ABSTRACT
Introduction:
Lung cancer is most common cause of cancer-related mortality worldwide. Non-small-cell lung carcinoma (NSCLC) is disease with very low 5-year relative survival rate. For patients with non-small cell lung cancer, roles of current treatments are to prolong survival time and to improve quality of life.
Aim:
The aim of the work was to compare values of Glasgow Prognostic Score (GPS) before application of the chemotherapy medication with response to chemotherapy and toxic side effects associated with chemotherapy in patients treated with cisplatin-etopozid (PE) and cisplatin-gemcitabin (PG) in stages IIIb and IV of NSCLC. Testing role of Glasgow Prognostic Score as a possible predictor of response to therapy and toxic side effects of chemotherapeutic protocol was another aim of this work.
Patients and methods:
This prospective study included 60 patients in stages IIIb or IV of NSCLC, with ECOG ≤ 2. The patients were divided in two groups. First group contained 30 patients treated with chemotherapeutic protocol using cisplatin-etopozid (PE), and the same number of patients in the second group were treated with cisplatin-gemcitabin (PG).
Results:
Glasgow Prognostic Score (GPS) evaluation before the chemotherapy inclusion showed values of 1 (43.30:53.30), then 2 (40.00:36.70) and the lowest 0 (16.70:10.00) which supports the pathological values of GPS in developed lung cancer, i.e. most patients had pathological GPS value in both protocols (83.30:90.00). Monitoring of toxic side effects and response to chemotherapy was done after each cycle of treatment.
Discussion:
Results of this study revealed importance of GPS in selection of patients for treatment with chemotherapy. Patients with lower values of GPS treated using PE chemotherapeutic protocol had weaker response to therapy.
Conclusion:
Coefficient of correlation for therapy response in both chemotherapeutic protocol, compared with values of GPS before treatment, were not statistically significant, therefore GPS cannot be considered as a predictor of therapeutic on chemotherapy.
Keywords: Lung cancer, chemotherapy, Glasgow prognostic score
1. INTRODUCTION
Lung cancer is most common cause of cancer-related mortality worldwide. With help of modern medical therapies, median survival time in patients with advanced lung carcinoma is between 8 to 10 months. One-year relative survival rate is about 30-40%. (1, 2) The main reason for high mortality rate in patients with lung cancer is delay with diagnosing of disease, resulting in more than 40% of patients diagnosed at advanced stage of disease (IIIB and IV). Non-small-cell lung carcinoma (NSCLC) is disease with very low 5-year relative survival rate. Roles of current treatments in patients with NSCLC are to prolong survival time, to improve disease-related symptoms and quality of life (3). According to NCCN (National Comprehensive Cancer Network) good prognostic factors of survival are including: diagnosis in the early stages of the disease, Eastern Cooperative Oncology Group (ECOG) performance status 0, 1, or eventually 2, non-significant weight loss (lower than 5%), female sex (4). Parameters of Glasgow prognostic score (GPS) are including monitoring serum levels of albumin and C-reactive protein (CRP). GPS is useful as predictor of survival in patients with advanced NSCLC (5, 6).
2. AIMS
To compare values of GPS before application of the chemotherapy medication with response to chemotherapy and toxic side effects associated with chemotherapy in patients treated with cisplatin-etopozid (PE) and cisplatin-gemcitabin (PG) in stages IIIb and IV of NSCLC.To define role of GPS as a possible predictor of response to therapy and toxic side effects of chemotherapeutic protocol.
3. PATIENTS AND METHODS
This prospective study included 60 patients in stages IIIb or IV of NSCLC, with ECOG ≤ 2. The patients were divided in two groups. First group contained 30 patients treated with chemotherapeutic protocol using cisplatin-etopozid (PE), and the same number of patients in the second group were treated with cisplatin-gemcitabin (PG). Monitoring of toxical side effects after each cycle of chemotherapy was based on anamnesis, clinical examination, parameters of blood (7) (level of hemoglobin, leukocyte, erythrocyte, neutrophils, and platelet count), parameters of renal function (levels of urea and creatinine) parameters of liver function (levels of alanine aminotransferase–ALT, and aspartate aminotransferase–AST), according to „Common Terminology criteria for adverse events” (8). Response to chemotherapy was defined in concordance with RECIST (Response Evaluation Criteria in Solid Tumors) as: complete response (CR), partial response (PR), progressive disease (PD), stationary disease (SD) (9). Data analysis was performed using SPSS software, version 15 (SPSS, Inc., Chicago, IL).
