Abstract
The Dgcr14/Es2 gene is located in a chromosomal region the loss of which has been associated with DiGeorge syndrome, a cause of immunodeficiency, heart defects, and skeletal abnormalities. However, the role of DGCR14 protein remains to be elucidated. Here, I found that DGCR14 protein acts as a coactivator of RORγt in TH17 cells. Biochemical purification of the RORγ coregulator complex allowed me to identify the associated DGCR14 protein by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Overexpression of Dgcr14 mRNA enhanced RORγt-mediated transcriptional activity and facilitated TH17 cell differentiation. Furthermore, knockdown of Dgcr14 reduced Il17a mRNA expression. I also found that DGCR14 associated with ribosomal S6 kinase 2 (RSK2, also called RpS6ka3) and BAZ1B, both of which were recruited to the Il17a promoter during TH17 cell differentiation. Knockdown of Baz1b or RpS6ka3 also reduced Il17a mRNA expression, and Baz1b knockdown increased transcriptional suppressive histone marks (histone H3K9me3) on the Il17a promoter. My findings showed the roles of DGCR14, RSK2, and BAZ1B in the transcriptional regulation of Il17a mRNA during TH17 cell differentiation.
INTRODUCTION
Retinoid-related orphan nuclear receptor gamma (RORγ, also called Rorc or Nr1f3) is a member of the nuclear hormone receptor (NR) superfamily. RORγ regulates gene transcription by binding as a monomer to specific ROR response elements (ROREs) consisting of the consensus core motif RGGTCA preceded by a 6-bp A/T-rich sequence (1). RORγ controls circadian rhythm, lymphocyte development, and lipid and glucose homeostasis. RORγ expression exhibits an oscillatory pattern (low levels during the day and maximal levels at night) in the liver, brown adipose tissue, and kidneys (2). Mice deficient in RORγ exhibit improved insulin sensitivity and glucose tolerance because of reduced hepatic gluconeogenesis, particularly during the daytime (3).
More importantly, RORγ knockout mice lack peripheral and mesenteric lymph nodes and Peyer's patches (4). Furthermore, RORγt, which is an isoform encoded by the Rorc gene, is highly expressed in lymphocytes and acts as a key regulator in the development of TH17 cells (5). The N-terminal region amino acid sequence of RORγt differs from that of RORγ, but the DNA- and ligand-binding regions are conserved. RORγt knockout mice have diminished numbers of TH17 cells and are protected against experimental autoimmune encephalomyelitis (2). Because TH17 cells play a pivotal role in autoimmune diseases, suppression of the transcriptional activities of RORγt is critical for developing therapeutics for TH17-mediated autoimmune disorders, including multiple sclerosis and rheumatoid arthritis. Recent studies have described the synthesis of inverse agonists of RORγ to abrogate TH17 cell function (6–9). However, the molecular mechanism of RORγ-dependent transcriptional regulation is not fully understood.
In general, transcriptional control by NRs depends on multiprotein coregulatory complexes (10, 11). After chromatin remodeling and decreased nucleosome density, NRs bind to DNA elements. The associated transcriptional coactivators/corepressors are specific and depend on DNA elements and other transcriptional factors' context. Recent studies showed that corepressors are also necessary for recruiting coactivators (12). Moreover, the association and dissociation of coregulators constitute a transcriptional cycle (13). Thus, the identification of associated transcriptional coregulators for RORγt in CD4+ T cells would be beneficial for understanding the regulation of its transcriptional activity.
Here, I purified and identified transcriptional coregulators of RORγ in T-lymphocyte-related cells. Among the identified known coregulators, I found that DGCR14 acts as a coactivator of RORγ function, although it does not have any known functional domain. I also identified proteins that associated with DGCR14. Among them, RSK2 and BAZ1B associated with DGCR14 protein on the Il17a promoter. These results showed the importance of the DGCR14/RSK2/BAZ1B pathway for TH17 cell differentiation and autoimmune disease.
MATERIALS AND METHODS
Cell culture.
Cells of the murine T-lymphocyte-related line 68-41 were provided by Masato Kubo (Research Center for Allergy and Immunology, Yokohama, Japan) and cultured as described previously (14). 68-41 cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 50 U penicillin, 50 μg ml−1 streptomycin, and 100 nM nonessential amino acids. For Rorc and Il17a mRNA induction, cells were stimulated with 1 μg ml−1 anti-CD3ε antibody in the presence or absence of 10 ng ml−1 recombinant interleukin-6 (IL-6; Peprotech) and 2 ng ml−1 transforming growth factor β (TGF-β; Peprotech) as described above. 293T cells were maintained in Dulbecco's modified Eagle's medium with 10% FBS, 50 U penicillin, and 50 μg ml−1 streptomycin.
Protein purification and mass spectrometry.
For RORγ complex purification, 68-41 cells were incubated with anti-CD3ε (1 μg ml−1) antibody, 2 ng ml−1 TGF-β, and 10 ng ml−1 IL-6 for 8 h, after which nuclear extracts were prepared as previously described (15). Extracts were fractionated with protein G-Sepharose and eluted with 0, 100, 200, 300, 500, and 1,000 mM NaCl in buffer D (20 mM HEPES [pH 7.8], 20% glycerol, 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 0.5 mM dithiothreitol [DTT]). RORγ-containing fractions (100 to 300 mM NaCl in buffer D) were collected and bound to an anti-RORγ antibody resin column prepared as previously described (16), washed with binding buffer (20 mM HEPES [pH 7.8], 250 mM KCl, 0.2 mM EDTA, 0.1% NP-40, 0.2 mM PMSF), and eluted with 0.1 M Tris-glycine (pH 3.0). After elution, proteins were separated by SDS-PAGE and then identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Ultraflex TOF/TOF; Bruker) as previously described (16). For database searches by MS-Fit (University of California, San Francisco), the following parameters were used. Trypsin max missed cleavages = two, constant modification was carbamidomethyl (C), masses were monoisotopic, mass tolerance was 50 to 75 ppm, the instrument was MALDI-TOF/TOF, and the data format was PP M/Z intensity charge.
For DGCR14 complex purification, I generated a retroviral vector expressing DGCR14-Flag and transduced it into 68-41 cells. Cultured DGCR14-Flag-expressing 68-41 cells were treated for nuclear extract preparation. Nuclear extracts were then fractionated by glycerol density gradient centrifugation (10 to 40% glycerol in buffer D, 18,000 rpm, 4°C, 20 h). After Western blotting (see Fig. 4A), DGCR14-Flag-expressing fractions were collected and precipitated with anti-Flag M1 agarose affinity gel (Sigma) and then eluted with 100 μg ml−1 Flag peptide (Sigma) in buffer D.
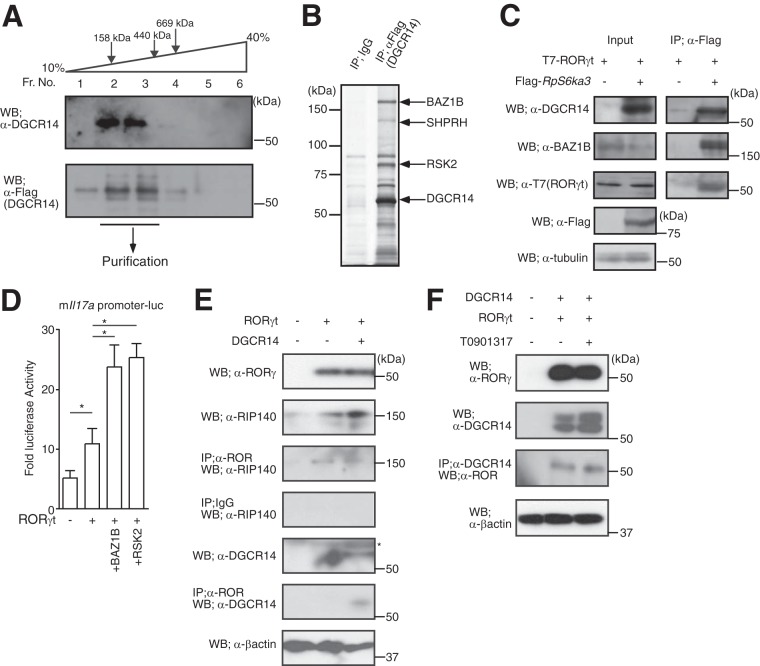
FIG 4.
