ABSTRACT
Introduction:
Postmenopausal uterine bleeding is a „cancer until proven otherwise”. Endometrial cancer is a typical disease among postmenopause woman, because every bleeding in this age etiology associated with endometrial cancer (10-30%). The lifespan of women today has been extended and post menopause today last one third of a woman’s life. Early diagnosis of endometrial cancer has a very high cure rate. Screening for this cancer has limits in practice and is necessary given the definition of high-risk groups would be subject to primary and secondary prevention.
Goal:
Primary to evaluate the leading causes of postmenopausal uterine bleeding among patients at risk for endometrial cancer (diabetes, obesity, nulliparity, late menopause (after 55 years) and compared them with the causes of postmenopausal uterine bleeding patients without this risk.
Material and methods:
A retrospective, descriptive study with a targeted sample of 50 consecutive patients who had registered postmenopausal uterine bleeding in high-risk groups (cohorts) and the same number of patients with postmenopausal uterine bleeding that does not belong to the risk group (control group). Each patient underwent clinical examination, then fractionated curettements and its histopathological verification and assessment of treated clinical stage of disease with PH analysis of the resected specimens.
Results:
The patients of the studied risk group were significantly affected by endometrial cancer compared with the control group (RR=2.45, 95% CI 1.2 4.6, p=0.005). Endocervical pathology did not differ between groups. Clinical forms of bleeding: for those that are profuse bleeding cancer was present in 54.6% of cases. With intermittent bleeding cancer is verified in the 33.3% of patients. Risk patient groups with cancer frequently suffer from clinically more advanced stages of histologically aggressive endometrial cancer (serous adenocarcinoma–type II, low differentiated cancer).
Keywords: postmenopausal bleeding, endometrial cancer, risk factors, clinical forms of bleeding
1. INTRODUCTION
Menopause is the last physiological bleeding from the uterus. Postmenopause is according to the definition of the World Health Organization (WHO) defined as the time from the end of the menstrual period onwards. It is divided into early and late–senium (after 70 years). Both of these conditions establish retrograde if the period from the last day of bleeding was one year ago (1, 2, 3).
Improving living conditions, socioeconomic status, women today spend nearly a third of life in postmenopausal period. Casuistry morbidity in this age group dominates the prevention, early detection and treatment of malignant diseases.
WHO estimates that until 2030 approximately 1.2 billion of women will be aged over 50 years. Due to these facts, each country invited to the health needs of women in menopause incorporated as an essential item in health research and public health programs (1, 3).
1.1. Postmenopausal uterine bleeding
Postmenopause uterine bleeding is etiologically associated with endometrial cancer in 10 to 30% of cases (4, 5, 6, 7). Other causes of these hemorrhages are hyperplasia with atypia as premalignant changes and atrophy of the endometrium (8, 9, 10).
1.2. Endometrial cancer
It is estimated that every woman in postmenopause before of 75 years has 2-3% chance of developing endometrial cancer. Endometrial cancer in Europe and the United States is the most common cancer of female genital tract (11, 12, 13). In our country, according to the frequency it is behind cervical cancer.
1.3. Risk factors for endometrial cancer
There are multiple risk factors for endometrial cancer. These are situations when there is chronic exposure of endometrium to neoponated (progesterone) action of endogenous or exogenous estrogen source (6), then older age, nulliparity, obesity (21,22), diabetes, late onset of menopause, reduced physical activity (23).
Therapeutic use of selective estrogen receptor modulators (SERMs tamoxifen) and others at high risk of endometrial cancer are women who are carriers of mutations associated with hereditary nonpolyposis colon cancer (HNPCC) (24).
1.4. The diagnosis of endometrial cancer
Fractional explorative curettage is currently the most reliable diagnostic procedure in premalignant and malignant diseases with symptoms of endometrial uterine bleeding after menopause (4, 7). Unexpected risk of endometrial cancer was significantly lower among women who underwent fractionated curettage (D&C), compared to those who had a biopsy of the endometrium (33).
