ABSTRACT
Introduction:
To investigate the efficacy of platinum-based chemotherapy in patients with recurrent high-grade glioma (HGG) who had received previous alkylating line of chemotherapy.
Material and methods:
Case notes of patients who had received chemotherapy with carboplatin or cysplatin for recurrent HGG between June 2006 and July 2012 were reviewed. Baseline characteristics and outcomes after treatment were recorded.
Results:
Forty-eight patients received carboplatin/cysplatin as second line chemotherapy for recurrent HGG (grade III n=6; grade IV n=42). The median number of cycles completed was 4. Fifteen patients (28%) had at least minor response, 22 (49%) had stable disease and 11 (23%) had progressive disease. Six month progression-free survival was 30% (52% in patients with grade III glioma and 18% in patients with grade IV glioma). The median time to disease progression from the first treatment with platinum drug was 3.2 months. The median survival was 8 months (10 months for patients with grade III glioma and 7 months for patients with grade IV glioma). Among patients with either stable disease or a partial response, the median survival was 12 months compared with 3 months in patients with progressive disease. No survival or response rate differences were noted regarding the type of previous chemotherapy, nitrosoureas or temozolomide.
Conclusions:
Single-agent carboplatin/cysplatin has modest activity in patients with recurrent HGG previously treated with one line of chemotherapy, nitrosoureas or temozolomide. Despite the improvement of median survival of patients achieving stable disease or a partial response to treatment, more effective regimens are required for this patient population.
Keywords: Key-words: Recurrent high-grade glioma, carboplatin, survival
1. INTRODUCTION
The incidence of high-grade gliomas (HGG) is increasing, especially in the elderly population. Even with aggressive surgical resection, radiation therapy, and chemotherapy, the median survival of patients with glioblastoma (GBM) is less than 15 months (1). Despite the curative intent of these treatments, the vast majority of patients will relapse and a proportion of these patients will remain of suitable performance status to receive further treatment. The prognosis for patients with HGG is very poor, ranging from 4.6 to 58.6 months on the basis of clinico-pathologic prognostic factors. (2). This unfavorable prognosis is mainly due to the high propensity for tumor recurrence. The addition of chemotherapy (especially alkylating agents such as Temozolomide and nitrosoureas) to radiation therapy and surgery seemed clearly improved. For patients who progress after both nitrosorueas and temozolomide, effective thirdline chemotherapeutic options are less well defined. There have been no phase III randomized controlled trials and evidence, at best, is drawn from phase II trials. One possible agent for relapsed HGG is carboplatin (3). Platinum compounds were long considered active agents for the treatment of gliomas besides nitrosoureas (4), with reported response rates (RR) of approximately 15% (5). Cisplatin was shown to reduce AGAT activity in vitro (6), suggesting that it could enhance the single-agent activity of TMZ. The aim of this study was to investigate the effectiveness of carboplatin in a series of patients with relapsed HGG.
2. METHODS
2.1. Eligibility
We performed a retrospective review of the neuro-oncology database of our department. Selection’s criteria were histologically proven grade III and IV glioma, age >18 years, Karnofsky Performance status (KPS)>60, normal baseline counts for neutrophils and platelets, transaminases, and creatinine levels, and previous surgery followed standard radiotherapy (60Gy/30fractions) concomitantly and/or followed by nitrosoureas or temozolomide. A radiologically recurrent disease was defined as any new contrast enhancing area after complete resection of all contrast-enhancing tumor areas.
2.2. Treatment regimen
Carboplatin with an area under the curve (AUC) of 5 was given as an intravenous infusion over 1 h every 4 weeks to a maximum of six cycles. In the absence of hematologic toxicity, the carboplatin dose was escalated to AUC of 6 or 7. Cysplatin was given at 70mg/m2 as an intravenous infusion over 1 h. Subsequently, patients with tumor response or stable disease continued chemotherapy until clinical or radiological progression.
2.3. Response evaluation
Patients underwent routine neuroradiological and clinical evaluation for tumor response every 2 or 3 cycles or earlier, when clinical deterioration occurred. Radiological response was assessed according to the Mac Donald criteria (7). We defined minimal response as a 25% to 49% reduction in the largest cross-sectional tumor area. A radiologically proven progressive disease was defined as any increase of contrast enhancing tumor area over 25% or an additional contrast enhancing area. Clinical progression was defined as the occurrence of significant neurological deterioration (e.g. disabling hemiparesis, aphasia, and deterioration of the general condition).
An independent central review of computed tomography and MRI scans was made for patients achieving complete (CR) or partial (PR) response or disease stabilization (SD).
Patients were withdrawn from the study if they had progressive disease, unacceptable toxicity, or retracted their consent. Patients who interrupted treatment before the first radiologic evaluation were considered assessable for toxicity but not for response.
2.4. Study endpoints
Study endpoints included evaluation of progression free and overall survival. Progression free survival was defined as the interval from start of carboplatin treatment to radiological or clinical progression or death, whichever occurred first. Overall survival was defined as the time span from start of Carboplatin/cysplatin until death for any reason.
