Abstract
Synthesis of α and β chains of hemoglobin was studied in vitro in intact reticulocytes and bone marrow cells. The cells were from rabbits having a variant form of hemoglobin in which L-isoleucine is in the α but not in the β chains. This characteristic permitted a selective inhibition of α-chain synthesis to be produced by addition to the incubation medium of L-O-methylthreonine, an inhibitor of protein synthesis that is a specific antagonist of L-isoleucine.
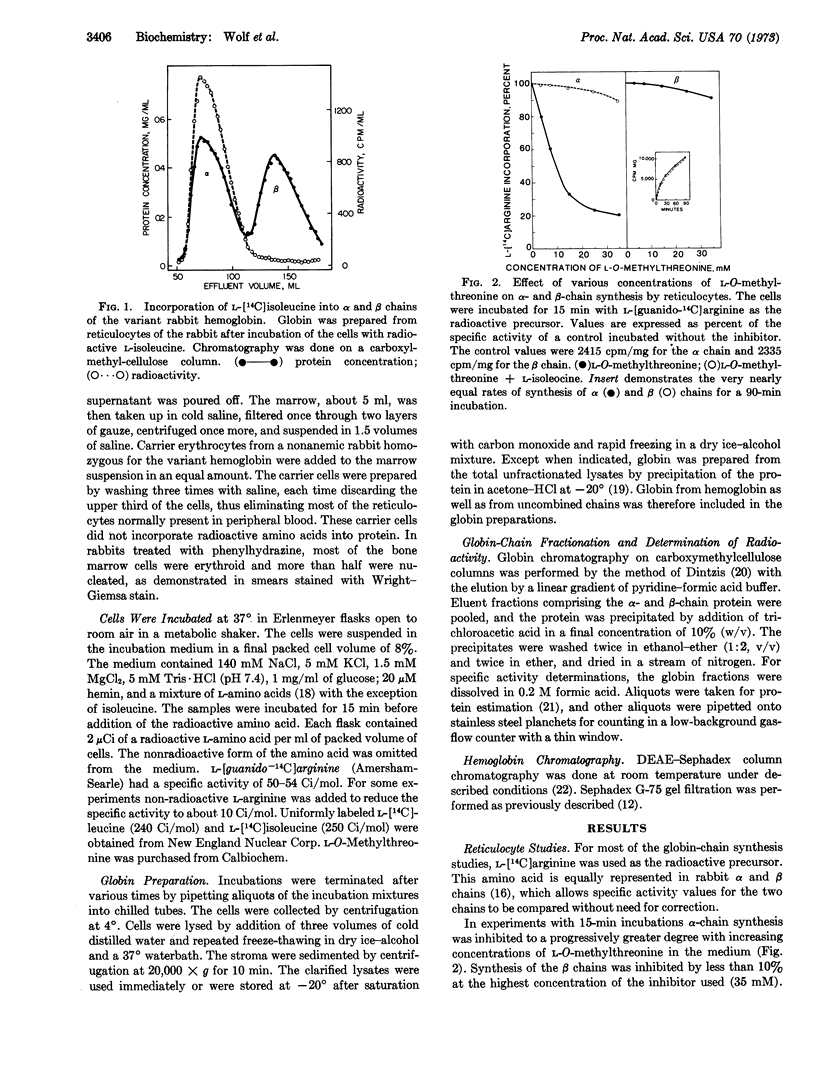
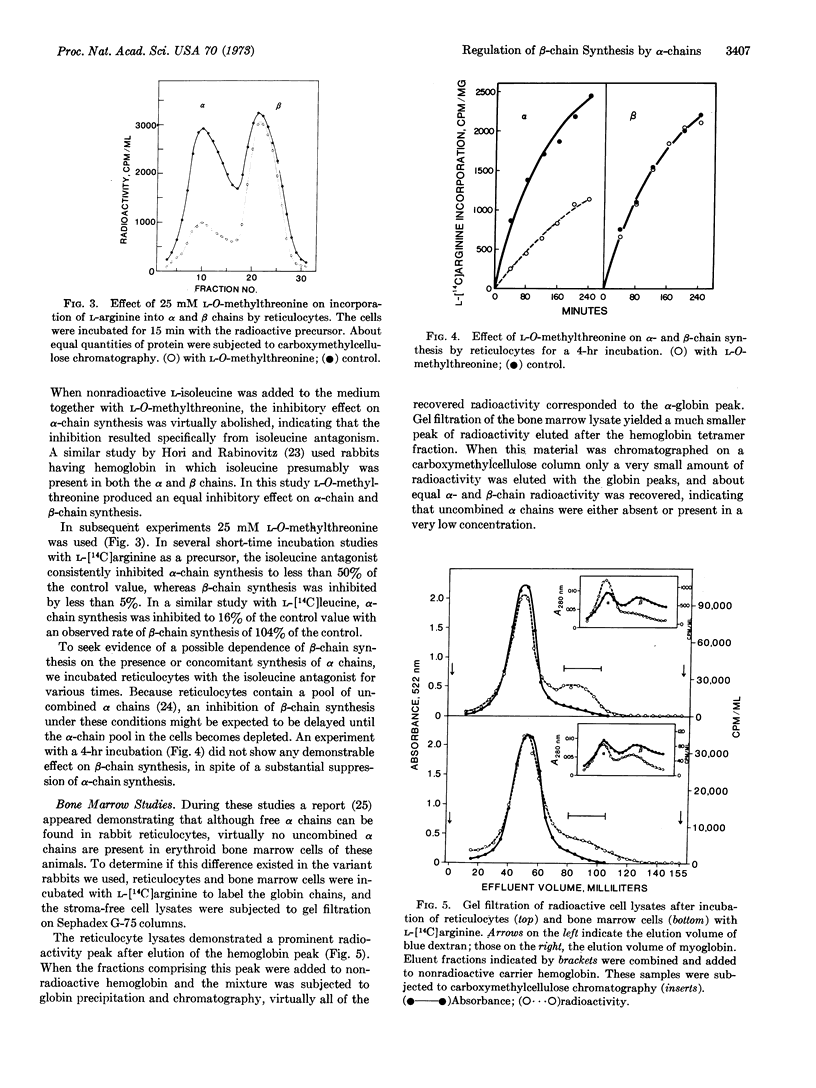
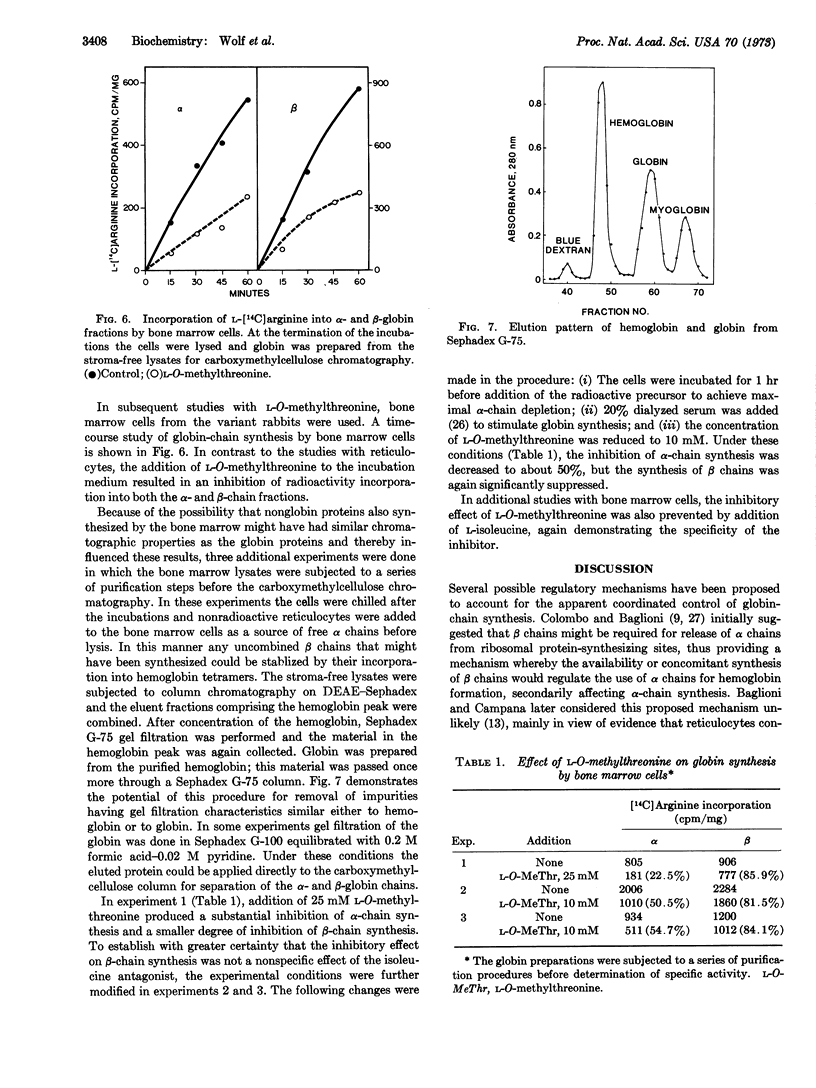
In studies with reticulocytes, 25 mM L-O-methylthreonine produced a 60-70% inhibition of α-chain synthesis, but β-chain synthesis was unaffected even after incubation times for 4 hr. Because reticulocytes contain a pool of uncombined α chains which might have obscured the demonstration of an α chain-dependent mechanism for β-chain synthesis, subsequent studies were done with bone marrow cells. The latter had little or no detectable α-chain pool. A substantial inhibition of α-chain synthesis by the bone marrow cells was produced by the isoleucine antagonist but was also accompanied by a significantly decreased rate of β-chain synthesis.
These findings suggest that the coordinated synthesis of the complementary α- and β-globin chains of hemoglobin may reflect in part a modifying effect of α-chain synthesis on the synthesis of β chains.
Keywords: globin synthesis, reticulocytes, L-O-methylthreonine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. G., 3rd, Winter W. P., Rucknagel D. L., Spencer H. H. Biosynthesis of hemoglobin Ann Arbor: evidence for catabolic and feedback regulation. Science. 1972 Jun 30;176(4042):1427–1429. doi: 10.1126/science.176.4042.1427. [DOI] [PubMed] [Google Scholar]
