Abstract
A cytoplasmic, microsomal bound RNA-dependent RNA polymerase has been purified 2500-fold from rabbit reticulocyte lysates. The synthesis of RNA with the purified enzyme is absolutely dependent on the addition of an RNA template. The best template is hemoglobin messenger RNA, while bacteriophage RNA and poly(A,G) are less active, and DNA is completely inactive as a template. With poly(A,G) as a template, only UTP and CTP are incorporated into polynucleotide chains, indicating that the RNA polymerase is an RNA replicase and not a terminal transferase. With messenger RNA as a template, all four ribonucleoside triphosphates are required for maximal activity. The RNA-dependent RNA polymerase reaction is extremely sensitive to low concentrations of heme, rifamycin AF/013, and ribonuclease and resistant to actinomycin D and DNase. The discovery of RNA-directed RNA synthesis in reticulocytes offers an additional site for control of gene expression in mammalian cells and provides a possible mechanism for amplification of the expression of specific genes.
Keywords: RNA replicase, hemoglobin mRNA, heme
Full text
PDF
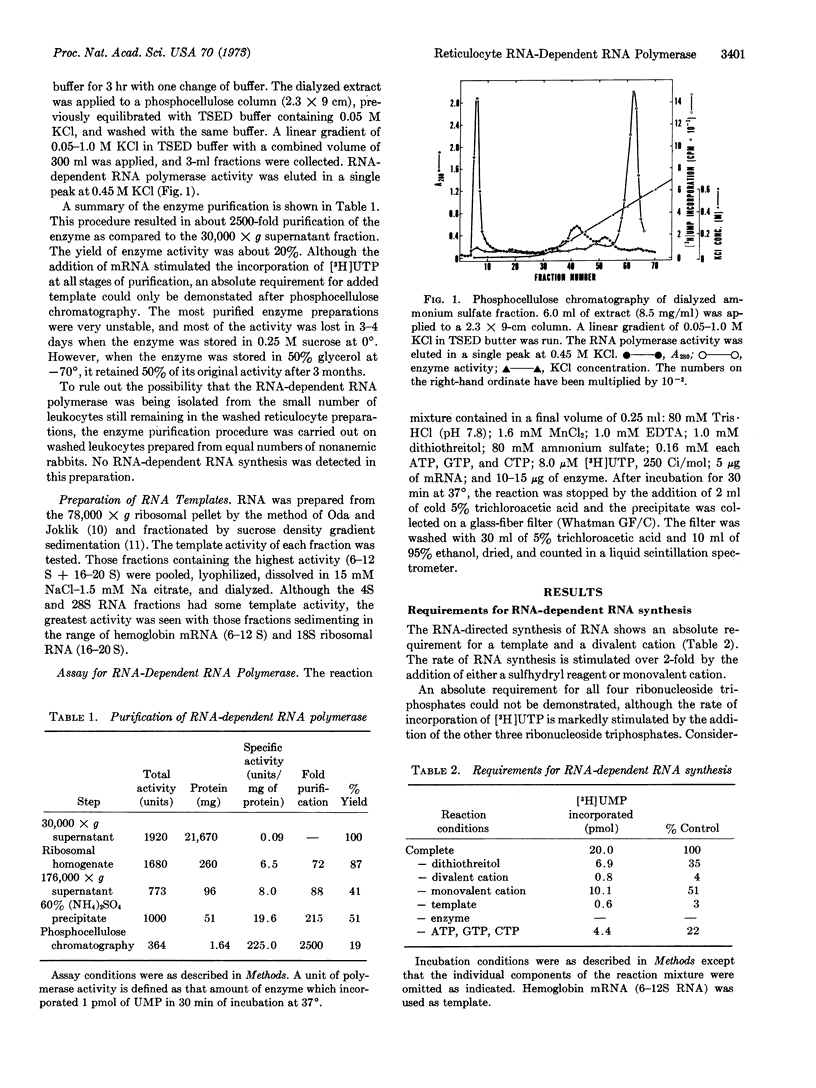
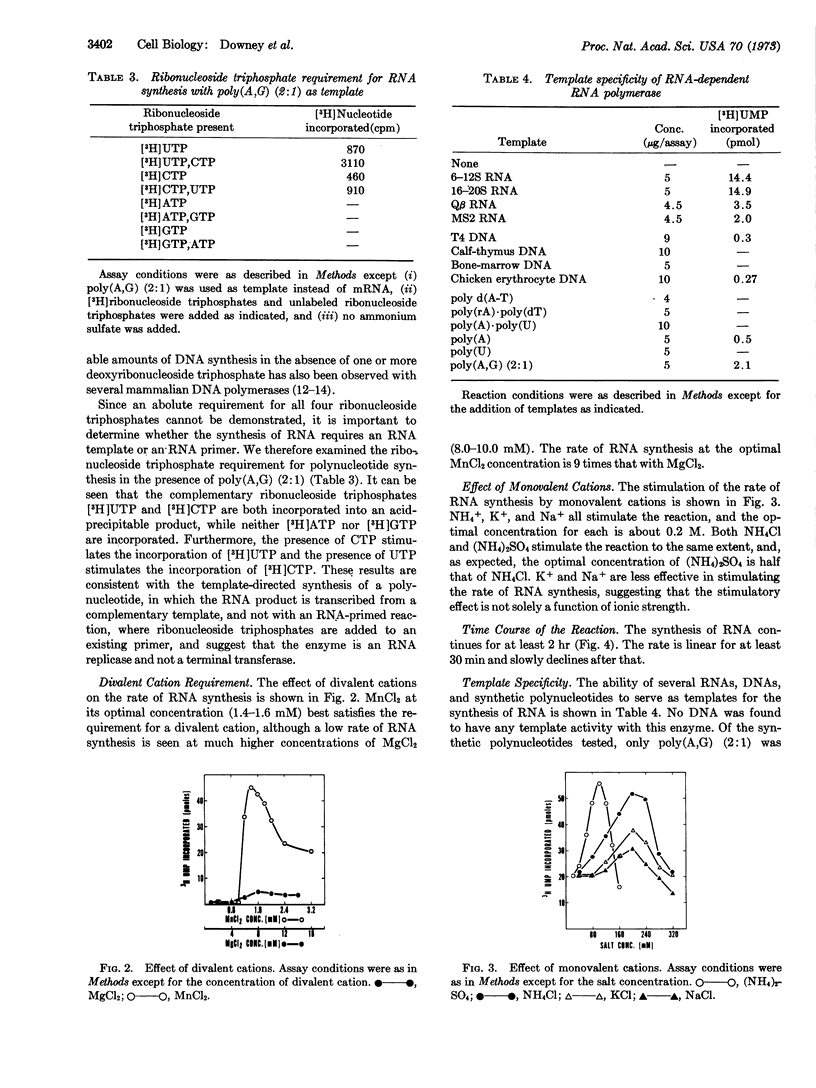
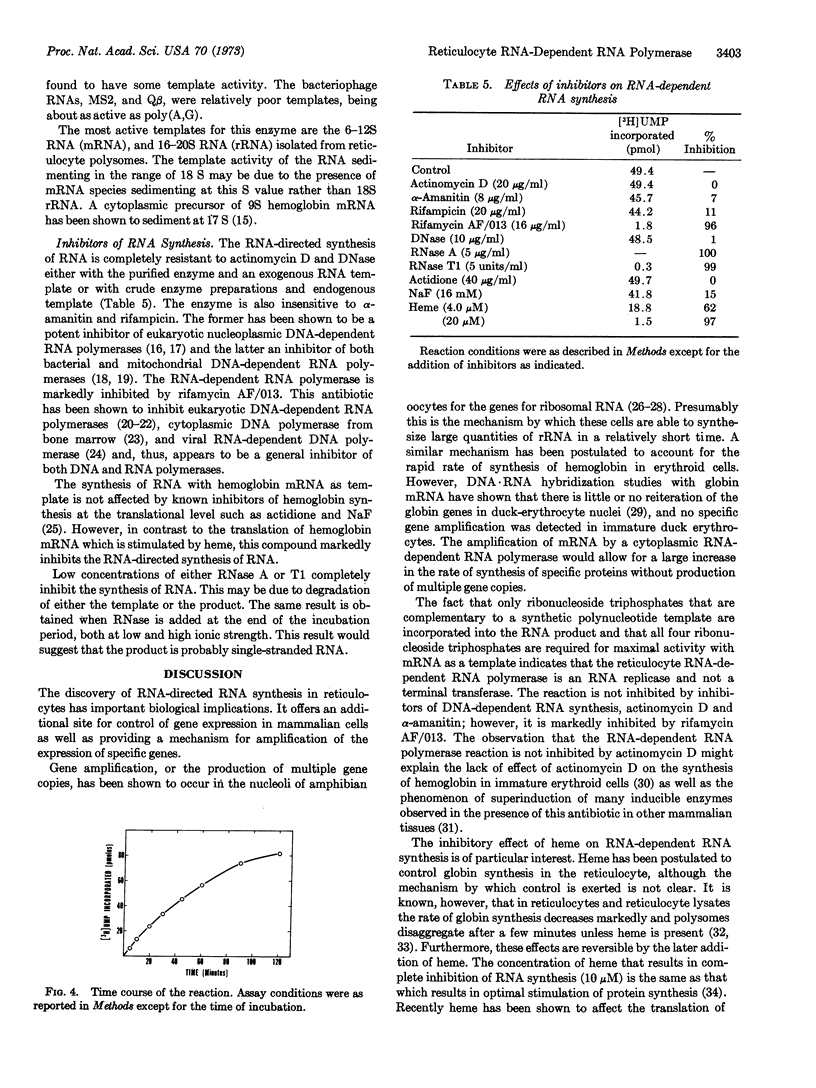

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman R., Schultz L. D., Hall B. D. Transcription in yeast: separation and properties of multiple FNA polymerases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1702–1706. doi: 10.1073/pnas.69.7.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSOOK H., DEASY C. L., HAAGENSMIT A. J., KEIGHLEY G., LOWY P. H. Incorporation in vitro of labeled amino acids into proteins of rabbit reticulocytes. J Biol Chem. 1952 May;196(2):669–694. [PubMed] [Google Scholar]
