ABSTRACT
Introduction:
Alprazolam is a triazolobenzodiazepine used in panic disorders and other anxiety states. Target organ of Alprazolam is CNS, causing depression of respiration and consciousness.
Aim:
This study aimed to estimate the genotoxic potential of Alprazolam using Allium cepa test.
Methods:
Allium cepa is one of the most suitable plants for detecting different types of xenobiotics. The test enables the assessment of different genetic endpoints making possible damage to the DNA of humans to be predicted.
Results:
Alprazolam induced chromosomal (anaphase bridges, breaks, lagging and stickiness, abnormal spiralisation, multipolarity and polyploidy) and cytological aberrations, especially nuclear alterations (nuclear buds, fragmented nucleus and apoptotic bodies, cells without nucleus, binucleated and micronucleated cells), morphological alterations in shape and size of cells, spindle disturbance and polar deviation in root tip meristem cells of Allium cepa at all tested concentrations. Alprazolam also caused significant inhibition of mitotic index in these cells.
Conclusion:
These changes in cells are indicators of genotoxic potential of Alprazolam suggesting a need for further in vitro studies on animal and human lymphocytes as well as in vivo studies.
Keywords: Allium cepa test, Alprazolam, Genotoxicity, Apoptotic bodies
1. INTRODUCTION
The root tips several of plant species have been used for the study of induced chromosomal aberrations (CAs) and presence of micronuclei (MNi). Root tips of different Allium species are used the most frequent as experimental material. The plants possess some advantages over other organisms in certain circumstances. The plants usually have the large chromosomes and the low chromosome number. The root meristem contains a high proportion of cells in mitosis (1-3). Plant systems had a major part in early investigations of the genetic changes caused by mutagenic chemicals and radiation. One of the most suitable plants for detecting different types of xenobiotics is Allium cepa L. The first modification of the Allium cepa test for environmental monitoring was introduced by Fiskesjö (4, 5).
The chromosome assays on plants are rapid and inexpensive and do not require elaborate laboratory facilities and a wide range of genetic endpoints is available. The chromosomes of plants and animals are morphologically similar, and appear to respond to treatment with mutagens in a similar way to those of mammals and other eukaryotes. There are some important limitations as well, such as the longer life cycle of most plants than bacteria, yeast or Drosophila and some biochemical differences between plants and mammals. There are also fundamental differences in structure between plant and mammalian cells. The growing root tips of the onion, Allium cepa provide a readily available source of material for studying the damaging effects of chemicals on chromosomes. This plant is useful for evaluating DNA damages that express such as CAs, disturbances in the mitotic cycle, nuclear alterations (NAs) and presence of MNi in meristem cells.
The decrease in the mitotic index (MI) of Allium cepa meristem cells can be considered as a reliable method to determine the presence of cytotoxic agents in the environment. CAs are including changes in either chromosomal structure or in the total number of chromosomes. Structural chromosomal alterations may be induced by DNA breaks, inhibition of DNA synthesis and replication of altered DNA. The CAs, such as chromosome bridges and breaks, are indicators of a clastogenic action. The numerical CAs (aneuploidy and polyploidy) are consequences of abnormal segregation of chromosomes (chromosome losses, delays, adherence, multipolarity and C-metaphases) which can occur either spontaneously or by the action of aneugenic agents. Chromosome without telomeres become “sticky” and may fuse with other broken chromosome ends. The result of these chromosomal rearrangements are acentric fragments, dicentric bridges that can be observed in mitotic cells of the first cell cycle after mutagenic treatment or MNi in the interphase cell in the next cell cycle.
The Allium cepa test is used for screening and monitoring environmental chemicals with mutagenic and carcinogenic potential (6-10). Alprazolam is a newer benzodiazepine that is being used more commonly in overdose. Overdose in adults frequently involves co-ingestion of other central nervous system (CNS) depressants, which act synergistically to increase toxicity. Alprazolam is assumed to be capable of causing fatal harm and an increased risk if congenital abnormalities when administered to a pregnant woman during the first trimester.
The aim of this study was to evaluate the genotoxic potential of benzodiazepine drug Alprazolam. For estimate the genotoxicity of this benzodiazepine and for assessment of risks from exposure to Alprazolam we used the Allium cepa test.