DGCR14 was associated with BAZ1B and RSK2. (A) Western blotting of DGCR14 in 68-41 cells. For biochemical purification, nuclear extracts of DGCR14-Flag-expressing 68-41 cells were prepared. After fractionation by glycerol density gradient centrifugation, each fraction was analyzed by Western blotting with the antibodies indicated. Fractions (Fr. No.) 2 and 3 were subjected to further purification with anti-Flag M1 agarose affinity gel. (B) Biochemical purification of DGCR14-associated proteins. After the establishment of DGCR14-Flag-expressing 68-41 cells, nuclear extracts were prepared and purified with anti-Flag M1 agarose affinity gel. Samples were eluted by Flag peptide and subjected to SDS-PAGE and silver stained, and proteins were identified by MALDI-TOF MS (see Table S2 in the supplemental material). (C) Immunoprecipitation assays of DGCR14 and RSK2 in 293T cells. After transfection with T7-RORγt and/or Flag-RSK2 expression vectors, cells were incubated for 1 day and lysed. Samples were then immunoprecipitated with anti-Flag M1 agarose affinity gel and examined by Western blotting with the antibodies indicated. (D) Luciferase reporter assay of the mIl17a promoter in 293T cells. After transfection with a β-gal expression vector, a mIl17a promoter luciferase vector, and/or expression vectors for RORγt, Rps6ka3, or Baz1b, 293T cells were cultured for 36 h and collected and luciferase assays were performed. As an internal control, ONPG-dependent β-gal activities were measured with a microplate reader. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (E) Immunoprecipitation assays of DGCR14, RORγt, and RIP140 in 293T cells. After transfection with expression vectors for RORγt and/or Dgcr14, cells were incubated for 1 day and lysed. Samples were then immunoprecipitated with anti-ROR common and examined by Western blotting with the antibodies indicated. The asterisk indicates a nonspecific band. (F) Immunoprecipitation assays of DGCR14 and RORγt in 293T cells with or without T0901317. After transfection with expression vectors for RORγt and/or Dgcr14, cells were incubated for 1 day with or without 10 μM T0901317 and lysed. Samples were then immunoprecipitated with anti-DGCR14 and examined by Western blotting with the antibodies indicated.
Plasmid constructs.
The full-length mouse RORγt cDNA expression vector and mIl17a promoter luciferase vector used were described previously (14). Deletion mutant variants of mouse RORγt were amplified by standard PCR techniques and cloned with the CMV-T7 vector. Full-length cDNAs for DGCR14, RpS6ka1, RpS6ka2, and RpS6ka3 were amplified by standard PCR techniques and cloned with a Flag tag into pcDNA3 (Invitrogen). Retroviral vectors expressing Dgcr14 cDNA were amplified by standard PCR techniques and cloned into e-MIGR1 by XhoI and EcoRI. Retroviral vectors of short hairpin RNAs (shRNAs) were synthesized oligonucleotides (see Table S3 in the supplemental material) that were amplified and cloned into vectors (Open Biosystems EAV4678 and EAV4679) according to the manufacturer's protocol (17). The mouse Dgcr14 promoter (−1 to −2364) was amplified by standard PCR techniques and cloned into a luciferase reporter vector. The full-length Baz1b cDNA expression vector was a gift from S. Kato (Soma Central Hospital).
Primary T cell differentiation.
CD4+ CD25− CD44low CD62Lhigh naive T cells from spleens and lymph nodes were enriched through negative selection with a magnetic cell-sorting system (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) with biotin-conjugated anti-CD8.2 (53-6.7), anti-B220/CD44 (RA3-6B2), anti-CD11b (M1/70), anti-CD11c (N418), anti-CD49b (Dx5) (all from eBioscience, San Diego, CA), and anti-TER119 (BD Biosciences) antibodies, as well as streptavidin-conjugated magnetic beads (Miltenyi Biotec). Cells were then flow cytometrically sorted with a BD FACSAria cell sorter (BD Biosciences). The purity of the sorted CD4+ T cell populations was consistently >98%. T cells were maintained in a complete medium containing RPMI 1640 supplemented with 10% FBS, 50 U penicillin, 50 μg ml−1 streptomycin, 100 nM nonessential amino acids, 2 mM glutamine, and 0.05 mM 2-mercaptoethanol. The culture conditions for different TH cell subsets were as follows: 1 μg ml−1 anti-CD-3ε and 1 μg ml−1 anti-CD-28 for TH0 (neutral conditions); 1 μg ml−1 anti-CD-3ε, 1 μg ml−1 anti-CD-28, 1 μg ml−1 anti-IL-4, and 10 ng ml−1 IL-12 (Peprotech) for TH1; 1 μg ml−1 anti-CD-3ε, 1 μg ml−1 anti-CD-28, 1 μg ml−1 anti-gamma interferon (anti-IFN-γ), and 1 ng ml−1 IL-4 (Peprotech) for TH2; 1 μg ml−1 anti-CD-3ε, 1 μg ml−1 anti-CD-28, 1 μg ml−1 anti-IL-4, 1 μg ml−1 anti-IFN-γ, and 2 ng ml−1 TGF-β (Peprotech) for inducible Treg; and 1 μg ml−1 anti-CD-3ε, 1 μg ml−1 anti-CD-28, 1 μg ml−1 anti-IL-4, 1 μg ml−1 anti-IFN-γ, 10 ng ml−1 IL-6 (Peprotech), and 2 ng ml−1 TGF-β for TH17.
For transduction with retroviruses, the following procedure was utilized. Isolated naive CD4+ T cells were cultured under the appropriate conditions for 29 h, and concentrated viruses were transduced with 1.6 ng ml−1 Polybrene and centrifuged at 3,000 rpm at 37°C for 1.5 h. The cells were incubated at 37°C in 5% CO2 overnight, after which they were washed and cultured for 2 to 4 days under the condition designated.
Flow cytometric analysis.
For intracellular cytokine staining, cells were stimulated for 4 h in complete medium with phorbol 12-myristate 13-acetate (50 ng ml−1) and ionomycin (500 ng ml−1) (both from Sigma-Aldrich) in the presence of 3 μg ml−1 brefeldin A (eBioscience). Surface staining was then performed in the presence of Fc-blocking antibodies (2.4G2), followed by intracellular staining with anti-IL-17A antibody (eBioscience) or anti-IFN-γ antibody (eBioscience) with the Fixation and Permeabilization kit (eBioscience) according to the manufacturer's instructions. Data were acquired on a BD FACSCanto and analyzed with FlowJo software (TreeStar, Ashland, OR).
ELISA.
Supernatants were collected after the cell culture periods indicated and analyzed for IL-17A with an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience) and for IL-4 and IFN-γ with ELISA kits (R&D) according to the manufacturers' instructions.
Antibodies, Western blotting, immunoprecipitation, and ChIP.
For Western blotting and/or chromatin immunoprecipitation (ChIP) analysis, I used antibodies against RORγ (PP-H6437; Perseus Proteomics), ROR common (PP-H3925; Perseus Proteomics), DGCR14 (sc-86411; Santa Cruz), RSK2 (ab32133; Abcam), BAZ1B (ab50632; Abcam), RIP140 (sc-8997; Santa Cruz), Flag (F3165; Sigma-Aldrich), and H3K9Me3 (07-442; Millipore).
For immunoprecipitation of cells transfected with the plasmids indicated, 293T or 68-41 cells (about 5 × 107) were washed with ice-cold phosphate-buffered saline. Cells were collected and resuspended in 100 μl lysis buffer (20 mM Tris-HCl [pH 7.9], 1% NP-40, 1 mM EDTA, 150 mM NaCl, 2.5 mM MgCl2, 5% glycerol, 5 mM DTT, 10 mg ml−1 aprotinin, 1 mM PMSF), incubated on ice for 30 min, and centrifuged for 30 min at 12,000 × g. The resultant supernatants were diluted 10-fold with lysis buffer without NP-40 and used as whole-cell extracts for immunoprecipitation with the antibodies indicated with protein G-Sepharose (GE Healthcare) or Dynabeads Protein G (Life Technologies). After separation by SDS-PAGE, proteins were transferred to a PVDF membrane and Western blotting was performed.