- BRUNS G. P., LONDON I. M. THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun. 1965 Jan 18;18:236–242. doi: 10.1016/0006-291x(65)90746-1. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzard Y., Rodvien R., London I. M. Effect of hemin on the synthesis of hemoglobin and other proteins in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1022–1026. doi: 10.1073/pnas.70.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., So A. G. Bone marrow cytoplasmic deoxyribonucleic acid polymerase. Variation of pH and ionic environment as a possible control mechanism. Biochemistry. 1973 Oct 23;12(22):4378–4384. doi: 10.1021/bi00746a013. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase from rabbit bone marrow. Biochemistry. 1972 Mar 28;11(7):1264–1272. doi: 10.1021/bi00757a023. [DOI] [PubMed] [Google Scholar]
- Evans D., Birnstiel M. L. Localization of amplified ribosomal DNA in the oocyte of Xenopus laevis. Biochim Biophys Acta. 1968 Aug 23;166(1):274–276. doi: 10.1016/0005-2787(68)90517-0. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayzel A. I., Hörchner P., London I. M. The stimulation of globin synthesis by heme. Proc Natl Acad Sci U S A. 1966 Mar;55(3):650–655. doi: 10.1073/pnas.55.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R., Korn D. Partial purification and characterization of deoxyribonucleic acid polymerase from KB cells. J Biol Chem. 1970 Jan 25;245(2):254–261. [PubMed] [Google Scholar]
- Gross P. R. Biochemistry of differentiation. Annu Rev Biochem. 1968;37:631–660. doi: 10.1146/annurev.bi.37.070168.003215. [DOI] [PubMed] [Google Scholar]