2. MATERIAL AND METHODS
Alprazolam (Xanax, Helex, Alpronax) is a triazolobenzodiazepine used in panic disorder and other anxiety states. Alprazolam, like other benzodiazepines, binds to specific sites on the GABAA (gamma-amino-butyric acid) receptor. The chemical name of Alprazolam is 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-a] [1,4] benzodiazepine. The molecular formula is C17H13ClN4 which corresponds to a molecular weight of 308.76.
The root tip cells of onion were used to test the potentially genotoxic effects of Alprazolam. The test was carried out according to Fiskesjö protocol (4, 11-12) with some modifications. Common onion (Allium cepa L.) has eight pairs of relatively large chromosomes (2n = 16) that allows for the easy detection of CAs. The plant material is available all the year round, it is inexpensive and easily to grow and handled. The size of chromosomes makes Allium cepa tips as favourable material for the study of the effects of chemicals on frequency of CAs which are biomarkers of damage of genetic material. The root tip cells have a very low spontaneous aberration frequency and a stable chromosome number.
To perform this test we used onion bulbs that cultivated without the application of herbicides or fungicides. Healthy young onion bulbs that were the same size were used in the test. The outer scales are removed from bulbs and they scraped at the root to promote the emergence of new roots. Onion bulbs then have been grown in water at room temperature. The bulbs should remain in the dark. When the newly emerged roots are 2 cm in length, they are ready for treatment with the test drug. Adequate root growth should be obtained in 3-4 days. The bulbs should be transferred to petri dish with 30 ml of Alprazolam solution appropriate concentration for the treatments. We use group of 6 bulbs of Allium cepa for each treatment. Roots of Allium cepa have been treated with a series of concentration 100, 150, 200, 250 and 500µg/ml for 24h. Freshly prepared solutions of Alprazolam should be used. Treatments took place in the dark. A negative control has been treated with distilled water. After 24h, the roots of each bulb were extracted and fixed in freshly prepared and cool mixture (4-10 °C) containing 3 parts of methanol and 1 part of glacial acetic acid (3:1, v/v) for 24h. Roots can be stored in fixative for several days or weeks.
For preparation root tip chromosome slides acetorcein squash technique has been used to analyse MI, cytotoxic effects and CAs. The root tips of bulbs hydrolysed in 1N hydrochloric acid (HCl) at 60 ºC for 4-5 min. The cell wall has to dissolve by hydrolysis with acid. From HCl the roots are transferred to distilled water and left for a few minutes. The roots then were transferred on clean slide. Three roots tips were used for each slide. On the slide, tips were crushed in drop of 2% acetorcein (Gurr Orcein, BDH Chemicals Ltd., Poole, England) with the flat end of metal rod (taper) and squashed under a cover slip. The pressure was applied under several thickness of blotting filter paper during sideways movements of cover slip must be avoided. The two slides prepared for each of bulbs in each experimental group (concentration of the test drug). A thousand cell for MI and a hundred metaphase cells (except for concentration of 500µg/ml due to reduction in MI) for CAs were counted for each bulb per treatment group. Twelve slides were prepared for each concentration of Alprazolam and the control. For cytogenetic examination of mitotic cells was used a microscope (Jenaval) at1000x magnification.
The Mitotic Index (MI) represents the total number of dividing cells in relation to the number of analysed cells in cell cycle. A minimum of 1,000 cells were scored for MI and expressed as a percentage of total number of examined cells undergoing mitosis. The frequency of CAs was expressed as the number of aberrant cells per 100 cells examined. A hundred metaphase cells were scored for each bulb in experimental group and the number of aberrant cells in each experimental group is compared with the values from the control group. The Allium cepa test enables to estimate CAs in all phases of the cell cycle.
Data were expressed as the mean ± standard deviation (SD) of the means for MI and frequency of aberrant cells. For statistical analysis of variance (ANOVA) was used. Relationships between different concentrations of Alprazolam and MI or frequency of aberrant cells were investigated using Pearson’s correlation analysis and/or Spearman’s rank correlation. Analyses were done at P<0.05 and 95% confidence level using MedCalc Version 12.5.0.0 statistical software package.