Transvaginal sonography is the method of choice to assess the state of the endometrium. Endometrium thickness in women with bleeding in postmenopause is 4 mm with 96% sensitivity and 68% specificity according to the largest clinical study, so called Nordial study (33)
2. GOAL
Analyze the leading causes of uterine bleeding among postmenopause woman which belong to risk groups (with diabetes, obesity, nulliparous, with late onset of menopause) and compare them with the causes of uterine bleeding among postmenopausal women without these risk factors (control group);
Compare the incidence of malignant endometrial diseases among these groups of patients;
Compare results abradata cavum uterus in both groups of patients;
Compare the results of the findings of endocervical abradata of these patients;
For those diagnosed with endometrial malignancy compare clinical stage, degree of tumor differentiation, the degree of infiltration of the uterine wall;
Determine whether risk groups get histologically more aggressive types of endometrial cancer and less differentiated form of endometrial cancer compared to the control group.
3. MATERIAL AND METHODS
Study was performed on consecutive and retrospective sample of patients hospitalized at the Gynecology Ward of the General Hospital “Prim. Dr. Abdulah Nakas” in Sarajevo and Department of Obstetrics, Gynecology and Perinatology of Cantonal Hospital Zenica admitted with diagnosis of postmenopause bleeding. The sample consisted of 45 consecutive patients with anamnesis and clinically confirmed presence of risk factors (with diabetes, obese patients, nulliparous, in late menopause, with confirmed hypertension) as a risk group and 45 consecutive patients admitted with diagnosis of postmenopausal bleeding without risk factors, as a control group. In all patients was taken history, they underwent gynecological and ultrasound examination, exploration curettage (D&C) or possibly hysteroscopy sample. Women with diagnosed premalignant or malignant endometrial disease underwent surgery and PH verification of resected specimens.
The definition and selection of patients was performed in the following groups of patients (categorical variables): Risk and control group; Nulliparous and multiparous; Nutritional status: normal BMI (20 to 25.9), excessive weight (26-30) and obese (over 30); The menopause onset: early (under 40 years) and late (over 55 years); Early (<70 years) and late (> 70 years) Clinical forms of bleeding: abundant, poorly, intermittent, spotting.
The parameters of the research were obtained from the case history of patients in successive order, retrospectively, then reports on the histological processing of material from cavum and endocervical or an operative samples. The key test characteristics (variables) were:
Endometrial malignancy, histological type and stage;
Premalignant disease-endometrial hyperplasia with and without atypia;
Endometrial cancer confirmed-clinical stage (FIGO) (degree of infiltration of endometrial cancer histologic type, degree of differentiation);
Malignant or premalignant endocervical disease (mucosa).
Collecting of investigated parameters was performed using the survey which defined population characteristics and variables that are being investigated. Questionnaires were encrypted to protect privacy of each patient.
Statistical analysis and presentation of results:
The characteristics examined were: endometrial cancer, endometrial premalignant disease, clinical stage, degree of myometrial infiltration, histological type of the cancer, the degree of differentiation of the tumor, the presence or absence of tumor vascular lesions.
The outputs of the research were:
a) absolute risk (probability of disease in the study group); b) Relative risk (risk ratio compared to the control group); c) Attributable risk (the difference between two groups); d) OR (odd ratio-risk ratio); e) Diagnostic accuracy (sensitivity, specificity, PPV +/-); f) Incidence of studied characteristics in a defined population.
Given the high perceived etiologic association of postmenopausal uterine bleeding with premalignant and malignant diseases of the endometrium, or high prevalence of the studied characteristics of defined population in general (bleeding in postmenopausal women) the study included 90 subjects (45 with and 45 without the risk) as risk and the control group. Statistical evaluation of the results (categorical variables) was conducted by chi-square test. Level of statistical significance was determined by calculating the p-value with the accepted significance level of 95% (p <0.05). Statistical analysis is complemented by the so-called assessment of confidence interval of 95% (CI-confidence interval).
Table 1.
The incidence of endometrial cancer

4. RESULTS
Results of the research are presented in tabular form in accordance with the tested categorical variables.
The incidence of endometrial cancer in risk group
The incidence of endometrial cancer in the risk group was 30% and in the control group 12.2%, compared with 21.1% in both groups. The relative risk for occurrence of endometrial cancer in risk group compared to the control RR=2.45, 95% CI 1.2-4.6; P=0.005. Risk ratio is OR=3.07, 95% CI 1.41 to 6.68 (p=0.0045, Chi-square=8.54, p=0.0035. Respondents from risk group more often suffer from endometrial cancer.
Table 2.