4. RESULTS
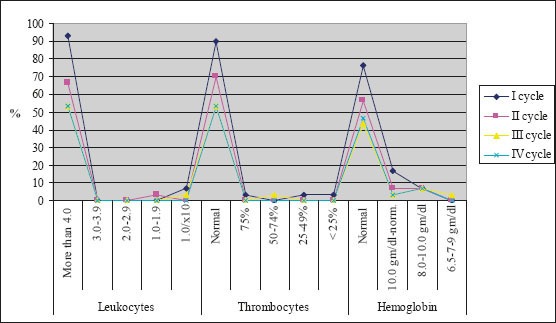
The majority of patients were male (88%), had squamous cell carcinoma (51%) and had stage IIIB disease. The average age of the examinees in PE protocol was 62.13±6.3, while in PG protocol it was 56.67±6.01. Figure 1 presents response rate after administration of chemotherapy for both protocols. After the first cycle of chemotherapy using PE protocol, 8 patients had progression of disease and there was one case of toxic death. Forth cycle of chemotherapy using PE protocol was prescribed in 16 patients, and after administration of chemotherapy 2 patients had progression of disease. Following the first cycle of chemotherapy using PG protocol 2 patients had progression of disease. Twenty-five patients were assigned to receive forth cycle of chemotherapy using PG protocol, and after administration of chemotherapy there were 4 cases with progression of disease. Figure 2 shows the incidence of chemotherapy-induced hematological toxicities in patients treated according to PE protocol. After the first cycle of chemotherapy 6,7% of patients experienced grade IV leukopenia, grade V thrombocytopenia was found in one patient, and five patients experienced grade I anemia. Following forth cycle of chemotherapy normal platelet count was recorded in 16 patients, and normal level of hemoglobin was found in 14 patients. Incidence of chemotherapy-induced hematological toxicities treated according to PG protocol are summarized in Figure 3. With the exception of lower values of hemoglobin found in 5 patients, there was no other hematological toxicities after the first cycle of chemotherapy. After 4 cycles of chemotherapy grade 2 leukopenia occurred in one patient, whereas normal platelet count was recorded in 22 patients, and normal level of hemoglobin was found in 17 patients. Table 1 summarizes values of GPS in patients treated according to both protocols. Value 1 of GPS has the highest incidence in both groups of patients.
Figure 1.
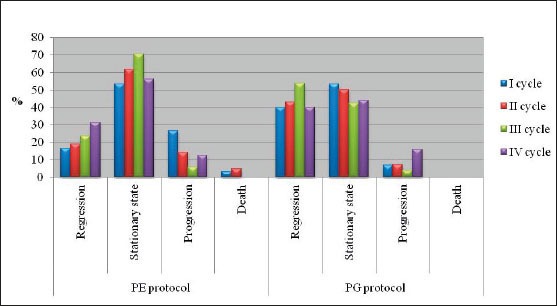
Chemoterapy reponse rate for both protocols
Figure 2.
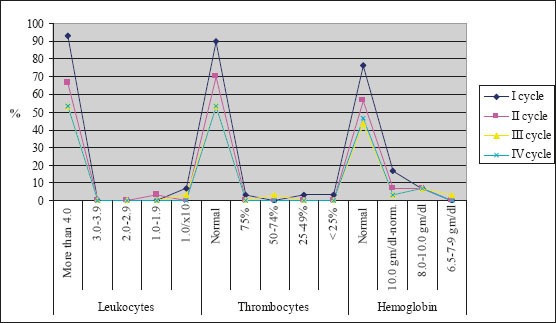
Incidence of hematological toxical effects in patients treated according to PE protocol
Figure 3.