- BAGLIONI C., COLOMBO B. CONTROL OF HEMOGLOBIN SYNTHESIS. Cold Spring Harb Symp Quant Biol. 1964;29:347–356. doi: 10.1101/sqb.1964.029.01.037. [DOI] [PubMed] [Google Scholar]
- Bargellesi A., Pontremoli S., Conconi F. Absence of beta-globin synthesis and excess of alpha-globin synthesis in homozygous beta-thalassemia. Eur J Biochem. 1967 Mar;1(1):73–79. doi: 10.1007/978-3-662-25813-2_13. [DOI] [PubMed] [Google Scholar]
- Best J. S., Flamm U., Braunitzer G. Haemoglobins, XVII. The primary structure of the beta-chain of rabbit haemoglobin. Hoppe Seylers Z Physiol Chem. 1969 May;350(5):563–580. doi: 10.1515/bchm2.1969.350.1.563. [DOI] [PubMed] [Google Scholar]
- Blum N., Kneip B., Schapira G. Influence de l'origine spécifique des chaînes alpha d'hémoglobine sur la biosynthèse de l'hémoglobine de lapin. Biochimie. 1972;54(9):1121–1128. doi: 10.1016/s0300-9084(72)80016-6. [DOI] [PubMed] [Google Scholar]
- Blum N., Maleknia M., Schapira G. Alpha- et bêta-globines libres et biosynthèse de l'hémoglobine. Biochim Biophys Acta. 1970 Jan 21;199(1):236–247. [PubMed] [Google Scholar]
- Blum N., Maleknia N., Schapira G. Alpha-hémoglobine libre et biosynthèse de l'hémoglobine. Biochim Biophys Acta. 1969 Apr 22;179(2):448–463. [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Eunson C. E. The distribution of nascent globin chains on human reticulocyte polysomes. Biochim Biophys Acta. 1971 Sep 30;247(1):109–112. doi: 10.1016/0005-2787(71)90813-6. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J. Haemoglobin synthesis in alpha-thalassaemia (haemoglobin H disease). Nature. 1967 Sep 16;215(5107):1241–1243. doi: 10.1038/2151241a0. [DOI] [PubMed] [Google Scholar]