ChIP was performed according to the manufacturer's protocol (Millipore). Briefly, cells were fixed with 1% formaldehyde (Sigma) and chromatin was sheared by sonication to average lengths of 300 to 500 bp. Chromatin was immunoprecipitated with control IgG or specific antibodies overnight at 4°C and then incubated with protein A-agarose-salmon sperm DNA (Millipore) for an additional 2 h. After washing and elution, protein-DNA cross-links were disrupted by heating at 65°C overnight. Immunoprecipitated DNA was purified with QIAquick spin columns (Qiagen) and analyzed by quantitative PCR (qPCR) with the CFX96 Touch real-time PCR detection system with SYBR green (Bio-Rad). The relative quantitation value is expressed as 2−ΔCT, where ΔCT is the difference between the mean CT value of triplicates of the sample and that of the input control. The sequences of the primers used are shown in Table S3 in the supplemental material.
RNA analysis.
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized from total RNA with the SuperScript III synthesis system (Invitrogen) and random hexamer primers. The gene expression levels were analyzed by reverse transcription (RT)-qPCR with the CFX96 Touch real-time PCR detection system with SYBR green (Bio-Rad). The relative quantitation value is expressed as 2−ΔCT, where ΔCT is the difference between the mean CT value of triplicates of the sample and that of the Gapdh control. The sequences of the primers used are shown in Table S3 in the supplemental material.
Luciferase reporter assays.
Luciferase assays were performed as previously described (18). Briefly, cells at 40 to 50% confluence were transfected with the plasmids indicated with the Lipofectamine Plus reagent (Invitrogen) in 24-well plates. The total amount of DNA was adjusted by supplementation with the empty vector up to 1.0 μg well−1. Luciferase activity was determined with the luciferase assay system (Promega). As a reference plasmid to normalize transfection efficiency, 10 ng well−1 of plasmid pCMV-βgal was cotransfected in all experiments and β-galactosidase (β-gal) assays were performed.
Statistical analysis.
Data are presented as means ± the standard deviations (SD). Differences between groups were assessed by Student's paired two-tailed t test. P values of <0.05 were considered significant. All of the error bars shown represent standard deviations.
RESULTS
DGCR14 promoted TH17 cell differentiation by coactivating RORγ/γt function.
To purify RORγ-associated proteins, I used the 68-41 murine T cell hybridoma cell line (19), which upregulates RORγ protein and Il17a mRNA expression when treated with anti-CD3ε antibody, TGF-β, and IL-6 (17) (Fig. 1A, left panel). After preparing nuclear extracts according to classical methods (15), RORγ interactants were purified by using an anti-RORγ antibody column and subsequently identified by MALDI-TOF MS (Fig. 1A, right panels). I identified several known transcriptional coregulators (see Table S1 in the supplemental material), including histone-modifying enzymes (JMJD1C and KDM2A), a subunit of the chromatin-remodeling complex (BAF60c) and other transcriptional coregulators (ASCC3L1, MYBBP, and RCOR3). Among the factors identified, I focused on the DGCR14/ES2 protein. Dgcr14/Es2 is one of the genes deleted in DiGeorge or 22q11.2 deletion syndrome (20, 21), which is characterized by various defects of the heart, thymus, and parathyroid gland (22). DGCR14 is a nuclear protein with a coiled-coil domain (21) that is conserved in lower species, including Drosophila melanogaster (23), Caenorhabditis elegans (24), and yeast (25). Although recent studies showed that DGCR14/ES2 associates with other nuclear proteins (26) or the splicing complex (27), the role of DGCR14 in T lymphocytes is not fully understood. Endogenous DGCR14 protein associates with RORγ in 68-41 cells stimulated with anti-CD3ε antibody, IL-6, and TGF-β (Fig. 1B).
FIG 1.
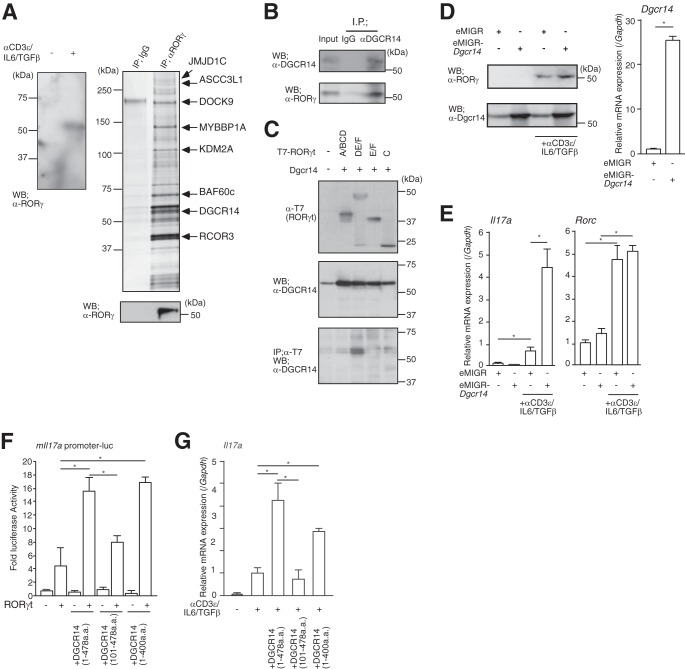
Identification of DGCR14 as a transcriptional coactivator of RORγ. (A) The left panel shows Western blotting for RORγ in 68-41 cells. After stimulation with or without anti-CD3ε antibody, IL-6, and TGF-β, nuclear extracts were prepared and Western blot (WB) assays were performed with the antibodies indicated. The right upper panel shows purification of RORγ-associated proteins from the 68-41 T cell hybridoma line. Large-scale cultures of 68-41 cells were stimulated with anti-CD3ε antibody, IL-6, and TGF-β for 8 h. Nuclear extracts were prepared and purified on anti-RORγ affinity columns and eluted with 0.1 M glycine, pH 3.0. Samples were separated by SDS-PAGE, silver stained, and identified by MALDI-TOF MS analysis (see Table S1 in the supplemental material). Eluted RORγ was detected by Western blotting with anti-RORγ antibody (right lower panel). IP, immunoprecipitation. (B) Immunoprecipitation assays with anti-DGCR14 antibody in 68-41 cells. After stimulation with anti-CD3ε antibody, IL-6, and TGF-β, cells were lysed and precipitated with rabbit IgG or anti-DGCR14 antibody. Western blot assays were performed with the antibodies indicated. (C) Immunoprecipitation assays with T7-tagged RORγt deletion mutant variants (A/BCD, DE/F, E/F, and C). After transfection with each expression vector, 293T cells were lysed and precipitated with anti-T7 antibody (MBL). Western blot assays were performed with the antibodies indicated. (D) The left panels show Western blotting for RORγ and DGCR14 in 68-41 cells with or without Dgcr14 cDNA retroviruses and stimulation with anti-CD3ε antibody, IL-6, and TGF-β. After infection of 68-41 cells with each virus, virus-expressing green fluorescent protein (GFP)-positive cells were sorted with a FACSAria (BD) and cultured. After stimulation with anti-CD3ε antibody, IL-6, and TGF-β, cells were lysed and Western blotting was performed with the antibodies indicated. The right panel shows RT-qPCR analysis for Dgcr14 mRNA in 68-41 cells transduced with Dgcr14 cDNA. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (E) RT-qPCR analyses for Il17a and Rorc mRNAs in 68-41 cells transduced with Dgcr14 cDNA. After stimulation with or without anti-CD3ε antibody, IL-6, and TGF-β, RNA was extracted and RT-qPCR was performed. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (F) Luciferase reporter assays with Dgcr14 deletion mutant variants and the mIl17a promoter-luciferase reporter vector. After the transduction of each Dgcr14 deletion cDNA expression vector and RORγt expression vector, 68-41 cells were stimulated with anti-CD3ε antibody, IL-6, and TGF-β. The cells were then lysed and used in luciferase reporter assays. As an internal control, o-nitrophenyl-β-d-galactopyranoside (ONPG)-dependent β-gal activities were measured with a microplate reader. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (G) RT-qPCR for assessment of Il17a mRNA in 68-41 cells as described for panel F. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05.
