ABSTRACT
Introduction:
Non-alcoholic (NAFLD) encompasses a spectrum of disease states, from steatosis (fatty liver) to non-alcoholic steatohepatitis (also called NASH steatosis with inflammatory changes) followed by progression to fibrosis and cirrhosis and hepatocellular carcinoma Excess liver fat is believed to be a manifestation of the metabolic syndrome and not surprisingly NASH is associated with obesity, insulin resistance, dyslipidemia and type 2 diabetes in humans.
Aim of the study:
is to establish anthropometric and biochemical specificities in patients with non-alcoholic steatohepatitis diagnosed with non-invasive diagnostic methods
Material and methods:
Study enrolled 170 participants, 130 with NASH steatosis. The non-alcoholic group (control), consisted of 40 normal weight patients without metabolic syndrome. Alcohol intake was estimated with established protocol. Routine biochemistry analysis were performed by standard laboratory procedures; serum levels of serum levels of fasting cholesterol and triglycerides, fasting glucose and insulin, insulin resistance estimated by HOMA index (Homeostasis model assessment), biochemistry tests and a liver ultrasound examination.
Results:
In study participants group, patients were more obese comparing with controls p < 0, 01, waist line extent also was of greater statistical significance in the non-alcoholic group fatty liver (p < 0, 01). Comparing biochemical parameter values, significant statistical deference has been noted in glaucosis and insulin levels, total cholesterol and gama-glutamil transferase levels, between groups (p<0, 01). Fasting glucose and insulin levels, HOMA-IR were significantly greater in study cohort group patients, as was significantly positive correlation between BMI and waist line extent.
Conclusion:
Patients with non-alcoholic fatty liver are excessively obese, have greater waist line extent, consequently insulin resistance and impaired glucose metabolism, insulin resistance, dyslipidemia, risk factors known to be associated with the development of cardiovascular disease.
Keywords: non-alcoholic fatty liver, obesity, body mass index, waist line extent, insulin resistance
1. INTRODUCTION
The definition of nonalcoholic fatty liver disease (NAFLD) requires that (a) there is evidence of hepatic steatosis, either by imaging or by histology and (b) there are no causes for secondary hepatic fat accumulation such as significant alcohol consumption, use of steatogenic medication or hereditary disorders (1). High serum triglyceride levels, low serum HDL levels and consequent presence of hepatic steatosis, are very common in patients with NAFLD, (histology verification >5% hepatocytes) (2). In summary, estimates of the worldwide prevalence of NAFLD in individuals with dyslipidemia attending lipid clinics was estimated to 6, 3% to 33%, with a median of 20% in general population, based on a variety of assessment method (2). In patients with severe obesity undergoing bariatric surgery, the prevalence of NAFLD can exceed 90% and up to 5% of patients may have unsuspected cirrhosis (3). Obesity is very common and well documented factor of risk for NAFLD. Parameters for the degree and obesity type estimation, excessive BMI Body mass index) and visceral obesity, defined as waist line, are recognized as factor of risk for NAFLD (4).
Insulin resistance (IR), common factor that links obesity, diabetes, hypertension and dyslipidemia with fatty liver leading into progressive form of liver disease, steatohepatitis, fibrosis cirrhosis and hepatocellular carcinoma (5, 6). Being almost always present in NAFLD insulin resistance is considered hepatic manifestation of the metabolic syndrome (7, 8).
Investigating pathophysiology background of the metabolic syndrome, insulin resistance and visceral obesity are recognized as main factors of risk. It could be pointed out that insulin resistance is core and obesity most frequent clinical manifestation (9). Early recognition of the metabolic syndrome is of great importance for atherogenesis which starts on the very beginning of the process, jointly with other multiple factors proceeds towards diabetes mellitus (DM) type 2, arteriosclerotic complications (10). Patients with non alcoholic fatty liver disease, adults and children usually meet diagnostic criteria for metabolic syndrome (abdominal fat deposition, hypertension, atherogenic dyslipidemia and hyperglycemia) respectively have factors of risk for cardiovascular disease development (5, 7, 9).