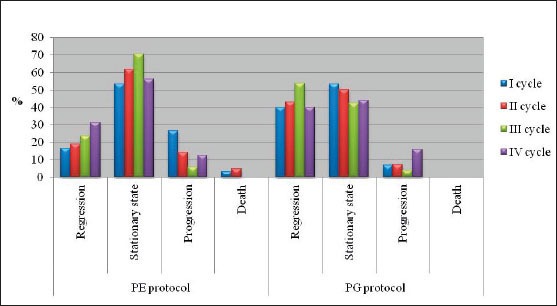
Incidence of hematological toxical effects in patients treated according to PG protocol
Table 1.
Values of GPS in patients treated according to both protocols
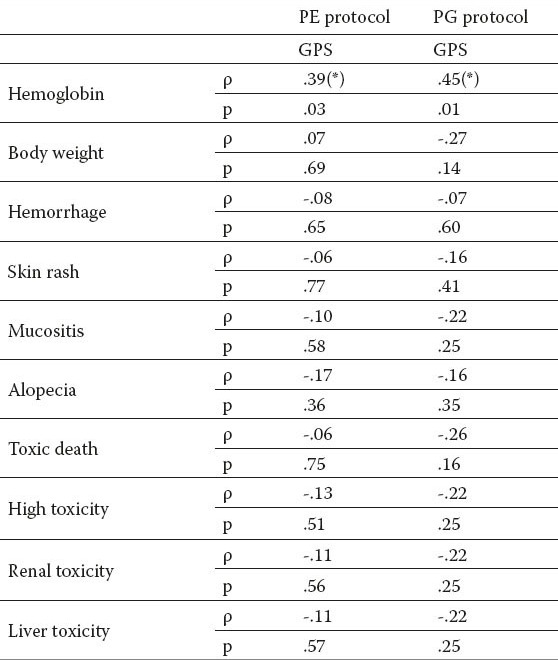
Correlation of toxic effects with GPS in both protocols of chemotherapy are shown in Table 2. There was found a statistically significant correlation between GPS and level of hemoglobin in both group of patients.
Table 2.
Correlation of toxic effects with GPS in both protocols of chemotherapy. *Correlation is significant at the 0.05 level
5. DISCUSSION
Roles of treatment strategies in patients with advanced NSCLC are to prolong survival time, to improve disease-related symptoms and quality of life. Effectiveness of the chemotherapy in the treatment of NSCLC has been a subject of full discussion of the benefits, due to limitations and toxicities of the treatment. To deliver the most suitable treatment, predictive and prognostic are included in pretreatment period. Cumulative prognostic score based on levels of C-reactive protein and albumin known as the Glasgow Prognostic Score (GPS), has been recognized as prognostic factor, independent from stage or primary localisation of cancer. Hypoalbuminemia is associated with more frequent chemotherapy-induced toxicity, because most of cytostatic drugs are bound to albumin in the bloodstream Due to lower levels of albumin, there are higher concentrations of cytostatic drug in the bloodstream and consequently there are higher possibilities for toxic effects.
CRP is used mainly as a marker of inflammation, but additionally is useful in determining disease progress or the effectiveness of treatments. CRP is a member of the class of acute-phase reactants, as its levels rise dramatically during inflammatory processes occurring in the body, therefore is important indicator of inflammation, tissue necrosis or trauma. Presence of systemic inflammatory response, as evidenced by elevated circulating concentrations of CRP, is connected with poor survival, independent of stage of tumor (10). Table 1 summarizes values of GPS in patients treated according to both chemotherapy protocols. Most of patients had pretreatment GPS score values of 1 (43.30:53.30), then 2 (40.00:36.70) and the lowest 0 (16.70:10.00) which supports the pathological values of GPS in developed lung cancer, i.e. most patients had pathological GPS value in both protocols (83.30: 0.00). This was confirmed in a study by Leung et al (11). Published data showed importance of pretreatment GPS values as a predictor of survival of patients with advanced NSCLC. Espinosa et al. (12) reported that serum levels of albumin are in positive correlation with better response on chemotherapy in patients with advanced NSCLC treated with cisplatin. In group of patients with inoperable NSCLC GPS values showed same prognostic role as performance status (6). Furthermore, in a study by Gupta et al. (13) measuring serum albumin levels in pretreatment period has been recognized as valuable prognostic importance in cancer patients. Therefore, level of serum albumin can be used in clinical trials, with a view to better define basic risk in cancer patients. In previous studies done by Wilop et al (14), and Proctor et al (15) had been recognized that measuring serum CRP levels offers additional prognostic information in patients with advanced NSCLC. The results of the present study suggest that the GPS can be used in selection of patients for chemotherapy and type of chemotherapy regimen that will be used. Patients with lower values of GPS treated according to PE protocol had weaker response to chemotherapy. In addition, we found more cases of chemotherapy-induced leukopenia and thrombocytopenia in patients treated according to PE protocol. Our results are consistent with the results of studies done by Srimuninnimint et al (16) and by Gioulbasanis et al (17). Previous studies have demonstrated that higher values of CRP before treatment and persisting high values during the treatment, are bad prognostic signs, and that this measured values can be used in assessment of toxicity caused by platinum-based drugs.