Curettements of the uterus
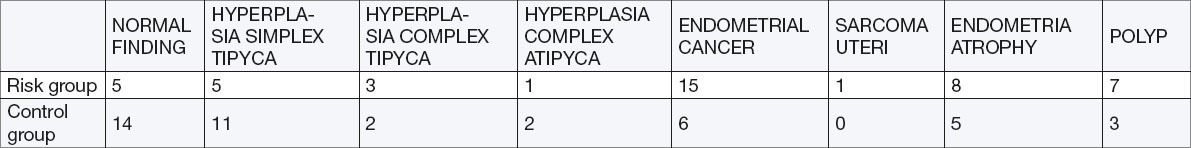
Table 3.
Risk group –Diabetes mellitus

Table 4.
Risk group – obese patients

Table 5.
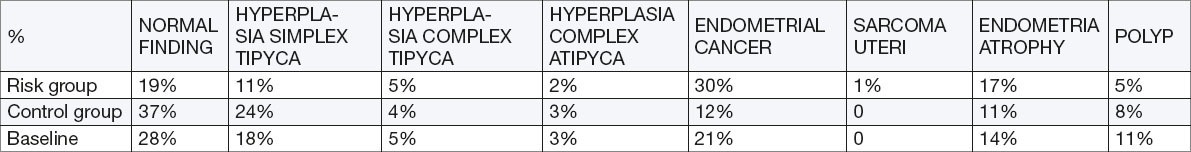
Hyperplasia with and without atypia
Curettements of the uterus both groups
The indicators were: OR=3.07 95%; CI 1.41 to 6.08 (p=0.0045), Chi Square test = 8.54, p= 0.0035). Patients fro, risk group had statistically significantly higher incidence of endometrial cancer. The incidence of endometrial cancer in both groups was 23.0%.
Risk group –Diabetes mellitus
RR=2.45, 95% CI 1.18 to 5.0; p=0.015. Patients with diabetes and have postmenopausal bleeding was significantly had higher incidence of endometrial cancer.
Risk group – obese patients
RR 2.41, 95% CI 1.07 to 4.30, p=0.031, Chi square test p=0.0275.
Obese patients with uterine bleeding in postmenopause had significantly higher incidence of endometrial cancer. The incidence of endometrial cancer in obese patients is 26.23%.
Premalignant changes in endometrial hyperplasia with and without atypia
Endometrial complex hyperplasia with atypia: there was no statistically significant difference between the risk and the control group–(RR 0.66, 95% CI 0.114-3.89, p=0.65). Complex Endometrial hyperplasia without atypia: no statistical significant difference compared to the control group–(RR 1.25, 95% CI 0.3 to 4.5, p=0733, OR 1.26). Endometrial hyperplasia simplex significantly more occurred in the control group (the lowest level of premalignant potential)–(RR 0.45, 95% CI 0.22-0.9, p=0.02, OR 0.38). Overall prevalence of premalignant lesions in both groups was 25.5%.
Table 6.
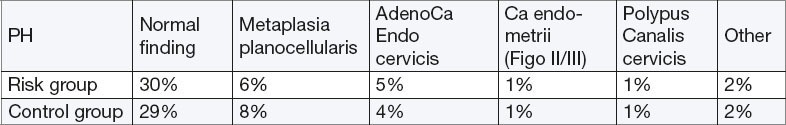
Endocervical pathology
Endocervical pathology
There was no statistically significant difference in the incidence of malignant and premalignant endocervical pathology between groups: a) adenocarcinoma of the cervix (RR 0.54, 95% CI 0.5-5.95, p = 0.62), b) Adenocarcinoma of the cervix and endometrium cancer (FIGO II)–(RR 2.0, 95% CI 0.62 to 6.4, p=024 R = 2.09). The total incidence of adenocarcinoma in both groups was 1.66%. Normal findings of endocervical abradata found in risk group 66.6% in the control virtually the same–65.5%.
Clinical stage (FIGO) in verified cancer
Patients of risk group with endometrial cancer more frequently had the more advanced clinical stage (FIGO)–(RR 1.22, 95% CI 0.40 to 3.68, p = 0.72).
Clinical cancer: degree of infiltration
Infiltration of myometrium showed no statistically significant difference between the risk and the control group (RR 1.12, 95% CI 0.74 to 1.67, p = 0.58, R = 1.65.)