3. RESULTS
The results this study showed concentration-dependent reduction in MI of Allium cepa root tip meristem cells treated with different concentrations of Alprazolam for 24h (Table 1). This difference was significant in Pearson’s correlation analysis where correlation coefficient (r) was -0.7454 with significance level P<0.0001 and 95% confidence interval (CI) for r -0.8700 to -0.5315. The similar results we obtained after using Spearman’s rank correlation. Spearman’s coefficient of rank correlation (rho) was -0.725 with significance level P<0.0001 and 95% CI for rho -0.859 to -0.500. Based on the statistical analysis using ANOVA test we observed inhibition of MI in meristem cells that was statistically significant at all tested concentrations (P<0.05) but not significant at 150µg/ml when compared with the control.
Table 1.
Mitotic index (MI) of Allium cepa root tip cells exposed to different concentrations of Alprazolam (mean ± SD)
The CAs in Allium cepa root tip meristem cells after 24h exposure to Alprazolam were anaphase bridges, breaks, chromosome lagging and stickiness, abnormal spiralisation of chromosomes, multipolarity and polyploidy (Table 2). Statistically significant increased number of CAs in the meristem cells with increasing concentration of Alprazolam was observed in Spearman’s rank correlation analysis (rho=0.524, P=0.0025 and 95% CI: 0.208 to 0.741). Statistically significant higher number of CAs were at all tested concentrations (P<0.05) except at 100µg/ml in comparison with the control when analysed with the ANOVA test. In this analysis we excluded cells treated with Alprazolam of 500µg/ml because a little number of dividing cells at this concentration. Figure 1 shows CAs at all phases of cell cycle that can be observed by the Allium cepa test.
Table 2.
Chromosome aberration assay in Allium root cells exposed to Alprazolam (100, 150, 200, 250 and 500µg/ml for 24h)

Figure 1.

Photomicrographs of CAs induced by Alprazolam in root tip cells of Allium cepa; (a) prolonged prophase and abnormal kinetics; (b) and (c) chromosome laggards and spindle disturbance at metaphase; (d) disturbed anaphase in a polyploid cell; (e) anaphase with chromosome laggards; (f) multipolar anaphase and spindle disturbance (magnification: 1000x).
Cytological aberrations observed at cell cycle after the treatments with Alprazolam were different NAs (nuclear buds, ghost cells–cells without nucleus, fragmented nucleus and apoptotic bodies), binucleated cells and micronucleus formation (Table 3). MNi were present in the different phases of cell cycle in root meristem cells of Allium cepa. The spindle disturbance and polar deviation can lead to abnormal kinetics of chromosome and prolonged prophase. Figure 2 shows nuclear abnormalities that can be found in Allium cepa meristem cells exposed to Alprazolam. Statistically positive correlations were found between concentrations Alprazolam and NAs after using Pearson’s correlation analysis (r=0.7866, P<0.0001 and 95% CI: 0.5994 to 0.8923) and Spearman’s rank correlation (rho=0.811, P<0.0001 and 95% CI: 0.640 to 0.905). These results were statistically significant except for concentration 100µg/ml when analysed with the ANOVA test (P<0.05) in comparison with the control.
Table 3.
Cytological effects of different concentrations of Alprazolam on root tip cells of Allium cepa (mean ± SD)
Figure 2.
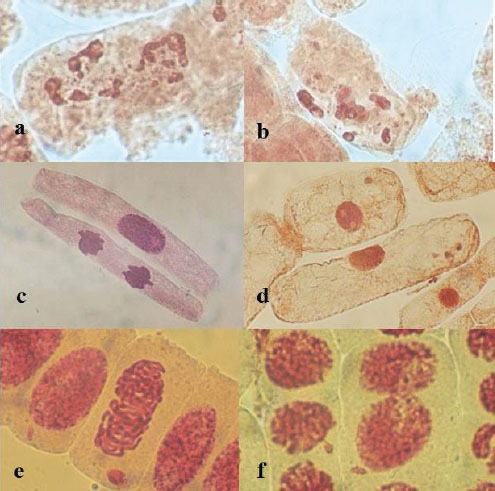
Photomicrographs of cytological aberrations in Allium cepa meristem cells exposed to Alprazolam; (a–b) cells with fragmented nucleus and apoptotic bodies, morphological alterations in shape and size of cells; (c) extended interphase and telophase cell, dislocation of spindle, stickiness; (d) extended cells, disturbed spindle and cytoplasm destruction; (e) micronucleus in prophase, stickiness; (f) cells with nuclear bud (magnification: 1000×).