In estimating cardiovascular risk, fat distribution, is of greater importance, than amount of body fat and adipocyte dysfunction. Visceral fatty tissue correlates with insulin resistance, which contrary to subcutaneous fatty tissue is metabolic active organ and exhibits numerous local and systemic effects through secretion of whole lot of variety peptides, some of them with altered function in obesity. Enlarged and inflamed visceral fatty tissue releases wide spectrum of the molecules potentially involved in development of the insulin resistance and atherosclerosis, including free fatty acids, interleukin 6, tumor necrosis factor α (TNF-α), monocytes chemotactic protein 1and other proinflammatory cytokines (7). Therefore recently modified definitions of the metabolic syndrome include waist line measurements as insulin resistance marker (11). Zhu and associates pointed out that waist line better predicts development of the cardiovascular diseases than BMI in patients of both gender.
A group of authors from our surrounding area, also support the attitude that waist line extent is a good indicator of the visceral obesity, that can independently point out health risk (12). Those values that indicate sudden rise of the risk factors for cardiovascular diseases for men are 96 cm, and for women 86 cm in waist line (13).
Notification of either of the disorders in patients with established disrupted AST/ALT (ratio-aspartate aminotransferase/alanine aminotransferase), ratio, obesity, especially morbid obesity (BMI>35), DM type 2 diagnosis, diagnosis of the metabolic syndrome, obstructive apnea, point out towards NAFLD (2). Body mass measurements (BMI index), waist line measurements (VC) waist-hip ratio (VHR), bio electrical impedance analysis (BIA) are widely recommended in patients with NFLD as diagnostic tools, for being comfortable and relatively at low cost (2). In raised suspicion for NAFLD ultrasonographic imaging is diagnostic method first to be used in liver steatosis detection, in order to avoid more invasive methods as liver biopsy (1, 2). Existing dogma posits that liver biopsy is the most reliable approach for identifying the presence of steato- hepatitis and fibrosis in patients with NAFLD, but it is generally acknowledged that biopsy is limited by cost, sampling error, and procedure-related morbidity and mortality. So, liver imaging is the common test used in liver steatosis screening (2). Imaging reveals lever of the homogenic structure, light with increased echogenicity (glittering liver) (15).
Aim of this study was to establish anthropometric and biochemical specificities for patients with non alcohol fatty liver, diagnosed by noninvasive diagnostic procedures.
2. MATERIAL AND METHODS
Prospective study conducted during 24 months (2009-2011) covered and analyzed 170 participants through clinical monitoring. Diagnosis of “non alcoholic fatty liver” confirmed by noninvasive imagining liver ultrasound procedure and by the AST/ALT ratio (1, 2).
Respondents were distributed in two groups:
Study group (consisted of responders with imagining confirmed fatty liver and AST/ALT pathological values. Group numbered 130 participants.
Controls (consisted from responders of the same age, slim with imagining that match normal liver findings, 40 of them.
Patients have been monitored in internists ambulances of the Clinical-Hospital Center of Prishtina, and through Health Centre of Gracanica, with already estimated protocols that included patient's name, generalities and valid anamnestic data. By those protocols anthropometric measurements have been carried out in order to estimate the degree of the nutritional status, mass, and the fatty tissue distribution.
Building up databases and processing was done in SPSS program version 10,0. Also standard descriptive methods were performed, as were analytical methods, t-test for related samples, t-test for independent samples and χ2 test. Correlation between biochemical and anthropometric characteristics tested with Pearson's correlation differences of p<0.05 taken as statistically significant.
3. RESULTS
Basic data on the respondents are listed on table 1. There were no big difference among respondents considering age, the average age of the study group was 56, 26 ± 4, 2 years and 56, 40 ± 4, 22 years in control.