- Colombo B., Baglioni C. Regulation of haemoglobin synthesis at the polysome level. J Mol Biol. 1966 Mar;16(1):51–66. doi: 10.1016/s0022-2836(66)80262-0. [DOI] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozy A. M., Kleihauer E. F., Huisman T. H. Studies on the heterogeneity of hemoglobin. 13. Chromatography of various human and animal hemoglobin types on DEAE-Sephadex. J Chromatogr. 1968 Feb 20;32(4):723–727. doi: 10.1016/s0021-9673(01)80551-3. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Hamilton R. W., Schwartz E., Atwater J., Erslev A. J. Acquired hemoglobin H disease. N Engl J Med. 1971 Nov;285(22):1217–1221. doi: 10.1056/NEJM197111252852202. [DOI] [PubMed] [Google Scholar]
- Honig G. R. Inhibition of synthesis of fetal hemoglobin by an isoleucine analogue. J Clin Invest. 1967 Nov;46(11):1778–1784. doi: 10.1172/JCI105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig G. R., Rowan B. Q., Mason R. G. Unequal synthesis of complementary globin chains of human fetal hemoglobin by the effect of L-O-methylthreonine. J Biol Chem. 1969 Apr 25;244(8):2027–2032. [PubMed] [Google Scholar]
- Hori M., Rabinovitz M. Polyribosomal changes during inhibition of rabbit hemoglobin synthesis by an isoleucine antagonist. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1349–1355. doi: 10.1073/pnas.59.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R. T., Hunter A. R., Munro A. J. Control of haemoglobin synthesis: a difference in the size of the polysomes making alpha and beta chains. Nature. 1968 Nov 2;220(5166):481–483. doi: 10.1038/220481a0. [DOI] [PubMed] [Google Scholar]
- Hunt T., Hunter T., Munro A. Control of haemoglobin synthesis: rate of translation of the messenger RNA for the alpha and beta chains. J Mol Biol. 1969 Jul 14;43(1):123–133. doi: 10.1016/0022-2836(69)90083-7. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Freedman M. L. The characterization of separated alpha and beta-chain polyribosomes in rabbit reticulocytes. J Biol Chem. 1968 Dec 25;243(24):6446–6450. