ABSTRACT
Introduction:
Gastric cancer is the fourth most common cancer and the second leading cause of death from cancer. Only complete resection of all gross disease with negative microscopic margins (R0 resection) provides a long-term survival benefit, and the overall 5-year relative survival rate is approximately 20%. To improve survival and quality of life, new therapeutic approaches have been introduced.
Material and methods:
A total of 277 patients (171 men, 106 women) were included in this analysis. The results from the preoperative EUS and MDCT were compared to the postoperative pathological findings. A radial scanning ultrasonic endoscope was used. In patients with early gastric cancer, especially in cases confined to mucosa, endoscopic resection is performed to avoid unnecessary surgical procedures. To achieve R0 resection for locally-advanced gastric cancer, neoadjuvant treatments have been investigated.
Results and discussion:
Laparoscopic surgery has been shown to improve quality of life for both early and locally advanced gastric cancer. Endoscopic ultrasonography (EUS), which is considered to be the most precise method for locoregional staging, was commonly used for differentiating mucosal lesions from submucosal lesions. By contrast, computed tomography (CT) was used to detect the presence of distant metastasis. The difference in accuracy between the ≤20-mm group and other groups was statistically significant for both EUS and MDCT (P = 0.026 and P = 0.044, respectively).
Conclusion:
However, recent technological advances with the helical and multi-detector scanners have provided better CT performance.
Keywords: gastric cancer, EUS, CT
1. INTRODUCTION
Gastric cancer is the fourth most common cancer and the second leading cause of death from cancer (1, 2). Only complete resection of all gross disease with negative microscopic margins (R0 resection) provides a long-term survival benefit, and the overall 5-year relative survival rate is approximately 20% (1, 3). To improve survival and quality of life, new therapeutic approaches have been introduced. In patients with early gastric cancer, especially in cases confined to mucosa, endoscopic resection is performed to avoid unnecessary surgical procedures (4). To achieve R0 resection for locally-advanced gastric cancer, neoadjuvant treatments have been investigated (5). Laparoscopic surgery has been shown to improve quality of life for both early and locally advanced gastric cancer (6, 7).
Endoscopic ultrasonography (EUS), which is considered to be the most precise method for locoregional staging, (8, 9) was commonly used for differentiating mucosal lesions from submucosal lesions. By contrast, computed tomography (CT) was used to detect the presence of distant metastasis (10). However, recent technological advances with the helical and multi-detector scanners have provided better CT performance (11, 12, 13).
With the introduction of new therapeutic options and the recent improvements in CT, further evaluation of the diagnostic accuracy for individual staging by EUS and multidetector-row computed tomography (MDCT) is needed. The present study was conducted to compare the staging accuracy of EUS with that of MDCT in series of patients and to evaluate their usefulness in association with the clinicopathological factors.
2. PATIENTS AND METHODS
In total, 277 patients with gastric lesions who underwent EUS and CT, hospitalized or outpatient treated at Department of gastroenterology and hepatology, Clinical Centre, University of Sarajevo, from January 2008 to December 2012, were analyzed. The results from the preoperative EUS and MDCT were compared to the postoperative pathological findings. A radial scanning ultrasonic endoscope was used. Our experienced endoscopists carried out planned procedures. The tumor infiltration depth was assessed at the time of the procedure using the standard criteria. Lymph nodes equal to or larger than 8 mm were considered positive for metastasis. When lymph node enlargement was found to be >3 cm from the primary lesion, stage N2 disease was diagnosed. Contrast material-enhanced CT examinations were performed using 16 or 64 detector row scanners. Tumor invasion depth was assessed according to previously reported criteria (15).
When endoscopic resection was indicated from the preoperative imaging, (4, 16) endoscopic mucosal resection or endoscopic submucosal dissection was performed. Patients who were not candidates for endoscopic resection or had residual disease after endoscopic resection underwent either a total or subtotal gastrectomy. The operative specimens were staged by experienced pathologists according to the Japanese Classification of Gastric Cancer (17).
The results from the preoperative EUS and MDCT were compared with the postoperative pathological staging. In cases with mixed pathology, the pathological type that mainly accounted for the lesion was selected. Papillary and tubular adenocarcinomas were considered differentiated gastric cancers, and poorly-differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma were considered undifferentiated carcinomas (17, 18). In the analysis of T-staging accuracy in relation to the clinicopathological features, χ2-test was used. A P-value of less than 0.05 was considered significant.