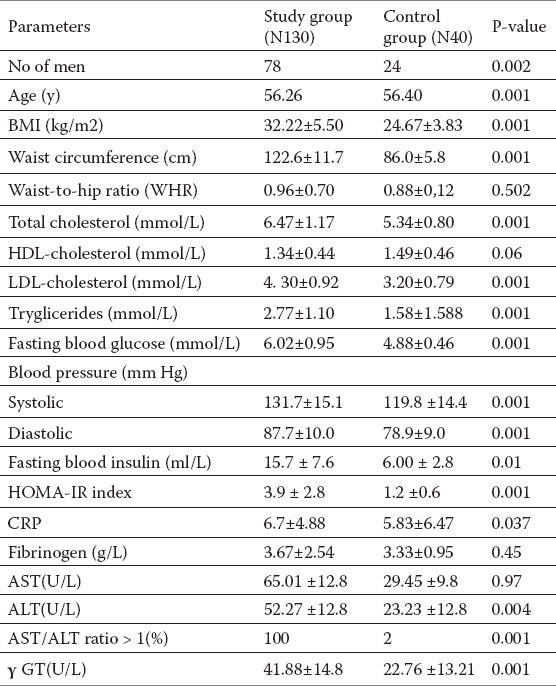
Table 1.
The values of assessed antropometric, clinical and laboratory data (expressed as X±SD) of patients with NAFLD and control group
In group with nonalcoholic fatty liver there were 78% of man or 60%, and 52 women or 44%, while controls consisted of 24 men or 60% and 16 women or 40%. Between study group and control group no statistical important difference between gender representation could be noted.
Table 1. shows the anthropometric characteristics of the subjects with NAFLD and control group also clinical and biochemical characteristics of the tested groups. Respondents of the study group were more obese at the level of significance compared to the controls, p < 0.01 (BMI (kg/m2)) (32.22±5.50 vs 24.67±3.83), waist line circumductio also showed statistically significant higher values in the group with NAFLD (p < 0.01). Waist/hip ratio was higher in the group with NAFLD, but there were no statistical significance among the groups (p =0.502).
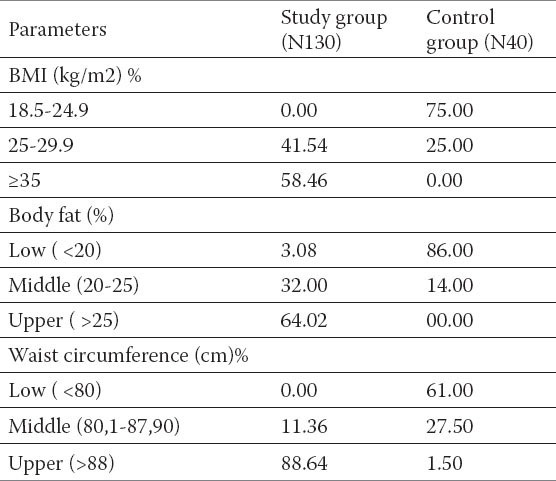
Comparing biochemical parameters between groups significant statistical importance could be established among the glucose and insulin values, total cholesterol values and glutamyltransferase (γ-GT) (p<0, 01) values. No statistically significant difference between AST i ALT was noted. Anthropometric characteristics, and their frequency among the tested groups are shown on the Table 2. and 58.46 % tested subjects of the group with NAFLD had BMI ≥35, what according to the WHO guidelines fall in obesity of second degree. Waist circumference > 88 cm had 88.64 % of the responders. These was followed by increased fat deposition, fat body value were detected in 32 % responders, beetwen 20 and 25, while values >25, had 64.02 % of them. Responders with NAFLD had a significantly higher incidence of disorders compared with those in controls.
Table 2.
Antropometric caracteristics, body fat and their frequency in tested groups.
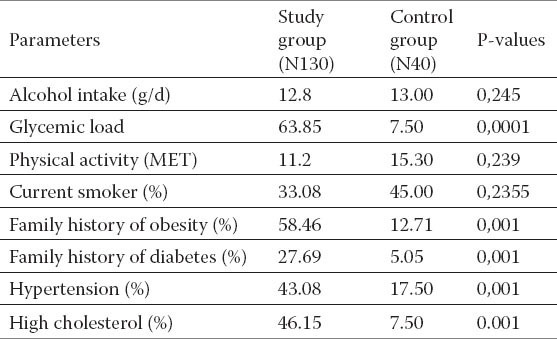
Comparing participants to the input of alcohol abuse and the factor of risk for the cardiovascular disease presence, we came out with results pointing on study group, which had significantly higher incidence of hypertension, diabetes, and hypercholesteremia (p<0.001), comparing with controls, also significantly higher incidence for obesity and diabetes was noted among close relatives (Table 3).