4. DISCUSSION
The Allium cepa test is important test in vivo, where the roots grow in direct contact with the substance of interest enabling possible damage to DNA of humans to be predicted. In this study, test enables the assessment of different genetic endpoints which occur as a result from exposure to Alprazolam. Alprazolam caused significant inhibition of MI in Allium cepa meristem cells and induced CAs, nuclear abnormalities and micronucleated cells (MNCs).
The decrease in the MI as the concentration of Alprazolam increased. The changes in MI of Allium cepa cells are indicators of cytotoxic and genototoxic potential and mitodepressive activity of Alprazolam. The number of CAs increased as concentration of Alparzolam increased.
The analysis of the different CA types, in all phases of the cell cycle, enables a better investigation of the effects of Alprazolam, concerning its clastogenic, aneugenic and tubergenic effects. Breakages may occur and subsequent inhibition of repair mechanisms may lead to base mismatch, mutation and CAs such as fragment chromosomes and DNA breaks (12-14). Examination of anaphase chromosomes for fragments and bridges is a useful for obtaining information on clastogenic activity. The presence of dicentric chromosomes and unequally exchanged chromatids undergoing translocation has been reported to be responsible for chromosomal bridges at anaphase.
The bridges, breaks, lagging and multipolar anaphase chromosomes were observed at all concentrations, except at the highest tested concentration. The results showed that higher concentrations were able to inhibit significantly cell division. Alprazolam cause lethal CAs in Allium cepa at higher concentrations. Stickiness is due to inter-chromosomal linkages of sub-chromatid strands coupled with excessive formation of nucleoproteins and inappropriate protein-protein interaction.
Nuclear abnormalities are characterized by morphological alterations in the interphase nuclei as a result from the exposure to Alprazolam. Aberrations observed at interphase stage of cell cycle were different NAs (nuclear buds, cells without nucleus–ghost cells, fragmented nuclei and apoptotic bodies), BNCs and micronucleus formation. Also, we observed alterations in shape and size of cells (gigantic and extended cells). The presence of fragmented nuclei and polynuclear cells can indicate a cell death process and this may lead to aneuploidy and then to cell death. The number of cytological aberrations increased with increasing concentration.
MNi are formed as a consequence of chromosome breakage (clastogenic agent) or whole chromosomes (aneugenic agent) that were not incorporated to the main nucleus during the cell division cycle (15-16). The frequency of cells with MNi is indicator of the cytogenetic effects of tested chemicals. MNi were observed to be mostly caused by Alprazolam at 500µg/ml suggesting its greater genotoxic and tubergenic effects on Allium cepa when compared with lower concentrations of drug. Inhibition of cytokinesis following telophase is responsible for binucleated cell formation.
Long half-life benzodiazepines, such as diazepam or clonazepam, accumulate with repeated doses and clearance is significantly increased with age. Alprazolam is intermediate half-life benzodiazepine and it’s the usual starting dose varies between 0.5 and 1 mg per day. Benzodiazepines produce dose-dependent adverse effect and long half-life benzodiazepines use may induce physiological dependence (17). Musanovic et al. (18) reported cytotoxic and genotoxic effects of lower concentrations of Alprazolam against Allium cepa root meristem cells. According to Isbister et al. (19) Alprazolam was significantly more toxic than other benzodiazepines.
5. CONCLUSION
In conclusion, the results of this study indicate that benzodiazepine drug Alprazolam induced chromosomal (anaphase bridges, breaks, lagging and stickiness, abnormal spiralisation, multipolarity and polyploidy) and cytological aberrations, especially nuclear alterations (nuclear buds, fragmented nucleus and apoptotic bodies, cells without nucleus, binucleated and micronucleated cells), morphological alterations in shape and size of cells, spindle disturbances and polar deviation in root tip meristem cells of Allium cepa at all tested concentrations. Alprazolam also caused significant inhibition of mitotic index in these cells and almost stopped the cell proliferation at the highest tested concentration.