To better assess the role of DGCR14, I mapped the interaction domain between RORγt and DGCR14 by immunoprecipitation assay. RORγt consists of an activation function 1 (AF1) region (A/B), a C4-type Zn finger domain (a DNA binding region; C), a hinge region (D), and a ligand and coactivator binding site including AF2 (E/F). I generated four RORγt deletion mutant variants (A/BCD, amino acids [aa] 1 to 244; DE/F, aa 74 to 495; E/F, aa 245 to 495; C, aa 4 to 74) and found that the DE/F region of RORγt associated with DGCR14 (Fig. 1C). This region is conserved between RORγ and RORγt. I then established a stably expressed Dgcr14 cDNA in 68-41 cells by transducing eMIGR-Dgcr14 retroviruses (Fig. 1D) and examined mRNA levels of Il17a by RT-qPCR. As shown in Fig. 1E, Dgcr14 overexpression strongly induced levels of Il17a but not Rorc mRNA.
In addition, luciferase reporter assays using the murine Il17a (mIl17a) promoter in 68-41 cells showed that the N-terminal domain of DGCR14 was required for DGCR14-dependent coactivation of RORγt function (Fig. 1F). Overexpression of these mutant DGCR14 proteins showed the significance of the N-terminal domain (aa 1 to 100) of DGCR14 in Il17a mRNA expression (Fig. 1G), although this domain does not show any similarities to known transcriptional coactivation domains.
Dgcr14 knockdown suppressed the transcriptional activity of RORγ/γt.
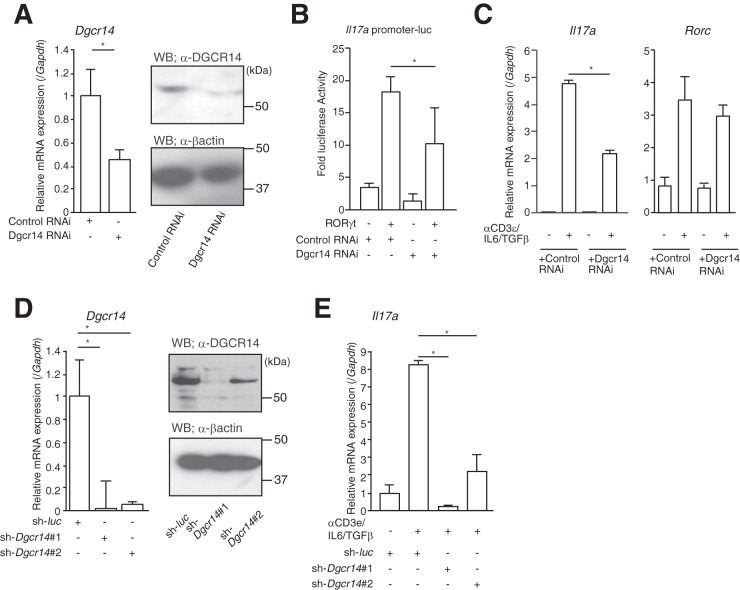
To investigate the effect of Dgcr14 on Il17a mRNA expression, I transfected vectors expressing interfering RNA (RNAi) for Dgcr14 into 68-41 cells and conducted luciferase reporter assays. As expected, Dgcr14 knockdown inhibited the transcriptional activity of RORγt on the mIl17a promoter (Fig. 2A and B). Moreover, endogenous Il17a mRNA levels were also reduced by Dgcr14 RNAi-expressing 68-41 cells stimulated with anti-CD3ε antibody, IL-6, and TGF-β (Fig. 2C). I then transduced a retrovirus vector expressing shRNA for Dgcr14 mRNA into 68-41 cells. I tested several shRNAs for Dgcr14 mRNA and found one that effectively downregulated Dgcr14 mRNA expression (Fig. 2D; see Table S3 in the supplemental material). This shRNA (sh-Dgcr14#1) also reduced mRNA levels of Il17a in 68-41 cells stimulated with anti-CD3ε antibody, IL-6, and TGF-β (Fig. 2E). These results clearly showed the role of DGCR14 as a transcriptional coactivator in T-lymphocyte-related cells.
FIG 2.
Knockdown of Dgcr14 mRNA suppressed the function of RORγt. (A) The left panel shows RT-qPCR for assessment of Dgcr14 in 68-41 cells transfected with control or Dgcr14 RNAi-expressing vectors. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. The right panels show Western blotting for DGCR14 proteins in 68-41 cells transfected with control or Dgcr14 RNAi-expressing vectors. (B) Luciferase reporter assay of 68-41 cells. After transfection with Dgcr14 RNAi expression vectors, an mIl17a promoter luciferase vector, a β-gal expression vector, and/or a RORγt expression vector, cells were cultured for 1 day. Cells were then harvested, and luciferase assays were performed. As an internal control, ONPG-dependent β-gal activities were measured with a microplate reader. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (C) RT-qPCR analysis of Dgcr14 RNAi-expressing 68-41 cells. After transfection with or without Dgcr14 RNAi expression vectors, 68-41 cells were cultured with or without anti-CD3ε antibody, IL-6, and TGF-β. RNA was extracted and RT-qPCR was performed. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (D) The left panel shows RT-qPCR assessment for Dgcr14 mRNA in 68-41 cells transduced with control (sh-luc)- or two Dgcr14 shRNA-expressing retroviruses (sh-Dgcr14#1 and sh-Dgcr14#2). The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. After transduction with each virus, GFP-positive cells were isolated with a FACSAria (BD) and cultured. Among six shRNAs for Dgcr14, two shRNAs targeting Dgcr14 reduced its mRNA levels. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. The right panels show Western blotting for DGCR14 in 68-41 cells transduced with control or Dgcr14 shRNA-expressing retroviruses. Western blot assays were performed with the antibodies indicated. (E) RT-qPCR assessment of Il17a mRNA in 68-41 cells transduced with control or Dgcr14 shRNA-expressing retroviruses. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05.
DGCR14 regulated Il17a mRNA expression in primary TH17 cells.
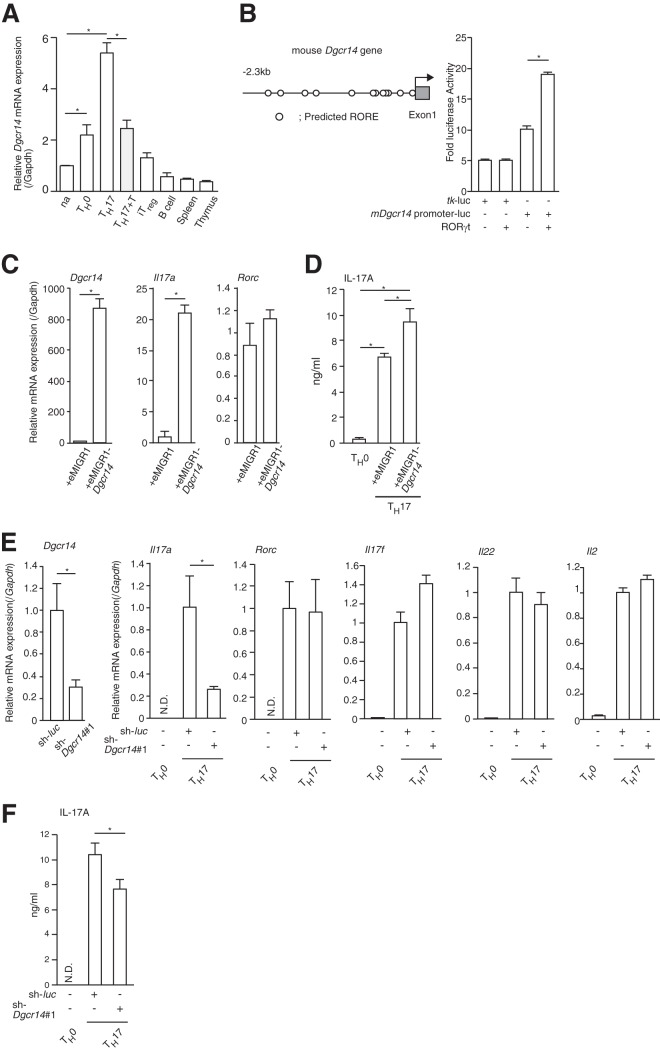
My results showed that DGCR14 coactivated the transcriptional activity of RORγt in 68-41 cells, but the role of DGCR14 in primary cultured TH17 cells remained unclear. Dgcr14 mRNA expression levels were higher in cultured primary TH17 cells than in naive T and TH0 cells (Fig. 3A). Interestingly, T0901317, a partial agonist of RORγ, reduced mRNA levels of Dgcr14 (Fig. 3A). This result raises the possibility that Dgcr14 mRNA expression was regulated by RORγ/γt. The mouse Dgcr14 promoter region contains several ROREs (5′-AGGTCA-3′), and I cloned the mDgcr14 promoter region and subjected it to luciferase reporter assays. As shown in Fig. 3B, RORγt overexpression modestly induced Dgcr14 gene promoter activities. These results show that RORγt partially regulates Dgcr14 mRNA expression.