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lodish H. F., Jacobsen M. Regulation of hemoglobin synthesis. Equal rates of translation and termination of - and -globin chains. J Biol Chem. 1972 Jun 10;247(11):3622–3629. [PubMed] [Google Scholar]
- Shaeffer J. R., Trostle P. K., Evans R. F. Inhibition of the biosynthetic completion of rabbit hemoglobin by isolated human hemoglobin chains. J Biol Chem. 1969 Aug 25;244(16):4284–4291. [PubMed] [Google Scholar]
- Shaeffer J. R., Trostle P. K., Evans R. F. Rabbit hemoglobin biosynthesis: use of human hemoglobin chains to study molecule completion. Science. 1967 Oct 27;158(3800):488–490. doi: 10.1126/science.158.3800.488. [DOI] [PubMed] [Google Scholar]
- Tabill A. S., Vanderhoff G. A., London I. M. The control of hemoglobin synthesis. A comparison of the role of heme in rabbit bone marrow and reticulocytes. J Biol Chem. 1972 Jan 25;247(2):326–333. [PubMed] [Google Scholar]
- Tavill A. S., Grayzel A. I., London I. M., Williams M. K., Vanderhoff G. A. The role of heme in the synthesis and assembly of hemoglobin. J Biol Chem. 1968 Oct 10;243(19):4987–4999. [PubMed] [Google Scholar]
- Waxman H. S., Freedman M. L., Rabinovitz M. Studies with 59Fe-labeled hemin on the control of polyribosome formation in rabbit reticulocytes. Biochim Biophys Acta. 1967 Sep 26;145(2):353–360. doi: 10.1016/0005-2787(67)90053-6. [DOI] [PubMed] [Google Scholar]
- Waxman H. S. Stimulation of globin synthesis: relative responsiveness of reticulocytes and nucleated erythroid cells. J Clin Invest. 1970 Apr;49(4):701–707. doi: 10.1172/JCI106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Naughton M. A. Globin synthesis in thalassaemia: an in vitro study. Nature. 1965 Dec 11;208(5015):1061–1065. doi: 10.1038/2081061a0. [DOI] [PubMed] [Google Scholar]