The changes in Allium cepa meristem cells are indicators of genotoxic potential of Alprazolam suggesting a need for safe dose administration of the drug in human medicine and further in vitro studies on animal and human lymphocytes as well as in vivo studies.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Kihlman BA. Root tips of Vicia faba for the study of the induction of chromosomal aberrations. In: Kilbey BJ, Legator M, Nichols W, Ramel C, editors. Handbook of mutagenicity test procedures. Amsterdam, New York, Oxford: Elsevier scientific publishing company; 1977. pp. 389–400. [Google Scholar]
- 2.Grant WF. The present status of higher plant bioassays for detection of environmental mutagens. Mutat Res. 1994;310:175–185. doi: 10.1016/0027-5107(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 3.Leme DM, Marin-Morales MA. Allium cepa test in environmental monitoring: A review on its application. Mutat Res. 2009;682(1):71–81. doi: 10.1016/j.mrrev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Fiskesjö G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102:99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 5.Trushin MV, Ratushnyak AY, Arkharova IA, Ratushnyak AA. Genetic Alterations Revealed in Allium cepa-Test System under the Action of Some Xenobiotics. World Applied Sciences Journal. 2013;22(3):342–344. [Google Scholar]
- 6.Sofradžija A, Hadžiselimovic R, Maslic E. Sarajevo: Svjetlost; 1989. Genotoksicnost pesticida. [Google Scholar]
- 7.Knasmuller S, Gottmann E, Steinkellner H, Fomin A, Pickl C, Paschke A, God R, Kundi M. Detection of genotoxic effects of heavy metals contaminted soils with plant bioassays. Mutat Res. 1998;420:37–48. doi: 10.1016/s1383-5718(98)00145-4. [DOI] [PubMed] [Google Scholar]
- 8.Ma TH. The role of plant systems for the detection of environmental mutagens and carcinogens. Mutat Res. 1999;437:97–100. [PubMed] [Google Scholar]
- 9.Cabaravdic M. Induction of Chromosome Aberrations in the Allium cepa Test System Caused by the Exposure of Cells to benzo(a)pyrene. Med Arh. 2010;64(4):215–218. [PubMed] [Google Scholar]
- 10.Sharma S, Vig AP. Genotoxicity of Atrazine, Avenoxan, Diuron and Quizalofop-P-ethyl Herbicides using the Allium cepa Root Chromosomal Aberration Assay. Terrestrial and Aquatic Environmental Toxicology. 2012;6(2):90–95. [Google Scholar]
- 11.Fiskesjö G. A 2-3 day plant test for toxicity assessment by measuring the mean root growth of onions (Allium cepa L.) Environ Toxicol Water Quality. 1993;8:461–470. [Google Scholar]
- 12.Fiskesjö G. Allium Test For Screening Chemicals;Evaluation of Cytological Parameters. In: Wang W, Gorsuch JW, Hughes JS, editors. Plants for Environmental Studies. New York, USA: Lewis; 1997. pp. 308–333. [Google Scholar]
- 13.Türkoğlu S. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat Res. 2007;626:4–14. doi: 10.1016/j.mrgentox.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Badr A, Ibrahim AG. Effect of herbicide Glean on mitosis, chromosomes and nucleic acids in Alium cepa and Vicia faba root meristems. Cytol. 1987;52:293–302. [Google Scholar]
- 15.Fenech M. Cytokinesis-block micronucleus cytome assay. Nature protocols. 2007;2(5):1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 16.Leme DM, de Franceschi de Angelis D, Marin-Morales MA. Action mechanisms of petroleum hydrocarbons present in waters impacted by an oil spill on the genetic material of Allium cepa root cells. Aquatic Toxicology. 2008;88:214–219. doi: 10.1016/j.aquatox.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Yousif WB. Microscopic Studies on the Effect of Alprazolam (Xanax) on the Liver of Mice. Pakistan Journal of Biological Sciences. 2002;5(1):1220–1225. [Google Scholar]
- 18.Musanovic J, Ramic N, Nefic H, Dzubur A. Chromosome aberration and irregular cell cycle in Allium cepa root cells caused by different concentrations of Alprazolam. International Journal of Collaborative Research on Internal Medicine & Public Health. 2013;5(6):407–418. [Google Scholar]
- 19.Isbister GK, O’Regan L, Sibbritt D, Whyte IM. Alprazolam is relatively more toxic than other benzodiazepines in overdose. Br J Clin Pharmacol. 2004;58(1):88–95. doi: 10.1111/j.1365-2125.2004.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