FIG 3.
DGCR14 induced IL17A expression in primary cultured TH17 cells. (A) RT-qPCR analysis of Dgcr14 mRNA in CD4+ helper T cells, B cells, thymus cells, and spleen cells. Isolated naive T cells (na) were cultured under TH0, iTreg, or TH17 conditions. TH17 cells treated with 10 μM T0901713 (TH17+T) reduced Dgcr14 mRNA expression. B cells were isolated with anti-CD19 microbeads with a MACS (Miltenyi Biotec). After RNAs were extracted and cDNAs were synthesized, real-time PCR was performed. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (B) Luciferase reporter assay of 293T cells. The mouse Dgcr14 gene promoter regions are shown in the left panel. After transfection with a tk-luc or mDgcr14 promoter-tk-luc reporter vector (mDgcr14 promoter-luc), a β-gal expression vector, and/or an RORγt expression vector, cells were cultured for 1 day. Cells were then harvested, and luciferase assays were performed. As an internal control, ONPG-dependent β-gal activities were measured with a microplate reader. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (C) RT-qPCR analysis of Dgcr14-overexpressing primary cultured CD4+ T cells under TH17 conditions. eMIGR1-control or eMIGR1-Dgcr14 retroviruses were transduced into primary cultured TH17 cells (anti-CD3ε, -CD28, -IFN-γ and -IL-4 antibodies; 10 ng ml−1 IL-6; and 1 ng ml−1 TGF-β). GFP-expressing cells were sorted with a FACSAria (BD), and RT-qPCR was performed. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (D) ELISA of mIL-17A in TH0, control, and Dgcr14-overexpressing primary TH17 cells. After cultivation under the conditions indicated, supernatants were collected and analyzed. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (E) RT-qPCR analysis of primary cultured TH17 cells transduced with control (sh-luc)- or sh-Dgcr14#1-expressing retroviruses. After transduction with each virus, GFP-positive cells were isolated with a FACS Aria (BD) and RT-qPCR was performed. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (F) ELISA for IL-17A protein in primary cultured TH0 cells and TH17 cells transduced with control (sh-luc)- or sh-Dgcr14#1-expressing retroviruses. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. N.D., not determined.
I then transduced retroviruses expressing Dgcr14 cDNA into primary cultured TH17 cells. I found that Il17a expression was consistently enhanced but Rorc mRNA levels were unchanged (Fig. 3C and D). As expected, Dgcr14 shRNA reduced Il17a mRNA and protein expression in cultured primary TH17 cells (Fig. 3E and F). These results indicated that DGCR14 mRNA is induced during CD4+ helper T cell differentiation, at least in part, by regulating the transcriptional activation of RORγ/γt.
RSK2 and BAZ1B coactivated the transcriptional activity of RORγ/γt.
In general, transcriptional coregulator function is achieved by forming a complex that includes a chromatin-remodeling factor and histone-modifying enzymes (28). DGCR14 protein does not have known domains for histone-modifying enzyme activity or chromatin-remodeling activity. In addition, I found that DGCR14 was associated with other transcriptional factors and regulated their functions (data not shown). Therefore, I hypothesized that DGCR14 regulated the transcriptional activity of several transcriptional factors by associating with other proteins. To characterize the function of DGCR14 in transcriptional regulation, I further enriched DGCR14-associated proteins by biochemical purification (Fig. 4A and B). I was unable to generate 68-41 cells expressing DGCR14 protein tagged with Flag peptide in the N-terminal region. Thus, I generated a retroviral vector expressing DGCR14 protein Flag tagged in the C-terminal region and established 68-41 cells stably expressing DGCR14-Flag. As shown in Fig. 4A, a nuclear extract was fractionated by glycerol gradient ultracentrifuge to enrich the DGCR14-expressing fraction (fractions 2 and 3, Fig. 4A). After elution with Flag peptide, BAZ1B, SHPRH, and RSK2 were identified as DGCR14-associated proteins (see Table S2 in the supplemental material). BAZ1B is a subunit of ATP-dependent chromatin remodelers and plays various roles in the nucleus, including participation in transcription (29) and DNA damage repair (30). SHPRH is an E3 ubiquitin ligase involved in DNA repair (31). RSK2 consists of a group of serine/threonine kinases that are constituents of the AGC subfamily in the human kinome (32). RSK isoforms play an important role in the mitogen-activated protein kinase signaling cascade. RSK2 regulates ligand-inducible estrogen receptor activity by phosphorylation (33).
Immunoprecipitation assays showed that RSK2 associated with DGCR14, RORγt, and BAZ1 (Fig. 4C) and RSK2 and BAZ1B coactivated the transcriptional activities of RORγt on the mIl17a promoter (Fig. 4D). Furthermore, DGCR14 overexpression did not interfere with the interaction between RORγt and RIP140 (34) (Fig. 4E), and T0901317 did not suppress the association of RORγt and DGCR14 (Fig. 4F). These results showed that DGCR14 regulated the transcriptional activity of RORγt through RSK2/BAZ1B in an AF2-independent manner.
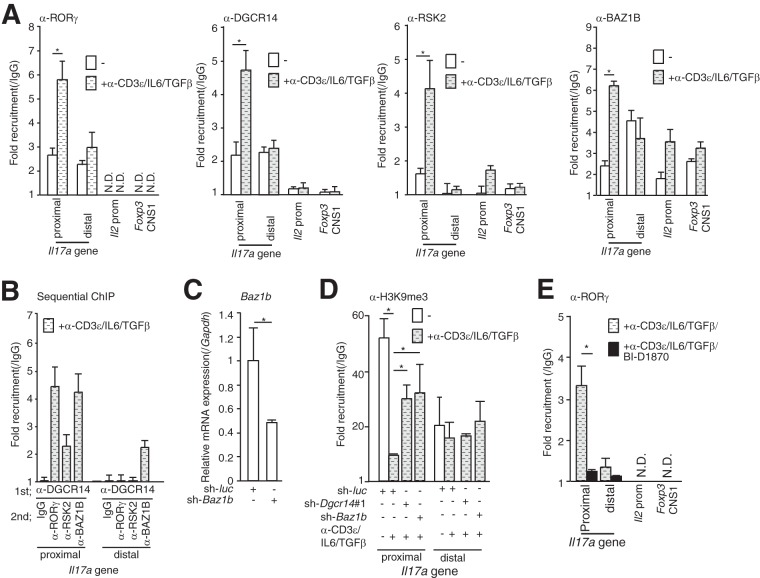
DGCR14, RSK2, and BAZ1B were bound to the Il17a promoter.