Histologic types of endometrial cancer
Respondents from risk group more often suffer from histologically aggressive form of endometrial cancer compared to the control group (RR =2.85, 95% CI = 0.39 to 20.54, p = 0.298)
Of the total number of confirmed endometrial cancer of well differentiated (grade I) we found 30% (8) of moderately differentiated (grade II) 55% (15), and poorly differentiated (grade III) 40 cases. Within the control group, there has never been a case of poorly differentiated cancer. We conclude that the risk group had significantly higher histological immature tumors with potentially greater malignancy (p = 0.35). The study attributes vascular invasion of cancer did not find significant differences in the incidence of vascular invasion and lymph vessels in both groups.
5. DISCUSSION
In our sample among patients of both groups we get a relatively high incidence of endometrial cancer of 21%. Analyzing the impact of risk factors on the incidence of endometrial cancer, we came to the conclusion that the patients exposed to known risk (diabetes and obesity) are significantly more often affected than those in the control group (p = 0.005). Diabetic patients in the risk group had the highest risk (p = 0.015), followed by obese patients (p = 0.031). Thus, a significant correlation between the analyzed risk factors with the incidence in this study leads to the conclusion that prevention of diabetes, lifestyle changes, reduction of obesity and physical activity can significantly reduce the risk of the emergence of this disease. Patients exposed to the analyzed risk factors are put in the focus of attention in the primary and secondary prevention of endometrial cancer.
Patients from risk group with confirmed endometrial cancer were more likely to have clinically severe stages of the disease. There were no significant differences in the extent and frequency of infiltration of the wall of the uterus. There is an apparent high incidence of infiltration of the wall of the uterus in both groups (78%) as an indicator of late detection of endometrial cancer. In our sample in both groups, cancer was detected in the first stage in 60% of cases, in the second stage in 32% and the third in 8% of cases. All cases of poorly differentiated endometrial cancer (biologically potent) are found in the risk group. There was no significant difference in the incidence of lymph-vascular invasion in both groups.
Subjects exposed to the risk factors have higher prevalence of aggressive histologic types of endometrial cancer.
Table 7.
Clinical stage of cancer
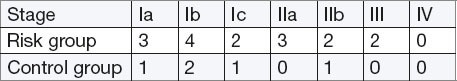
Table 8.
The degree of infiltration of the uterus wall
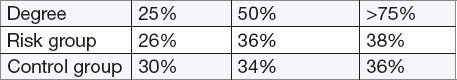
Table 9.
Types of endometrial cancer
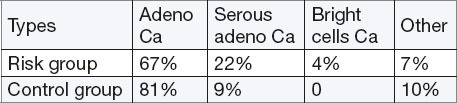
By analyzing the frequency of endocervical pathology the histologic evaluations did not show any significant differences between the two groups. These results coincide with the data of other authors. Results of studies by other authors, meta-analysis, cohort and case-control association studies of diabetes and risk of endometrial cancer showed statistically significant results (15, 16).
Obesity as a public health problem increases the risk of developing endometrial cancer (2, 4, 18). Research shows that obesity, especially abdominal obesity (waist circumference over 80 cm) in symptomatic postmenopausal women carries a high risk for endometrial cancer. Burbos N, Mosunda P. et al. in their research, developed a clinical predictive methods and models to assess the risk of endometrial cancer in post-menopausal period. It is a triad (FAD 31), where F is the frequency of bleeding, A = age, D = Diabetes and 31 is the cut off BMI score (14, 15).