Table 3.
Comparasion of the participants to the input of alchocol abuse and the factor of risk for the cardiovascular deseases presence.
Fasting glucose and fasting insulin values, insulin resistency (HOMA-IR) were significantly higher in respondents with NAFLD, also significantly positive correlation with BMI and waist circumference (Tables 4 and 5) established. Correlation between WHR relationship and insulin resistance was not confirmed (r =-0.08782; p=0.3167).
Table 4.
Corelation between BMI, glucosis values, triglicerides, total cholesterol values insulinemia and HOMA-IR index. Pearson`s correlation coeffitient p-level of the correlation significance
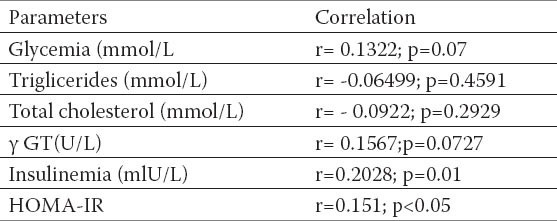
Table 5.
Corelation between waist circumference, glucosis values, triglicerides, total cholesterol values, insulinemia and HOMA-IR index. Pearson`s correlation coeffitient p-level of the correlation significance
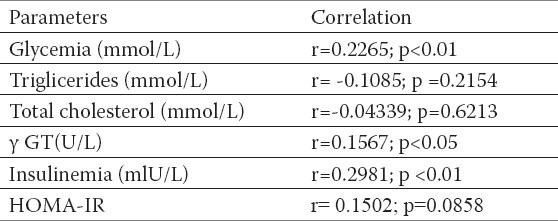
Table 6.
Corelation between WHR, glucosis values, triglicerides, total cholesterol values, insulinemia and HOMA-IR index. Pearson`s correlation coeffitient p-level of the correlation significance
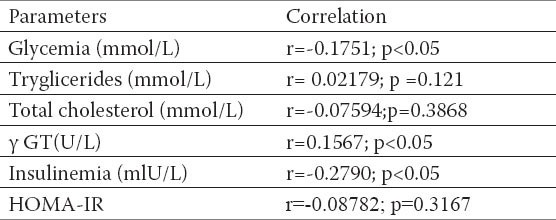
4. DISCUSSION
There is all the more evidence that NAFLD presents hepatic component of the “metabolic syndrome” defined with obesity, hypercholesteremia, peripheral insulin resistance, diabetes mellitus, hypertriglyceridemia and hypertension (5,6,26,27). There are not that many studies that dwelt with anthropometric measurements in subjects with non. alcoholic fatty liver so assessed data are conflicting. Obesity is well documented factor of risk for NAFLD. Excessive obesity defined as BMI >25 and visceral obesity, waist circumference of ≥88 cm in women and ≥102 cm in men have been recognized as factors of risk for NAFLD (21). Our results confirmed that responders in study group were more obese (BMI 32.22±5.50kg/m2 vs 24.67±3.83± 3, 51 kg/m2) than responders in controls which were. statistically highly significant ((p <0, 001). In group of steatosis also were assessed statistically higher values for waist circumference 122.6±11.7 vs 86.0±5.88 cm (p<0, 001). Comparing data from available literature, studies from the region confirm facts that subjects with liver steatosis are significantly more obese than respondents than responders without steatosis (26, 27). The impact of the excessive body mass on liver disorders appearance confirmed studies that based diagnosis on liver biopsy. Ratziu V and associates confirmed in their studies, that obesity is the factor of risk in development of severe fibrosis (28). Cheung O and a group of authors based on collected data, that the degree of abdominal obesity correlates with necroinflamatory liver activity (29). Uslusoy HS and associates on the contrary did not establish statistically significant correlation between body mass index, waist circumference and severe necroinflamatory inflammation, respectively fibrosis (30).