3. RESULTS
A total of 277 patients (171 men, 106 women) were included in this analysis.
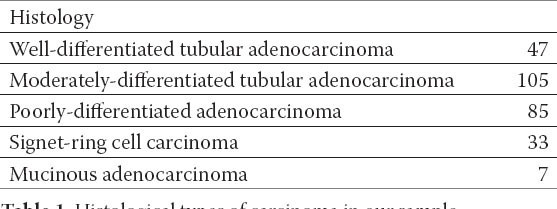
Among the 277 patients included in this analysis, the overall accuracy of EUS for T staging was 74.7%, and the rate of overstaging (13.7%) was higher than that of understaging (11.6%). On MDCT, the primary lesions were visualized in 141 of the 277 patients, which meant an overall detection rate of 50.9%. The sensitivity and specificity were 64% and 92.5% for EUS and 96% and 87.3% for MDCT. Table 1. shows the distribution of histology.
Table 1.
Histological types of carcinoma in our sample
Among the 141 patients with visualized primary lesions on MDCT, T-staging accuracy in relation to the clinicopathological features was analyzed (Table 2). The lesions at the angle revealed the lowest accuracy by EUS (41.7%), followed by lesions at the cardia (53.3%). When compared to other groups, the lesions at the angle showed a statistically significant difference (P = 0.037). For MDCT, the accuracy of the lesions at the cardia was the lowest (33.3%, P = 0.012), followed by lesions at the angle (58.3%). The performance of EUS and MDCT for the combination group of lesions at the cardia and angle had also significantly lower accuracy than the other groups (P = 0.019 and P = 0.031, respectively). With regard to size, the accuracy of both modalities tended to decline as the tumor increased. The difference in accuracy between the ≤20-mm group and other groups was statistically significant for both EUS and MDCT (P = 0.026 and P = 0.044, respectively).
Table 2.
Accuracy of EUS and MDCT for T staging and clinicopathological features.
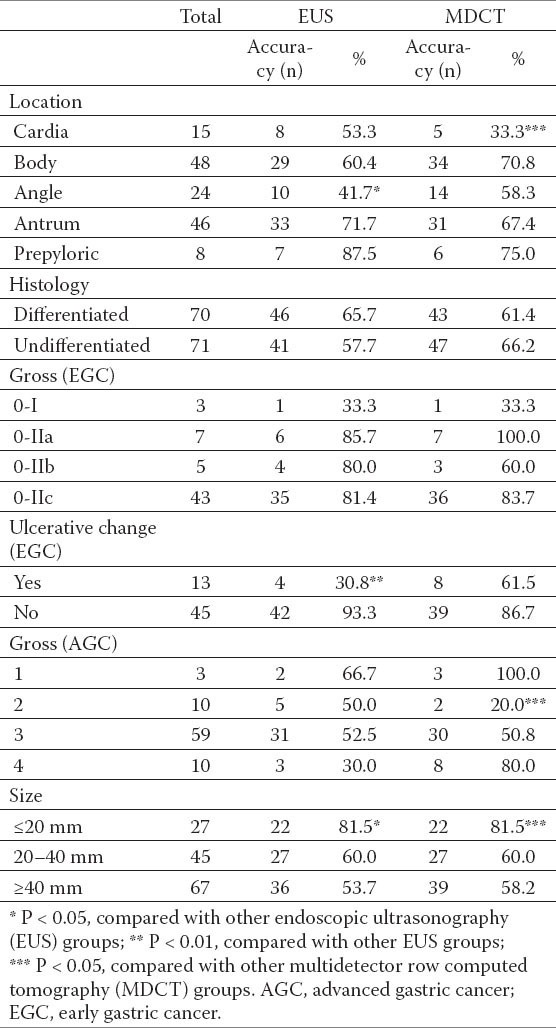
For early gastric lesions with ulcerative changes, EUS demonstrated a significantly lower accuracy rate when compared to lesions without ulcerative changes (P=0.00001). In contrast, the accuracy of MDCT for lesions with and without ulcerative changes was not significantly different.