6. CONSLUSIONS
By the analysis of the results we have come to the following conclusions:
Patients from risk group (diabetes, obesity) in our sample were significantly more often affected by endometrial cancer compared to the control group (post-menopausal bleeding in patients without risk); Patients with uterine bleeding in postmenopause suffering from diabetes mellitus and obese patients (BMI> 31) were significantly more likely to have endometrial cancer compared to the control group; There was no significant difference in the incidence of endometrial complex hyperplasia with and without atypia in risk group than in the control group;
There was no significant difference in the incidence of endocervical premalignant and malignant pathology between groups; Patients from risk group with endometrial cancer were significantly more affected by histologically aggressive form of cancer, as they more frequently had the disease in clinically more advanced stages; Infiltration of the wall of the uterus was present in 80% of patients with confirmed endometrial cancer in both groups, indicating a late detection.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Shaw LM, Shaw SL. Menopuse, evolution and changing cultures. Menopuse Int. 2009 Dec;15(4):175–179. doi: 10.1258/mi.2009.009044. [DOI] [PubMed] [Google Scholar]
- 2.Šimunic V. Ginekologija. Zagreb: Naklada Ljevak; 2001. Klimakterij, menopuza i postmenopuza. U: Šimunic V. & sur., ur; pp. 368–386. [Google Scholar]
- 3.Van Hanegem N, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeing: an evidence-based approach. Maturitas. 2011 Feb;68(2):155–164. doi: 10.1016/j.maturitas.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Strnad M, Znaor A. Epidemiologija gineloškog raka. In: Corušic A, Babic D, et al., editors. Ginekološka onkologija. Zagreb: Medicinska naklada, Zagreb; 2005. pp. 3–15. [Google Scholar]
- 5.Drljevic K, Mehmedbašic S, et al. II Kongres ginekologa i perinatologa u BiH. Sarajevo: Kjiiga sažetaka; 2009. Endometriji kod žena oboljelih od raka dojke; p. 80. [Google Scholar]
- 6.Ciglar S. Rak trupa materice/rak endometrija. In: Šimunic V, editor. Ginekologija. Zagreb: Naklada Ljevak; 2001. pp. 451–457. [Google Scholar]
- 7.Fader AN, et al. Endometrial cancer and obesity;: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol. 2009 Jul;114(1):121–127. doi: 10.1016/j.ygyno.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Schmandt RE, et al. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011 Jun 7; doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burbos N, Musonda P, et al. Estimating the risk of endometrial cancer in symptomatic postmenopausal woman: a novel clinical prediction model based on patients characteristics. Int J Gynecol Cancer. 2011 Apr;21(3):500–506. doi: 10.1097/IGC.0b013e31820c4cd6. [DOI] [PubMed] [Google Scholar]
- 10.Musonda P, Burbos N, et al. Coparing the performance of two clinical models in estimating the risk of endometrial cancer in symptomatic postmenopausal woman. Eur J Obstet Gynecol Reprod Biol. 2011 Oct 3; doi: 10.1016/j.ejogrb.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Korhonen MO. Epidemiological differences between adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecologic Oncology. 2004 Dec;:312–317. doi: 10.1016/0090-8258(80)90099-2. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JJB, Anthony M, Messina M, Garner SC. Effects of phyto-oestrogens on tissues. Nutr Res Rev. 1999;12:75–116. doi: 10.1079/095442299108728875. [DOI] [PubMed] [Google Scholar]
- 13.Balk JA, Whiteside DA, Naus G, DeFerrari E, Roberts JM. A pilot study of the effects of phytoestrogen supplementation on postmenopausal endometrium. J Soc Gynecol Investig. 2002;9:238–242. [PubMed] [Google Scholar]
- 14.Barnes S. Phytoestrogens and breast cancer. Baillieres Clin Endocrinol Metab. 1998;12:559–579. doi: 10.1016/s0950-351x(98)80004-9. [DOI] [PubMed] [Google Scholar]
- 15.Belcher SM, Zsarnovszky A. Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J Pharmacol Exp Ther. 2001;299:408–414. [PubMed] [Google Scholar]
- 16.Benassayag C, Perrot-Applanat M, Ferre F. Phytoestrogens as modulators of steroid action in target cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:233–248. doi: 10.1016/s1570-0232(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 17.Cassidy A, Bingham S, Setchell K. Biological effects of isoflavones in young women: importance of the chemical composition of soyabean products. Br J Nutr. 1995;74:587–601. doi: 10.1079/bjn19950160. [DOI] [PubMed] [Google Scholar]
- 18.File SE, Duffy R, Wiseman H. Improved memory and frontal lobe function in post-menopausal women after 3 months’ treatment with soya supplements. Eur J Neuropsychopharmacol. 2002;12:S406. [Google Scholar]
- 19.File SE, Hartley DE, Alom N, Rattray M. Soya phytoestrogens change cortical and hippocampal expression of BDNF mRNA in male rats. Neurosci Lett. 2003;338:135–138. doi: 10.1016/s0304-3940(02)01391-5. [DOI] [PubMed] [Google Scholar]
- 20.File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H. Eating soya improves human memory. Psychopharmacology (Berl) 2001;157:430–436. doi: 10.1007/s002130100845. [DOI] [PubMed] [Google Scholar]