Obesity is an independent risk factor for DMT2 development. Risk of developing the disease increases exponentially when body mass index crosses 25 kg/m (1, 2, 3). Our data indicate significant correlation between cardiovascular risk factors and risks for the development of diabetes with almost all anthropometric experimental procedures. Fasting glucose and fasting insulin values, insulin resistency (HOMA-IR) were significantly higher in responders with NAFLD, as was significantly positive correlation with BMI, waist circumference with other risk factors for the cardiovascular diseases development. The correlation between WHR relations and insulin resistency (r=-0.08782; p=0.3167) have not been confirmed. HOMA index is a simple method used to assess the function of pancreatic beta-cells, and the degree of metabolic insulin resistance from the basal (fasting) glucose values and insulin concentration. HOMA index was introduced in 1985 (9). Nowadays is widely recognized and useful assessment tool in clinical and epidemiological studies, revealing very valuable information on IR and beta–cell function (24). Our study data confirmed the existence of IR in subjects with NAFLD. The study group had a significantly higher values of fasting insulin 15.7 ± 7.6 mU/L comparing to controls were the average values of this parameter were 6.00 ± 2.8mU/L (p <0, 01). Beta-cell dysfunction was assessed as HOMA-IR index > 3, established in the group with NAFLD, with average 3.9 ± 2.8 vs 1.2 ±0.6 values, which was statistically significant. Results very similar to ours obtained Ana Lúcia Farias de Azevedo Salgado (32). In the preceding study biochemical analysis of the 116 patients with NAFLD biopsy verified diagnosis, were compared with the results of the healthy subjects. NAFLD patients had higher levels of insulin, glycemia and HOMA-IR values, compared to controls, even when they did not have diabetes or glucose intolerance.
In addition to related disorders of glucose metabolism and insulin resistance, we found a large frequency of the hypertension in patients with NAFLD. Lopez-Suarez and ass (33) presented in their observation study, that percentage of the participants with hypertension were 21, 2% higher comparing to those without NAFLD (95% CI, 11.8–30.6, P < 0.0005). Numerous studies of other authors do not justify the importance of elevated values of the liver enzymes and their impact for the diagnosis of nonalcoholic fatty liver, one of those is study conducted by Noguchi and ass (34), while several cross sectional studies found firm connection with elevated ALT, γ-GT values, and metabolic syndrome, diabetes and NAFLD (35, 36, 37, 38, 39). Investigating the importance of the liver enzymes disorders Goessling and assoc. state that in general population high aminotransferase (a surrogate marker for NAFLD) increase long term risk for diabetes, metabolic syndrome and cardiovascular incidents (36). Degree of enzyme is not determined, it is usually between 1 and 4 times over upper limit. Although amino- transferase (ALT) values are higher than aspartate aminotransferase (AST) levels, in most cases (37) the AST levels temporarily could be higher than levels of AST, especially in cirrhosis (38). All the same, the fact is that AST / ALT ratio never exceeds 2.
Kotronen and associates in their research pointed out that AST and ALT values were higher in patients with DM type 2, from 40 up to 200 % comparing to those without diabetes and fatty liver (39). Also studies with conducted by Duvnjak et al. Most of the patients had ALT levels higher than AST, although with the development of fibrosis, AST could be found significantly increased (40). One of the requirements for inclusion in this study was disrupted AST/ALT ratio. Data from our study implicate higher γ-GT values, statistically high significant in patients with NAFLD. New American guidelines, unfortunately does not support non invasive biomarkers for identification and prediction NAFLD (1) despite great interest and ongoing numerous studies.
Finally, by analyzing the above mentioned facts, it can be observed that despite the presence of disturbed anthropometric parameters, responders with non alcoholic fatty liver have also disrupted specific biochemical findings. Presented data confirmed significant deviation from the normal values in levels of glucose, liver enzymes, γ GT, insulin but lipids too. Patients with NAFLD have disrupted lipid status, concluded by several studies (41, 42, 43) as for our results they are in accordance to the cited studies. Accumulation of triglyceride in hepatocytes is considered the main pathogenic trigger in the development of NAFLD (44). Cholesterol metabolism in patients with NAFLD is still insufficiently explored, but it is well known fact that insulin resistance is associated with increased cholesterol synthesis (45). In these patients in order to maintain lipid homeostasis, synthesis of VLDL (very-low density lipoproteins) increases, increased levels of VLDL leads to rise of LDL cholesterol levels (46). The results of our study go along with the last statement in the line, raised levels of the cholesterol of LDL cholesterol was established in patients with NAFLD (47). In order to assess the size of the total fat mass, we used reference values by Bray, so referent values FAT% for men were 12-20%, and for women were 20-30%. This method confirmed that participants with nonalcoholic fatty liver have significantly greater amount of the fat mass, than the controls.
