Abstract
Human immunoglobulin M was reduced with concentrations of 2-mercaptoethylamine chosen so that approximately 40% of the immunoglobulin M was reduced to 7S subunits. Under these conditions, which selectively cleave intersubunit disulfides, J chain was released. The 7S subunits of immunoglobulin M so produced did not contain J chain. The high-molecular-weight immunoglobulin M remaining after treatment with 2-mercaptoethylamine had a sedimentation coefficient of 18.0 S and molecular weight of 1 million, and dissociated in 4 M guanidine into subunits similar in size to the 7S subunits. J chain was found in the 18.0S, noncovalently linked immunoglobulin M from which it was released only after more complete reduction with 10 mM dithiothreitol. The results are consistent with the hypothesis that J chain participates in the formation of the intersubunit linkages. However, it need not necessarily be directly involved in all of the intersubunit disulfides. It may play an important role in modulating the assembly of immunoglobulin M subunits, perhaps by inducing conformational changes that lead to noncovalent interactions between the subunits.
Keywords: 2-mercaptoethylamine, disulfide cleavage
Full text
PDF
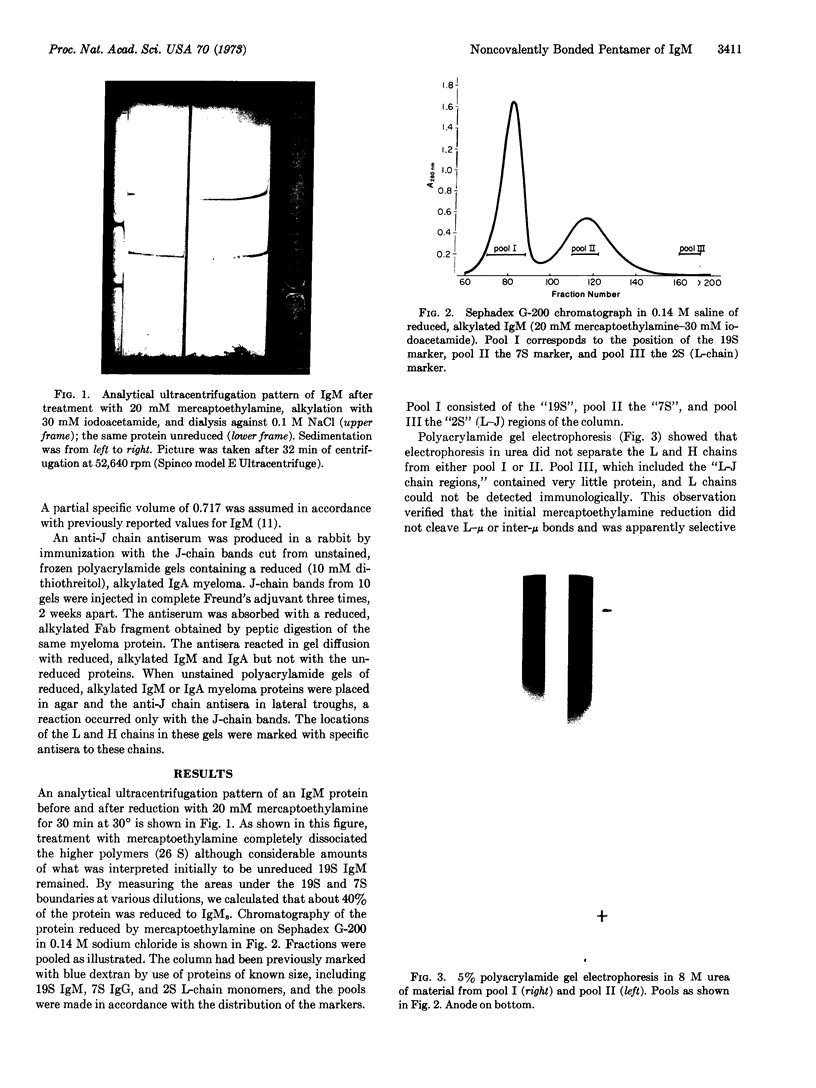
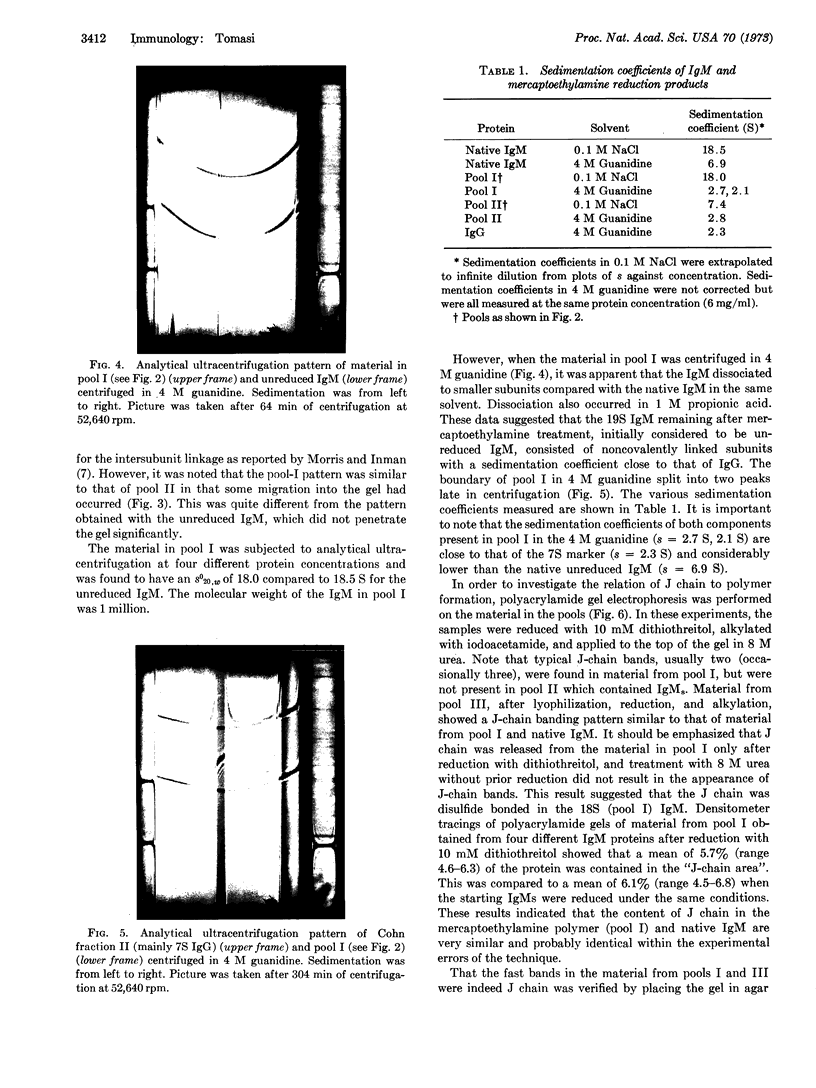