4. DISCUSSION
Since 1990, EUS has been accepted as the most reliable imaging method for T staging (10, 11, 12). It was reported to have very high T-staging accuracy. There have been reported few studies directly comparing the accuracy of EUS and conventional CT, EUS has been considered more accurate than CT (19, 20). Two reports comparing single-/two-detector helical CT and EUS demonstrated the increased accuracy of CT, but the accuracy of EUS was still higher than CT (12, 14). Recently, studies using MDCT for T staging of gastric cancer have shown improved accuracy, approaching that of EUS. In the studies performed with the 16 or 64 MDCT alone, the T-staging accuracy has been reported to be up to 89%. Some authors have suggested that the accuracy of MDCT for T staging had almost caught up with that of EUS, and that MDCT might replace EUS for preoperative staging (21, 22).
Considering prior studies, it was suggested in a previous report that the presence of non-visualized primary lesions on MDCT might reflect the presence of early gastric cancer lesions without regional lymph node metastasis, (23) of which the results are consistent with the present study. Therefore, to correct the underestimation for the appropriate comparison of EUS with MDCT, we analyzed the performance for T staging. The results are consistent with recent studies on the accuracy of MDCT, which show it to be very close to that of EUS (11, 13, 15). The rate of early gastric lesions (41%) in the visualized lesion group was also adequate when compared to those reported in previous studies, from 46% to 53% (24, 25).
For the analysis of N staging, the criterion of 8 mm, used in previous studies, (12, 14) was chosen for both EUS and MDCT. For evaluating the depth of gastric invasion, the presence of ulcerative change, size, location, and histology have been established as important factors that influence the staging accuracy of EUS (26, 27, 28, 29, 30, 31).
In the present study, the performance of both modalities was analyzed in relation to the clinicopathological factors (Table 2). With regard to location and size, both scanning modalities showed a similar tendency. For early gastric lesions with ulcerative changes, the accuracy of EUS was significantly low; this finding is consistent with previous studies (30, 31). In cases with a diffuse infiltrative morphology (Bormann type 4), the performance of EUS was also low, although the result was not statistically significant. Seven of 10 diffused infiltrative lesions were very large, over 100 mm, and the low accuracy can be explained by the difficulty of a thorough examination of very large lesions with EUS. When a lesion with ulceration or a large, diffused infiltrative lesion is suspected upon preoperative evaluation, MDCT might be more accurate than EUS. However, this requires further study in a larger sample for confirmation. Among the 10 lesions classified as Bormann type 2, only two lesions were correctly staged by MDCT. For differentiating mucosal lesions from submucosal lesions, EUS is the first-line imaging modality; this is because it shows more detail of the five-layer structure of the gastric wall than CT. However, in determining the individual T and N stage, the present study showed that the accuracy of MDCT was very close to that of EUS. When a large lesion or a lesion at the angle or cardia is examined by both modalities, cautious interpretation is necessary for T staging. Early gastric lesions with ulceration should be also meticulously interpreted by EUS.
5. CONCLUSION
Both EUS and MDCT are useful, complementary modalities for the preoperative evaluation of gastric cancer.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Martin RC, 2nd, Jaques DP, Brennan MF, Karpeh M. Achieving RO resection for locally advanced gastric cancer: is it worth the risk of multiorgan resection? J Am Coll Surg. 2002;194:568–577. doi: 10.1016/s1072-7515(02)01116-x. [DOI] [PubMed] [Google Scholar]
- 4.Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561–569. doi: 10.1097/01.mog.0000239873.06243.00. [DOI] [PubMed] [Google Scholar]
- 5.Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 6.Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A. Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer. 1999;2:230–234. doi: 10.1007/s101200050069. [DOI] [PubMed] [Google Scholar]
- 7.Lehnert T, Rudek B, Kienle P, Buhl K, Herfarth C. Impact of diagnostic laparoscopy on the management of gastric cancer: prospective study of 120 consecutive patients with primary gastric adenocarcinoma. Br J Surg. 2002;89:471–475. doi: 10.1046/j.0007-1323.2002.02067.x. [DOI] [PubMed] [Google Scholar]