5. CONCLUSION
Our results revile that patients with nonalcoholic fatty liver excessively obese, have enlarged waist circumference, consequential insulin resistance, and impaired glucose metabolism, impaired lipid profile, and thus the risk of developing cardiovascular diseases.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012 Jun;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.World Gastroenterology Organisation Global Guidelines, Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis, June. 2012 doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 3.Boza C, Riquelme A, Ibanez L, Duarte I, Norero E, Viviani P, Soza A, Fernandez JI, Raddatz A, Guzman S, Arrese M. Predictors of non-alcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15:1148–1153. doi: 10.1381/0960892055002347. [DOI] [PubMed] [Google Scholar]
- 4.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 6.Neуschwander-Tetri BA, Caldwell SH. Nonalcoholic Steatohepatitis: Sуmmary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 7.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 8.Paschos P, Paletas K. Non alcoholic fatty liver and metabolic syndrome. Hippokratia. 2009;13(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- 9.Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 10.Hastreiter Lj, Micić D. Lečenje metaboličkog sindroma. Srp Arh Celok Lek. 2006;134(11-12):550–558. [PubMed] [Google Scholar]
- 11.Abbasi F, Brown BW, Lamendola C, et al. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 12.Srdic B, Stokic E, Polzovic A, Babovic S. Abdominalno masno tkivo: znacaj i metode njegove detekcije. Med Pregl. 2005;58(5-6):258–264. doi: 10.2298/mpns0506258s. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Z, Wang Z, heshka S, et al. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey. Clinical action thresholds Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 14.Younossi Y. Current management of non-alcoholic fatty liver disease and non-alcoholic steatohepatits. Aliment Pharmacol & Ther. 2008;28:2–12. doi: 10.1111/j.1365-2036.2008.03710.x. [DOI] [PubMed] [Google Scholar]
- 15.James OFW. Textbook of Gastroenterology (Yamada) 2nd ed. Lippnicott Williams & Wilkins; 2003. Nonalcoholic fatty liver disease. [Google Scholar]
- 16.Ewing J.A. Detecting Alcoholism: The CAGE questionnaire. Journal of the American Medical Association. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Physical status: the use and interpretation of anthropometry. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 18.World Health Organisation. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. WHO Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 19.Rezende F, Rosado L, Franceschinni S, Rosado G, Ribeiro R, et al. Critical revision of the available methods for evaluate the body composition in population-based and clinical studies. Arch Latinoam Nutr. 2007;57:327–334. [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Medicine. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Geneva: WHO; 1999. World Health Organisation. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and clasiffication of diabetes mellitus. [Google Scholar]
- 22.Bray GA. Newton, PA: Handbooks in Health Care Co; 1998. Contemporary diagnosis and measurement of obesity; pp. 5–104. [Google Scholar]
- 23.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2010 Jan;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Helsinki Declaration - editions 1964-2000. [Accessed on 15.01.2012]. Last version at- http://www.wma.net/en/30publications/10policies/b3 .