The Il17a promoter contains binding elements for ROR and other transcriptional factors, including IRF4 (35), RUNX1 (36), NFATc (37), Foxp3 (14), and STAT3 (38). I performed ChIP analyses with anti-DGCR14 antibody on the Il17a gene (proximal, −179 to −72; distal, −9818 to −9670), the Il2 promoter, and the conserved noncoding DNA sequence 1 (CNS1) region of the Foxp3 locus (18). ChIP analysis of 68-41 cells treated with or without anti-CD3ε antibody, IL-6, and TGF-β showed that DGCR14, BAZ1B, and RSK2 were recruited to the Il17a promoter region in a manner dependent on anti-CD3ε antibody, IL-6, and TGF-β (Fig. 5A). I then performed sequential ChIP analyses of the Il17a and Il2 promoters and Foxp3 CNS1 in 68-41 cells stimulated with anti-CD3ε antibody, IL-6, and TGF-β. I first performed ChIP analysis with anti-DGCR14 antibody and then performed re-ChIP analysis with IgG and anti-RORγ, -RSK2, and -BAZ1B antibodies. As shown in Fig. 5B, DGCR14 was associated with RORγ, BAZ1B, and RSK2 in the mIl17a promoter region. Moreover, DGCR14 was not recruited to the mIl2 promoter or the Foxp3 CNS1 region but BAZ1B was bound to both regions. These results showed that DGCR14 regulated transcriptional activation by associating with RSK2 and BAZ1B as a transcriptional coactivator of RORγ but BAZ1B has distinct functions in the expression of other genes.
FIG 5.
ChIP analysis of the mIl17a promoter. (A) ChIP analysis of the Il17a promoter with anti-RORγ, anti-RSK2, anti-BAZ1B, and anti-DGCR14 antibodies in 68-41 cells. After cultivation of cells that were treated with or without anti-CD3ε antibody, IL-6, and TGF-β, cells were lysed and ChIP analyses were performed with the antibodies indicated. qPCR was performed with the primers indicated (see Table S3 in the supplemental material). (B) Sequential ChIP analysis of the mIl17a promoter in 68-41 cells treated with anti-CD3ε antibody, IL-6, and TGF-β. After immunoprecipitation with anti-DGCR14 antibodies, proteins were eluted with 10 mM DTT, ChIP analysis was conducted with the antibodies indicated, and qPCR was performed. The Il2 promoter and Foxp3 CNS1 were also examined, but a signal was not detected (data not shown). (C) RT-qPCR analysis of Baz1b in 68-41 cells transduced with the control (sh-luc)- or Baz1b shRNA (sh-Baz1b)-expressing retroviruses used in panel D. After transduction with each virus, GFP-positive cells were isolated with a FACS Aria (BD) and cultured. (D) ChIP analysis of the mIl17a promoter in sh-luc-, sh-Dgcr14#1-, or sh-Baz1b-expressing 68-41 cells. After cultivation with or without anti-CD3ε antibody, IL-6, and TGF-β, cells were lysed and ChIP analysis was performed with the anti-histoneH3K9Me3 antibody. A quantitative PCR assay was then performed with each primer. (E) ChIP analysis of the mIl17a promoter in 68-41 cells treated with anti-CD3ε antibody, IL-6, and TGF-β with or without BI-D1870. Cells were lysed, and ChIP analysis was performed with anti-RORγ antibody. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. N.D., not determined.
I next investigated whether shRNA for Baz1b (Fig. 5C) or Dgcr14 affected the recruitment of a transcriptionally suppressive histone mark (histone H3K9me3) in the mIl17a promoter. As shown in Fig. 5D, both shRNAs increased histone H3K9me3 levels in the mIl17a promoter region in 68-41 cells stimulated with anti-CD3ε antibody, IL-6, and TGF-β. I then performed ChIP analysis of the Il17a promoter in 68-41 cells with an RSK inhibitor (BI-D1870). As shown in Fig. 5E, the pan-RSK inhibitor BI-D1870 reduced the recruitment of RORγ to the mIl17a promoter. These results showed that RSK activity and BAZ1B affected the recruitment of RORγ to the mIl17a promoter.
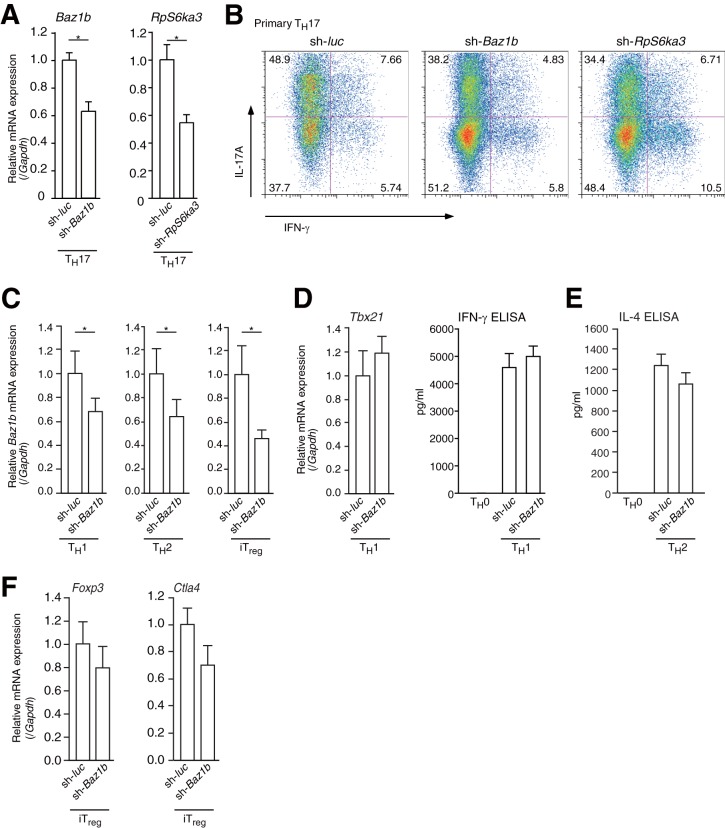
BAZ1B and RSK2 regulated Il17a mRNA expression in primary TH17 cells.
My results showed that RSK2 and BAZ1B were recruited to the mIl17a promoter and coactivated the function of RORγ/γt. In vivo, RSK2-deficient mice have reduced Il2 mRNA expression in CD4+ T cells (39) and Baz1b mutant mice have craniofacial features reminiscent of Williams syndrome (40). However, the role of RSK2 and BAZ1B in cultured primary TH17 cells remains unclear.
I transduced Baz1b or Rsk2 (RpS6a3) shRNA into primary TH17 cells (Fig. 6A) and conducted flow cytometric analysis. Both of the shRNAs reduced IL17A protein levels in TH17 cells (IFN-γ− IL-17A+ sh-luc TH17 cells, 48.9%; sh-Baz1b TH17 cells, 38.2%; sh-RpS6a3 TH17 cells, 34.4%) (Fig. 6B). Furthermore, I transduced Baz1b shRNA-expressing retroviruses into primary TH1, TH2, and iTreg cells and performed RT-qPCR and ELISA (Fig. 6C to F). As shown in Fig. 6D to F, cell differentiation was not affected by Baz1b knockdown. These results showed that RSK2 and BAZ1B mainly promoted TH17 cell differentiation.
FIG 6.
shRNA for RpS6ka3 or Baz1b reduced TH17 cell differentiation. (A) RT-qPCR analysis of Rps6a3 and Baz1b mRNA expression in primary cultured TH17 cells expressing shRNAs for Rps6a3 (sh-RpS6ka3) or Baz1b (sh-Baz1b). The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (B) Effect of Baz1b or Rps6ka3 shRNA on TH17 differentiation. After transduction with control (sh-luc), Baz1b shRNA (sh-Baz1b), or Rps6ka3 shRNA (sh-Rps6ka3) retrovirus, primary CD4+ T cells were cultured under TH17 conditions (anti-CD3ε, anti-CD28, anti-IFN-γ, and anti-IL-4 antibodies; 10 ng ml−1 IL-6; and 1 ng ml−1 TGF-β) for 4 days. Cells were then analyzed by flow cytometry. (C) RT-qPCR analysis of Baz1b mRNA expression in primary cultured TH1, TH2, and iTreg cells expressing shRNA for Baz1b (sh-Baz1b). mRNA levels of Baz1a were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (D to F) RT-qPCR analysis of mRNA expression and ELISA for IFN-γ and IL-4 in primary cultured TH1, TH2, and iTreg cells expressing shRNA for Baz1b (sh-Baz1b). The mRNA level of each gene was normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05.