6. CONCLUSIONS
Patients with lower values of GPS (values 1 and 2) treated using PE chemotherapeutic protocol had weaker response to therapy, higher incidence of hematological and non-hematological toxicities. Coefficient of correlation for therapy response in both chemotherapeutic protocol, compared with values of GPS before treatment, were not statistically significant, therefore GPS cannot be considered as a predictor of therapeutic response on chemotherapy.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Jemal A, Siegel R, Ward E. Cancer statistics 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Stinnett S, Williams L, Johnson DH. Role of chemotherapy for palliation in the lung cancer patient. J Support Oncol. 2007 Jan;5(1):19–24. [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J. Global cancer statistics 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an Eastern Cooperative Oncology Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1986;4(5):702–729. doi: 10.1200/JCO.1986.4.5.702. [DOI] [PubMed] [Google Scholar]
- 5.Leung Elaine YL, ChB MB, Scott Hazel R, McMillan DC. Clinical Utility of the Pretreatment Glasgow Prognostic Score in Patients with Advanced Inoperable Non-small Cell Lung Cancer. Prognostic/Predictive Factors. Journal of Thoracic Oncology. 2012;7(4):655–662. doi: 10.1097/JTO.0b013e318244ffe1. [DOI] [PubMed] [Google Scholar]
- 6.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia. Risks, consequences and new directions for its management. Cancer. 2004;100:228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 8.Common Terminology criteria for adverse events ver.4. 2009. Available from; http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm .
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) European Journal of Cancer. 2009;4(5):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Cur Oncol Rep. 2002;4:250–255. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 12.Espinosa E, Feliu J, Zamora P, González Barón M, Sánchez JJ, Ordón ez A, Espinosa J. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 1995;12(1-2):67–76. doi: 10.1016/0169-5002(95)00407-r. [DOI] [PubMed] [Google Scholar]
- 13.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;22(9):69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilop S, Crysandt M, Bendel M, Mahnken AH, Osieka R, Jost E. Correlation of C-reactive protein with survival and radiographic response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Onkologie. 2008;31(12):665–670. doi: 10.1159/000165054. [DOI] [PubMed] [Google Scholar]
- 15.Proctor MJ, Morrison DS, Talwar D, Balmer SM, O’Reilly DSJ, Foulis AK, Horgan PG, McMillan DC. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104(4):726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srimuninnimit V, Ariyapanya S, Nimmannit A, Wonglaksanapimon S, Akewanlop C, Soparattanapaisarn N. C-reactive protein as a monitor of chemotherapy response in advanced non-small cell lung cancer (CML study) J Med Assoc Thai. 2012;95(Suppl 2):S199–207. [PubMed] [Google Scholar]
- 17.Gioulbasanis I, Pallis A, Vlachostergios PJ, Xyrafas A, Giannousi Z, Perdikouri IE, Makridou M, Kakalou D, Georgoulias V. The Glasgow Prognostic Score (GPS) predicts toxicity and efficacy in platinum-based treated patients with metastatic lung cancer. Lung Cancer. 2012;77(2):383–388. doi: 10.1016/j.lungcan.2012.04.008. [DOI] [PubMed] [Google Scholar]


