carrasco D, Shammas M, Auclair D, Davies F, Mitsiades N, Mitsiades C, Kim RS, Li C, Rajkumar SV, Fonseca R, Bergsagel L, Chauhan D, Anderson KC. 2004. Identification of genes modulated in multiple myeloma using genetically identical twin samples. Blood 103:1799–1806. doi: 10.1182/blood-2003-02-0402. [DOI] [PubMed] [Google Scholar]
- 11.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, Yamamoto N, Yamamoto M. 2003. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol 38:605–614. doi: 10.1016/S0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix M, Leclercq G. 2004. About GATA3, HNF3A, and XBP1, three genes co-expressed with the oestrogen receptor-alpha gene (ESR1) in breast cancer. Mol Cell Endocrinol 219:1–7. doi: 10.1016/j.mce.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Tozlu S, Girault I, Vacher S, Vendrell J, Andrieu C, Spyratos F, Cohen P, Lidereau R, Bieche I. 2006. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr Relat Cancer 13:1109–1120. doi: 10.1677/erc.1.01120. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y, Singh B, Hewitt S, Liu A, Gomez B, Wang A, Clarke R. 2006. Expression patterns among interferon regulatory factor-1, human X-box biniding protein-1, nuclear factor kappa B, nucleophosmin, estrogen receptor-alpha and progesterone receptor proteins in breast cancer tissue microarrays. Int J Oncol 28:67–76. doi: 10.3892/ijo.28.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Gu Z, Lee RY, Skaar TC, Bouker KB, Welch JN, Lu J, Liu A, Zhu Y, Davis N, Leonessa F, Brunner N, Wang Y, Clarke R. 2002. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to Faslodex (ICI 182,780). Cancer Res 62:3428–3437. [PubMed] [Google Scholar]
- 16.Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, Rudland PS, Sibson DR. 2008. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. Int J Cancer 123:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 17.Ding L, Yan J, Zhu J, Zhong H, Lu Q, Wang Z, Huang C, Ye Q. 2003. Ligand-independent activation of estrogen receptor alpha by XBP-1. Nucleic Acids Res 31:5266–5274. doi: 10.1093/nar/gkg731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riggins RB, Zwart A, Nehra R, Clarke R. 2005. The nuclear factor kappa B inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant breast cancer cells. Mol Cancer Ther 4:33–41. doi: 10.1186/1476-4598-4-33. [DOI] [PubMed] [Google Scholar]
- 19.Chen LF, Greene WC. 2004. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 20.Xiao G, Rabson AB, Young W, Qing G, Qu Z. 2006. Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev 17:281–293. doi: 10.1016/j.cytogfr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Nehra R, Riggins RB, Shajahan AN, Zwart A, Crawford AC, Clarke R. 2010. BCL2 and CASP8 regulation by NF-kappaB differentially affect mitochondrial function and cell fate in antiestrogen-sensitive and -resistant breast cancer cells. FASEB J 24:2040–2055. doi: 10.1096/fj.09-138305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghantous A, Gali-Muhtasib HF, Vuorela HF, Saliba N, Darwiche FAU, Darwiche N. 2010. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today 15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Brunner N, Boulay V, Fojo A, Freter CE, Lippman ME, Clarke R. 1993. Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Res 53:283–290. [PubMed] [Google Scholar]
- 24.Brunner N, Boysen B, Jirus S, Skaar TC, Holst-Hansen C, Lippman J, Frandsen T, Spang-Thomsen M, Fuqua SA, Clarke R. 1997. MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182,780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res 57:3486–3493. [PubMed] [Google Scholar]
- 25.Bronzert DA, Greene GL, Lippman ME. 1985. Selection and characterization of a breast cancer cell line resistant to the antiestrogen LY 117018. Endocrinology 117:1409–1417. doi: 10.1210/endo-117-4-1409. [DOI] [PubMed] [Google Scholar]
- 26.Clarke R, Brunner N, Katz D, Glanz P, Dickson RB, Lippman ME, Kern FG. 1989. The effects of a constitutive expression of transforming growth factor-alpha on the growth of MCF-7 human breast cancer cells in vitro and in vivo. Mol Endocrinol 3:372–380. doi: 10.1210/mend-3-2-372. [DOI] [PubMed] [Google Scholar]
- 27.Butler WB, Fontana JA. 1992. Responses to retinoic acid of tamoxifen-sensitive and -resistant sublines of human breast cancer cell line MCF-7. Cancer Res 52:6164–6167. [PubMed] [Google Scholar]
- 28.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frandsen T, Boysen BE, Jirus S, Spang-Thomsen M, Dano K, Thompson EW, Brunner N. 1992. Experimental models for the study of human cancer cell invasion and metastasis. Fibrinolysis 6:71–76. [Google Scholar]
- 30.Tan M, Fang HB, Tian GL, Houghton PJ. 2002. Small-sample inference for incomplete longitudinal data with truncation and censoring in tumor xenograft models. Biometrics 58:612–620. doi: 10.1111/j.0006-341X.2002.00612.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr. 1997. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 17:3629–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, Lee J, Fisher SJ, White MF, Biddinger SB, Ozcan U. 2011. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med 17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, Mai J, Shen H, Hu DZ, Adoro S, Hu B, Song M, Tan C, Landis MD, Ferrari M, Shin SJ, Brown M, Chang JC, Liu XS, Glimcher LH. 2014. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature 508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook KL, Shajahan AN, Warri A, Jin L, Hilakivi-Clarke LA, Clarke R. 2012. Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness. Cancer Res 72:3337–3349. doi: 10.1158/0008-5472.CAN-12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loi S, Haibe-Kains B, Desmedt C, Lallemand F, Tutt AM, Gillet C, Ellis P, Harris A, Bergh J, Foekens JA, Klijn JG, Larsimont D, Buyse M, Bontempi G, Delorenzi M, Piccart MJ, Sotiriou C. 2007. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol 25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 36.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M. 2006. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 37.Symmans WF, Hatzis C, Sotiriou C, Andre F, Peintinger F, Regitnig P, Daxenbichler G, Desmedt C, Domont J, Marth C, Delaloge S, Bauernhofer T, Valero V, Booser DJ, Hortobagyi GN, Pusztai L. 2010. Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol 28:4111–4119. doi: 10.1200/JCO.2010.28.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. 2010. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med 16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. 2010. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med 16:438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo F, Lin EA, Liu P, Lin J, Liu C. 2010. XBP1U inhibits the XBP1S-mediated upregulation of the iNOS gene expression in mammalian ER stress response. Cell Signal 22:1818–1828. doi: 10.1016/j.cellsig.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida H, Uemura A, Mori K. 2009. pXBP1(U), a negative regulator of the unfolded protein response activator pXBP1(S), targets ATF6 but not ATF4 in proteasome-mediated degradation. Cell Struct Funct 34:1–10. doi: 10.1247/csf.06028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.