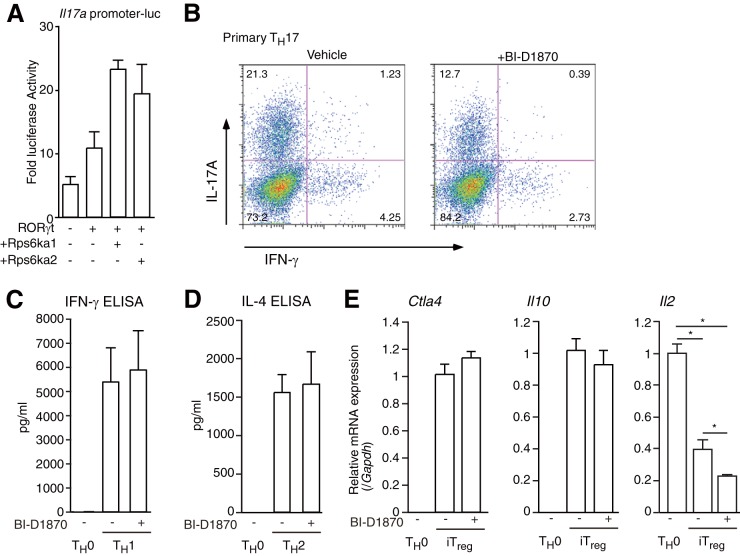
RSK inhibitor BI-D1870 suppressed TH17 differentiation.
I identified DGCR14 as a transcriptional coactivator of RORγ/γt in T cells through BAZ1B/RSK2. I also used a pan-RSK inhibitor (BI-D1870 [41]) to investigate the effect of RSKs in helper T cell differentiation. The reason for using the pan-RSK inhibitor was that RSK1 (Rps6ka1) and RSK3 (Rps6ka2) also coactivated the RORγt function on the Il17a promoter (Fig. 7A). First, I treated cells with BI-D1870 during TH17 cell differentiation and found that BI-D1870 reduced the number of TH17 cells (Fig. 7B) but did not change cell proliferation (Fig. 7C). I then treated primary TH1, TH2, and iTreg cells with BI-D1870 and performed RT-qPCR and ELISA. As shown in Fig. 7C and D, BI-D1870 did not affect IFN-γ (TH1) or IL-4 (TH2) expression. For iTreg cells, BI-D1870 reduced Il2 expression but did not regulate Il10 and Ctla4 mRNA levels. These results showed that RSKs affected TH17 differentiation.
FIG 7.
Effect of BI-D1870 on TH17, TH1, TH2, and iTreg differentiation. (A) Luciferase reporter assays of the mIl17a promoter in 68-41 cells. After transfection with a β-gal expression vector, an mIl17a promoter luciferase vector, and/or an expression vector for RORγt, Rps6ka1, or Rps6ka2, 68-41 cells were cultured for 36 h and collected and luciferase assays were performed. As an internal control, ONPG-dependent β-gal activities were measured with a microplate reader. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (B) Flow cytometric analysis of primary cultured CD4+ T cells under TH17 conditions (anti-CD3ε, -CD28, -IFN-γ, and -IL-4 antibodies; 10 ng ml−1 IL-6; and 1 ng ml−1 TGF-β) with or without 10 μM BI-D1870 for 4 days. The cells were collected, and flow cytometric analysis was performed. (C) ELISA of IFN-γ protein in primary cultured TH0 cells and TH1 cells treated with or without BI-D1870. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (D) ELISA of IL-4 protein in primary cultured TH0 cells and TH2 cells treated with or without BI-D1870. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (E) Real-time RT-PCR analysis of iTreg cells treated with or without 10 μM BI-D1870. After the isolation of RNAs from each tissue type, RT-qPCRs were performed. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05.
DISCUSSION
In this study, I purified RORγ-associated proteins from T-lymphocyte-related cells. The cofactors identified, including histone-modifying enzymes and chromatin-remodeling factors, should be investigated further. Among them, I first characterized the role of DGCR14 as a transcriptional coregulator through RSK2 and BAZ1B. My findings revealed that BAZ1B plays pivotal roles in the recruitment and transactivation of RORγ/γt. I could not elucidate the precise molecular mechanism of DGCR14-dependent transcriptional activation. Deletion analysis of DGCR14 revealed that its N-terminal domain is critical for the coactivation of RORγt function. Thus, this N-terminal domain of DGCR14 might regulate the transcriptional activity. RORγ associates with many known transcriptional coactivators (2), and recent studies identified HSP90 and RIP140 as RORγ interactants in HepG2 hepatocarcinoma cells (34). These factors might regulate RORγt transcriptional activities synergistically with DGCR14, RSK2, and BAZ1B in TH17 cells.
My study was unable to identify RSK2 phosphorylation sites associated with transcriptional activity. RSK phosphorylates R/KXRXXS/T or RRXS/T motifs (32). RSK phosphorylation sites in DGCR14 and BAZ1B have been conserved among species (DGCR14 [Thr382] and BAZ1B [Ser1240] in mice), but this is not the case for RORγ/γt. However, I could not determine the RSK2-dependent phosphorylation site affecting the transcriptional activity of RORγt (data not shown). The effects of an RSK2 inhibitor imply that RSK2 has a role in TH17 cell differentiation, and further experiments are required to clarify this point.
Dgcr14 mRNA is expressed in many tissues, and DGCR14 protein regulates the transcriptional activity of other transcriptional factors such as PPARγ and c-Fos (data not shown). Thus, the function of Dgcr14 is expected in other tissues. The generation of knockout mice is required for further analysis.
In summary, I found novel RSK2- and BAZ1B-mediated transcriptional activation by DGCR14. These results provide key information for new approaches to autoimmune disease therapeutics. The combined use of RSK inhibitors and other autoimmune disease drugs such as RORγ/γt inverse agonists should effectively inhibit the development of autoimmune disease.
Supplementary Material
ACKNOWLEDGMENTS
I thank A. Yoshimura, M. Makishima, and S. Nakagawa for critical discussions. I particularly thank A. Yoshimura, who assisted me in this project. I also thank Y. Yogiashi, R. Tanikawa, and S. Fujiyama-Nakamura for helping with protein purification and proteomics. I thank R. Nakagawa, T. Shichita, and all laboratory members for useful discussions.
This work was supported by a grant-in-aid for Basic Research on Priority Areas (Dynamics of Extracellular Environments and Immunological Self), a grant-in-aid for Young Scientists (B; 23791662), a grant-in-aid for Scientific Research (C; 25462382), the Cell Science Research Foundation, the Kanae Foundation for the Promotion of Medical Science, and the Mochida Memorial Foundation.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00926-14.