- 8.Willis S, Truong S, Gribnitz S, Fass J, Schumpelick V. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surg Endosc. 2000;14:951–954. doi: 10.1007/s004640010040. [DOI] [PubMed] [Google Scholar]
- 9.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107–2116. doi: 10.1200/JCO.2006.09.5224. [DOI] [PubMed] [Google Scholar]
- 10.Botet JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:426–432. doi: 10.1148/radiology.181.2.1924784. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari S, Shim CS, Kim JH, et al. Usefulness of three-dimensional, multi-detector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest. Endosc. 2004;59:619–626. doi: 10.1016/s0016-5107(04)00169-5. [DOI] [PubMed] [Google Scholar]
- 12.Habermann CR, Weiss F, Riecken R, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230:465–471. doi: 10.1148/radiol.2302020828. [DOI] [PubMed] [Google Scholar]
- 13.Yang DM, Kim HC, Jin W, et al. 64 multidetector-row computed tomography for preoperative evaluation of gastric cancer: histological correlation. J Comput Assist Tomogr. 2007;31:98–103. doi: 10.1097/01.rct.0000234072.16209.ab. [DOI] [PubMed] [Google Scholar]
- 14.Polkowski M, Palucki J, Wronska E, Szawlowski A, Nasierowska-Guttmejer A, Butruk E. Endosonography versus helical computed tomography for locoregional staging of gastric cancer. Endoscopy. 2004;36:617–623. doi: 10.1055/s-2004-814522. [DOI] [PubMed] [Google Scholar]
- 15.Chen CY, Hsu JS, Wu DC, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT - correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. [DOI] [PubMed] [Google Scholar]
- 16.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer A. Japanese classification of gastric carcinoma—2nd english edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 18.Adachi Y, Yasuda K, Inomata M, Sato K, Shiraishi N, Kitano S. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer. 2000;89:1418–1424. [PubMed] [Google Scholar]
- 19.Ziegler K, Sanft C, Zimmer T, et al. Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut. 1993;34:604–610. doi: 10.1136/gut.34.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg J, Durkin M, Van Drunen M, Aranha GV. Computed tomography or endoscopic ultrasonography in preoperative staging of gastric and esophageal tumors. Surgery. 1994;116:696–701. [PubMed] [Google Scholar]
- 21.Kumano S, Murakami T, Kim T, et al. T staging of gastric cancer: role of multi-detector row CT. Radiology. 2005;237:961–966. doi: 10.1148/radiol.2373041380. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu K, Ito K, Matsunaga N, Shimizu A, Kawakami Y. Diagnosis of gastric cancer with MDCT using the water-filling method and multiplanar reconstruction: CT-histologic correlation. AJR Am J Roentgenol. 2005;185:1152–1158. doi: 10.2214/AJR.04.0651. [DOI] [PubMed] [Google Scholar]
- 23.Yu JS, Choi SH, Choi WH, Chung JJ, Kim JH, Kim KW. Value of nonvisualized primary lesions of gastric cancer on preoperative MDCT. AJR Am J Roentgenol. 2007;189:W315–319. doi: 10.2214/AJR.07.2672. [DOI] [PubMed] [Google Scholar]
- 24.Park JC, Lee YC, Kim JH, et al. Clinicopathological aspects and prognostic value with respect to age: an analysis of 3,362 consecutive gastric cancer patients. J Surg Oncol. 2009;99:395–401. doi: 10.1002/jso.21281. [DOI] [PubMed] [Google Scholar]
- 25.Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92:1099–1102. doi: 10.1002/bjs.4952. [DOI] [PubMed] [Google Scholar]
- 26.Bentrem D, Gerdes H, Tang L, Brennan M, Coit D. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol. 2007;14:1853–1859. doi: 10.1245/s10434-006-9037-5. [DOI] [PubMed] [Google Scholar]
- 27.Meining A, Dittler HJ, Wolf A, et al. You get what you expect?A critical appraisal of imaging methodology in endosonographic cancer staging. Gut. 2002;50:599–603. doi: 10.1136/gut.50.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim AY, Kim HJ, Ha HK. Gastric cancer by multi-detector row CT: preoperative staging. Abdom Imaging. 2005;30:465–472. doi: 10.1007/s00261-004-0273-5. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Song KS, Youn YH, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66:901–908. doi: 10.1016/j.gie.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Hizawa K, Iwai K, Esaki M, Matsumoto T, Suekane H, Iida M. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973–978. doi: 10.1055/s-2002-35851. [DOI] [PubMed] [Google Scholar]
- 31.Akashi K, Yanai H, Nishikawa J, et al. Ulcerous change decreases the accuracy of endoscopic ultrasonography diagnosis for the invasive depth of early gastric cancer. Int J Gastrointest Cancer. 2006;37:133–138. doi: 10.1007/s12029-007-9004-9. [DOI] [PubMed] [Google Scholar]