- Hunt T., Ehrenfeld E. Cytoplasm from poliovirus-infected HeLa cells inhibits cell-free haemoglobin synthesis. Nat New Biol. 1971 Mar 17;230(11):91–94. doi: 10.1038/newbio230091a0. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Specific action of alpha-amanitin on mammalian RNA polymerase protein. Nature. 1970 Jan 3;225(5227):60–62. doi: 10.1038/225060b0. [DOI] [PubMed] [Google Scholar]
- Juhasz P. P., Benecke B. J., Seifart K. H. Inhibition of RNA polymerases from rat liver by the semi-synthetic rifampicin derivatives. FEBS Lett. 1972 Oct 15;27(1):30–34. doi: 10.1016/0014-5793(72)80402-2. [DOI] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Inhibition of cellular protein synthesis by double-stranded RNA: inactivation of an initiation factor. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1222–1226. doi: 10.1073/pnas.70.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Weissmann C. Possible mechanism for transition of viral RNA from polysome to replication complex. Nat New Biol. 1971 May 12;231(19):42–46. doi: 10.1038/newbio231042a0. [DOI] [PubMed] [Google Scholar]
- Labrie F. Isolation of an RNA with the properties of haemoglobin messenger. Nature. 1969 Mar 29;221(5187):1217–1222. doi: 10.1038/2211217a0. [DOI] [PubMed] [Google Scholar]
- MARKS P. A., WILLSON C., KRUH J., GROS F. Unstable ribonucleic acid in mammalian blood cells. Biochem Biophys Res Commun. 1962 Jun 19;8:9–14. doi: 10.1016/0006-291x(62)90225-5. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Burka E. R., Schlessinger D. PROTEIN SYNTHESIS IN ERYTHROID CELLS, I. RETICULOCYTE RIBOSOMES ACTIVE IN STIMULATING AMINO ACID INCORPORATION. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2163–2171. doi: 10.1073/pnas.48.12.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Protein synthesis: its control in erythropoiesis. Science. 1972 Mar 3;175(4025):955–961. doi: 10.1126/science.175.4025.955. [DOI] [PubMed] [Google Scholar]