REFERENCES
- 1.Medvedev A, Yan ZH, Hirose T, Giguere V, Jetten AM. 1996. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene 181:199–206. doi: 10.1016/S0378-1119(96)00504-5. [DOI] [PubMed] [Google Scholar]
- 2.Jetten AM. 2009. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda Y, Kang HS, Freudenberg J, DeGraff LM, Jothi R, Jetten AM. 2014. Retinoic acid-related orphan receptor gamma (RORgamma): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet 10:e1004331. doi: 10.1371/journal.pgen.1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. 2000. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci U S A 97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Kumar N, Lyda B, Chang MR, Lauer JL, Solt LA, Burris TP, Kamenecka TM, Griffin PR. 2012. Identification of SR2211: a potent synthetic RORgamma-selective modulator. ACS Chem Biol 7:672–677. doi: 10.1021/cb200496y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, Schurer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. 2011. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Kumar N, Nuhant P, Cameron MD, Istrate MA, Roush WR, Griffin PR, Burris TP. 2010. Identification of SR1078, a synthetic agonist for the orphan nuclear receptors RORalpha and RORgamma. ACS Chem Biol 5:1029–1034. doi: 10.1021/cb100223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. 2010. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol 77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stavreva DA, Varticovski L, Hager GL. 2012. Complex dynamics of transcription regulation. Biochim Biophys Acta 1819:657–666. doi: 10.1016/j.bbagrm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannister AJ, Kouzarides T. 2011. Regulation of chromatin by histone modifications. Cell Res 21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. 2004. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 116:511–526. doi: 10.1016/S0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 13.Métivier R, Reid G, Gannon F. 2006. Transcription in four dimensions: nuclear receptor-directed initiation of gene expression. EMBO Rep 7:161–167. doi: 10.1038/sj.embor.7400626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. 2008. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem 283:17003–17008. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 15.Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takada I, Tsuji N, Youn MY, Fujiyama S, Okada M, Imai Y, Kondo S, Kitakawa H, Yasuda H, Kato S. 2010. Purification and identification of estrogen receptor alpha co-regulators in osteoclasts. Ann N Y Acad Sci 1192:201–207. doi: 10.1111/j.1749-6632.2009.05215.x. [DOI] [PubMed] [Google Scholar]
- 17.Ichiyama K, Sekiya T, Inoue N, Tamiya T, Kashiwagi I, Kimura A, Morita R, Muto G, Shichita T, Takahashi R, Yoshimura A. 2011. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-beta is mediated by suppression of eomesodermin. Immunity 34:741–754. doi: 10.1016/j.immuni.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Wakabayashi Y, Tamiya T, Takada I, Fukaya T, Sugiyama Y, Inoue N, Kimura A, Morita R, Kashiwagi I, Takimoto T, Nomura M, Yoshimura A. 2011. Histone 3 lysine 9 (H3K9) methyltransferase recruitment to the interleukin-2 (IL-2) promoter is a mechanism of suppression of IL-2 transcription by the transforming growth factor-beta-Smad pathway. J Biol Chem 286:35456–35465. doi: 10.1074/jbc.M111.236794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubo M, Kincaid RL, Webb DR, Ransom JT. 1994. The Ca2+/calmodulin-activated, phosphoprotein phosphatase calcineurin is sufficient for positive transcriptional regulation of the mouse IL-4 gene. Int Immunol 6:179–188. doi: 10.1093/intimm/6.2.179. [DOI] [PubMed] [Google Scholar]
- 20.Gong W, Emanuel BS, Galili N, Kim DH, Roe B, Driscoll DA, Budarf ML. 1997. Structural and mutational analysis of a conserved gene (DGSI) from the minimal DiGeorge syndrome critical region. Hum Mol Genet 6:267–276. doi: 10.1093/hmg/6.2.267. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay EA, Rizzu P, Antonacci R, Jurecic V, Delmas-Mata J, Lee CC, Kim UJ, Scambler PJ, Baldini A. 1996. A transcription map in the CATCH22 critical region: identification, mapping, and ordering of four novel transcripts expressed in heart. Genomics 32:104–112. doi: 10.1006/geno.1996.0082. [DOI] [PubMed] [Google Scholar]
- 22.Gennery AR. 2012. Immunological aspects of 22q11.2 deletion syndrome. Cell Mol Life Sci 69:17–27. doi: 10.1007/s00018-011-0842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsay EA, Harvey EL, Scambler PJ, Baldini A. 1998. ES2, a gene deleted in DiGeorge syndrome, encodes a nuclear protein and is expressed during early mouse development, where it shares an expression domain with a Goosecoid-like gene. Hum Mol Genet 7:629–635. doi: 10.1093/hmg/7.4.629. [DOI] [PubMed] [Google Scholar]
- 24.Rizzu P, Lindsay EA, Taylor C, O'Donnell H, Levy A, Scambler P, Baldini A. 1996. Cloning and comparative mapping of a gene from the commonly deleted region of DiGeorge and Velocardiofacial syndromes conserved in C. elegans. Mamm Genome 7:639–643. doi: 10.1007/s003359900197. [DOI] [PubMed] [Google Scholar]
- 25.Taricani L, Tejada ML, Young PG. 2002. The fission yeast ES2 homologue, Bis1, interacts with the Ish1 stress-responsive nuclear envelope protein. J Biol Chem 277:10562–10572. doi: 10.1074/jbc.M110686200. [DOI] [PubMed] [Google Scholar]
- 26.Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, Vijayadamodar G, Pochart P, Machineni H, Welsh M, Kong Y, Zerhusen B, Malcolm R, Varrone Z, Collis A, Minto M, Burgess S, McDaniel L, Stimpson E, Spriggs F, Williams J, Neurath K, Ioime N, Agee M, Voss E, Furtak K, Renzulli R, Aanensen N, Carrolla S, Bickelhaupt E, Lazovatsky Y, DaSilva A, Zhong J, Stanyon CA, Finley RL Jr, White KP, Braverman M, Jarvie T, Gold S, Leach M, Knight J, Shimkets RA, McKenna MP, Chant J, Rothberg JM. 2003. A protein interaction map of Drosophila melanogaster. Science 302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 27.Hegele A, Kamburov A, Grossmann A, Sourlis C, Wowro S, Weimann M, Will CL, Pena V, Luhrmann R, Stelzl U. 2012. Dynamic protein-protein interaction wiring of the human spliceosome. Mol Cell 45:567–580. doi: 10.1016/j.molcel.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld MG, Lunyak VV, Glass CK. 2006. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 29.Cavellán E, Asp P, Percipalle P, Farrants AK. 2006. The WSTF-SNF2h chromatin remodeling complex interacts with several nuclear proteins in transcription. J Biol Chem 281:16264–16271. doi: 10.1074/jbc.M600233200. [DOI] [PubMed] [Google Scholar]
- 30.Barnett C, Krebs JE. 2011. WSTF does it all: a multifunctional protein in transcription, repair, and replication. Biochem Cell Biol 89:12–23. doi: 10.1139/O10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unk I, Hajdu I, Fatyol K, Szakal B, Blastyak A, Bermudez V, Hurwitz J, Prakash L, Prakash S, Haracska L. 2006. Human SHPRH is a ubiquitin ligase for Mms2-Ubc13-dependent polyubiquitylation of proliferating cell nuclear antigen. Proc Natl Acad Sci U S A 103:18107–18112. doi: 10.1073/pnas.0608595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romeo Y, Zhang X, Roux PP. 2012. Regulation and function of the RSK family of protein kinases. Biochem J 441:553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 33.Clark DE, Poteet-Smith CE, Smith JA, Lannigan DA. 2001. Rsk2 allosterically activates estrogen receptor alpha by docking to the hormone-binding domain. EMBO J 20:3484–3494. doi: 10.1093/emboj/20.13.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang ZM, Wu J, Jia ZC, Tian Y, Tang J, Tang Y, Wang Y, Wu YZ, Ni B. 2012. Identification of interacting proteins of retinoid-related orphan nuclear receptor gamma in HepG2 cells. BMB Rep 45:331–336. doi: 10.5483/BMBRep.2012.45.6.249. [DOI] [PubMed] [Google Scholar]
- 35.Mudter J, Yu J, Zufferey C, Brustle A, Wirtz S, Weigmann B, Hoffman A, Schenk M, Galle PR, Lehr HA, Mueller C, Lohoff M, Neurath MF. 2011. IRF4 regulates IL-17A promoter activity and controls RORgammat-dependent TH17 colitis in vivo. Inflamm Bowel Dis 17:1343–1358. doi: 10.1002/ibd.21476. [DOI] [PubMed] [Google Scholar]
- 36.Zhang F, Meng G, Strober W. 2008. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol 9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Rodriguez J, Sahu N, Handon R, Davidson TS, Anderson SM, Kirby MR, August A, Schwartzberg PL. 2009. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity 31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. 2006. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A 103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin JX, Spolski R, Leonard WJ. 2008. Critical role for Rsk2 in T-lymphocyte activation. Blood 111:525–533. doi: 10.1182/blood-2007-02-072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashe A, Morgan DK, Whitelaw NC, Bruxner TJ, Vickaryous NK, Cox LL, Butterfield NC, Wicking C, Blewitt ME, Wilkins SJ, Anderson GJ, Cox TC, Whitelaw E. 2008. A genome-wide screen for modifiers of transgene variegation identifies genes with critical roles in development. Genome Biol 9:R182. doi: 10.1186/gb-2008-9-12-r182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, Cohen P, Alessi DR. 2007. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J 401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.