- 26.Novaković T, Inić-Kostić B, Milinić S, Jovićević Lj, Dželetović G. Cardiovascular disease risk factors in patients with non-alcoholic fatty liver disease. Medicinski pregled. 2013;66(1-2):24–31. doi: 10.2298/mpns1302024n. [DOI] [PubMed] [Google Scholar]
- 27.Marović D. Ultrazvučni nalazi jetre radnika u tekstilnoj industriji za postavljanje dijagnoze oboljenja nealkoholne masne jetre. Srpski arhiv za celokupno lekarstvo. 2007;9-10:532–535. [Google Scholar]
- 28.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118(6):1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 29.Cheung O, Kapoor A, Puri P, Sistrun S, Luketic VA, Sargeant CC, et al. The impact of fat distribution on the severity of nonalcoholic fatty liver disease and metabolic syndrome. Hepatology. 2007;46(4):1091–1100. doi: 10.1002/hep.21803. [DOI] [PubMed] [Google Scholar]
- 30.Uslusoy HS, Nak SG, Gülten M, Biyikli Z. Non-alcoholic steatohepatitis with normal aminotransferase values. World J Gastroenterol. 2009;15(15):1863–1868. doi: 10.3748/wjg.15.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan JM, Stampfer MJ, Ribb EB, et al. Obesity, fat distribution and weight gain as risk factors for clinical diabetes in man. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 32.Lúcia Farias de Azevedo Salgado A, de Carvalho L, Claudia Oliveira A, Nascimento dos Santos V, Gilberto Vieira J, Roberto Parise E. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47:165–169. doi: 10.1590/s0004-28032010000200009. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Suarez A, Guerrero J.M, Elvira-Gonzalez J, Beltran-Robles M, Canas-Hormigo F, Bascunana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. European Journal of Gastroenterology and Hepatology. 2011;23(11):1011–1017. doi: 10.1097/MEG.0b013e32834b8d52. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi H, Tazawa Y, Nishinomiya F, Takada G. The relationship between serum transaminase activities and fatty liver in children with simple obesity. Acta Paediatr Jpn. 1995;37:621–625. doi: 10.1111/j.1442-200x.1995.tb03389.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen ZW, Chen LY, Dai HL, Chen JH, Fang LZ. Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease. Journal of Zhejiang University Science B. 2008;9:616–622. doi: 10.1631/jzus.B0720016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goessling W, Massaro JM, Vasan RS, D’Agostino RB, Sr, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135:1935–1944. doi: 10.1053/j.gastro.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonsuz A, Basaranoglu M, Ozbay G. Relationship between aminotransferase levels and histopathological findings in patients with nonalcoholic steatohepatitis (letter) Am J Gastroenterol. 2000;95:1370–1371. doi: 10.1111/j.1572-0241.2000.02046.x. [DOI] [PubMed] [Google Scholar]
- 38.Bacon BR, Farahvash MJ, Janney CG, Neuschwander Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 39.Kotronen A, Yki-Järvinen H, Männistö S, Saarikoski L, Korpi-Hyövälti E, Oksa H, Saltevo J, et al. Non-alcoholic and alcoholic fatty liver disease—two diseases of affluence associated with the metabolic syndrome and type 2 diabetes: the FIN-D2D survey. BMC Public Health. 2010;10:237. doi: 10.1186/1471-2458-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duvnjak M, Virović L. Nealkoholni steatohepatitis. Acta Medica Croatica. 2003;57:189–199. [PubMed] [Google Scholar]
- 41.von Tacer K, Rozman D. Nonalcoholic Fatty Liver Disease: Focus on Lipoprotein and Lipid Deregulation. Journal of Lipids. 2011:14. doi: 10.1155/2011/783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Archives of Medical Research. 2005;36(3):232–240. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Voshol PJ, Haemmerle G, Ouwens DM, et al. Increased hepatic insulin sensitivity together with decreased hepatic triglyceride stores in hormone-sensitive lipase-deficient mice. Endocrinology. 2003;144:3456–3462. doi: 10.1210/en.2002-0036. [DOI] [PubMed] [Google Scholar]
- 44.Cheol SC, Savage DB, Kulkarni A, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. Journal of Biological Chemistry. 2007;282(31):22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 45.Pihlajamaki J, Gylling H, Miettinen TA, Laakso M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. Journal of Lipid Research. 2004;45(3):507–512. doi: 10.1194/jlr.M300368-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Haidari M, Leung N, Mahbub F, et al. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Journal of Biological Chemistry. 2002;277(35):31646–31655. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]
- 47.Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics. 2008;28(no. 1):13–24. doi: 10.1111/j.1365-2036.2008.03703.x. [DOI] [PubMed] [Google Scholar]