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J. P., Reichlin M., Tomasi T. B., Jr Studies on the chymotrypsin C and papain fragments of human immunoglobulin M. Biochemistry. 1969 Jun;8(6):2246–2254. doi: 10.1021/bi00834a003. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Koshland M. E. Noval subunit in secretory IgA. Nature. 1970 Dec 26;228(5278):1276–1278. doi: 10.1038/2281276a0. [DOI] [PubMed] [Google Scholar]
- Kehoe J. M., Tomasi T. B., Jr, Ellouz F., Capra J. D. Identification of "J" chain in a homogeneous canine IgA immunoglobulin. J Immunol. 1972 Jul;109(1):59–64. [PubMed] [Google Scholar]
- Mestecky J., Zikan J., Butler W. T. Immunoglobulin M and secretory immunoglobulin A: presence of a common polypeptide chain different from light chains. Science. 1971 Mar 19;171(3976):1163–1165. doi: 10.1126/science.171.3976.1163. [DOI] [PubMed] [Google Scholar]
- Morris J. E., Inman F. P. Isolation of the monomeric subunit of immunoglobulin M with its interchain disulfide bonds intact. Biochemistry. 1968 Aug;7(8):2851–2857. doi: 10.1021/bi00848a022. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Koshland M. E. Characterization of the J chain from polymeric immunoglobulins (IgA-IgM-immunological specificity-primary structure). Proc Natl Acad Sci U S A. 1972 Jan;69(1):124–128. doi: 10.1073/pnas.69.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daly J. A., Cebra J. J. Chemical and physicochemical studies of the component polypeptide chains of rabbit secretory immunoglobulin A. Biochemistry. 1971 Oct 12;10(21):3843–3850. doi: 10.1021/bi00797a007. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]