- Maroun L. E., Driscoll B. F., Nardone R. M. Possible cytoplasmic precursor of haemoglobin messenger RNA. Nat New Biol. 1971 Jun 30;231(26):270–271. doi: 10.1038/newbio231270a0. [DOI] [PubMed] [Google Scholar]
- Meilhac M., Tysper Z., Chambon P. Animal DNA-dependent RNA polymerases. 4. Studies on inhibition by rifamycin derivatives. Eur J Biochem. 1972 Jul 13;28(2):291–300. doi: 10.1111/j.1432-1033.1972.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Reid B. D., Parsons P. Partial purification of mitochondrial RNA polymerase from rat liver. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2830–2834. doi: 10.1073/pnas.68.11.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Gallo R. C. DNA-dependent DNA polymerases I and II from normal human-blood lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2879–2884. doi: 10.1073/pnas.69.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Korant B. D., Lonberg-Holm K. K. RNA virus gene expression and its control. Annu Rev Microbiol. 1972;26:467–502. doi: 10.1146/annurev.mi.26.100172.002343. [DOI] [PubMed] [Google Scholar]
- Terada M., Metafora S., Banks J., Dow L. W., Bank A., Marks P. A. Conservation of globin messenger RNA in rabbit reticulocyte monoribosomes after sodium fluoride treatment. Biochem Biophys Res Commun. 1972 May 26;47(4):766–774. doi: 10.1016/0006-291x(72)90558-x. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Gallo R. C. RNA-directed DNA polymerase and virus-induced leukemia in mice. Proc Natl Acad Sci U S A. 1973 May;70(5):1298–1302. doi: 10.1073/pnas.70.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]