PFS and OS were calculated using the Kaplan-Meier method censoring observations at the time of last follow-up if the respective event was not observed. Median follow-up was derived from the estimated censoring distribution. Prognostic factors (age, KPS, tumor burden, gross total resection, pretreatment with TMZ or nitrosoureas) were all fitted together in an exploratory fashion into a Cox regression model, both as continuous and dichotomised predictors.
3. RESULTS
3.1. Patients’ characteristics
Forty-eight patients were included into the study. 6 patients had a histologically proven anaplastic astrocytoma and 42 other patients received carboplatin as second line chemotherapy for recurrent HGG (grade III n=6; grade IV n=42). There were 29 (62%) men and 19 (38%) women with a median age of 52,5 years old (range: 25 to 72 years). Median KPS at start of carboplatin/cysplatin was 70 (range: 60-90). The operative diagnostic procedure was gross total resection in 24 patients, a partial resection in 17 and an open biopsy in 7 patients. All patients received post-operative radiotherapy. 11 patients received temozolomide concomitantly and following to irradiation, and the other patients received Temozolomide (20 patients) and PCV or CCNU alone (17pts) in an adjuvant setting.
Toxicity and responses to carboplatin/cysplatin
The median number of cycles completed was 4. More than 45% of patients overpassed 5 cycles. 3 patients developed leucopenia grade 3 and one patient developed an anemia grade IV. No death related to therapy was observed.
In 11 patients a partial response, in 4 patients a minor response and in 22 patients (49%) a stable disease was observed. 11 patients (23%) showed a progressive disease. Despite the fact that our responsive patients had also respond to previous chemotherapy, no differences in response rates according to the previous response status toward nitrosoureas or temozolomide were noted.
3.2. Progression and survival
With a median follow-up of 26 months, six month progression-free survival was 30% (52% in patients with grade III glioma and 18% in patients with grade IV glioma). The median time to disease progression from the first treatment with carboplatin/cysplatin was 3.2 months. The median survival was 8 months (10 months for patients with grade III glioma and 7 months for patients with grade IV glioma). Among patients with either stable disease or a partial response, the median survival was 12 months compared with 3 months in patients with progressive disease.
After disease progression, eleven patients went to receive further chemotherapy using either retreatment with temozolomide (three patients) and bevacizumab (eight patients).
No survival differences were noted regarding the type of previous chemotherapy, nitrosoureas or temozolomide. Considering the time from the operative diagnostic procedure to the start of carboplatin/cysplatin, and then, adding this time interval to the survival from carboplatin/cysplatin, the median overall survival was 18,2 months (20.4 months for grade III and 15.6 months for grade IV gliomas).
4. DISCUSSION
Surgery, radiotherapy and first line chemotherapy with PCV or temozolomide are considered as standard treatment for high grade glioma. The natural history of these tumors has showed that they will recur and limited treatment options (surgical re-intervention, re-irradiation and chemotherapy) are available. The optimal treatment of these recurrent tumors is not well-defined due to lack of uniform criteria for tumor recurrence, heterogeneity of patients (in term of diagnosis and treatments used). Considering systemic approaches, temozolomide and other chemotherapies have been used widely as single agent or combined treatments showing only modest benefit with a 6 months progression free survival rate of 15% for patients with recurrent glioblastoma (8).
Our retrospective study evaluated the efficacy of a single agent, carboplatin or cysplatin, in unselected patients with recurrent grade III and IV glioma pre-treated in front-line with Temozolomide (TMZ) or CCNU alone. Why we used platinum drug? As TMZ and CCNU are counteracted via the same resistance mechanism, we hypothesized that pre-treatment with either TMZ or CCNU could have led to a resistance against alkylating agents reducing the efficacy of CCNU or TMZ.
Table 1.
Trials investigating carboplatin alone in recurrent high-grade glioma
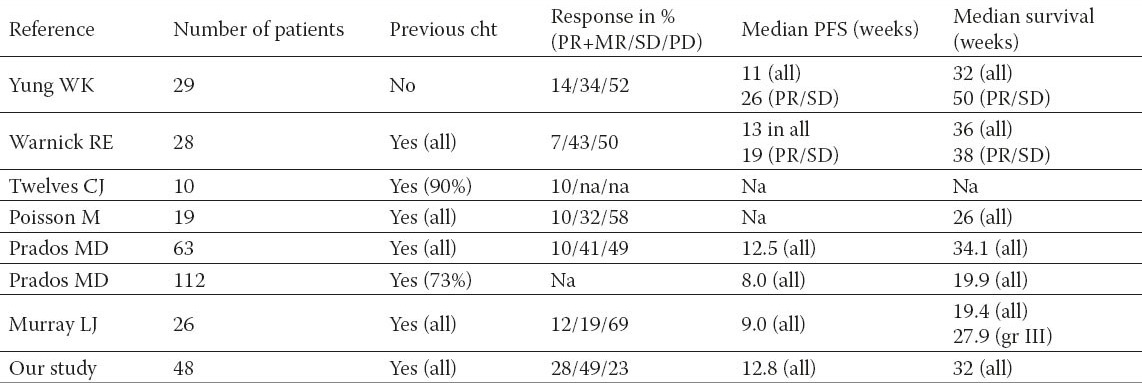
Median response duration was 8 months; six month progression-free survival was 30% (52% in patients with grade III glioma and 18% in patients with grade IV glioma). The median survival was 8 months (10 months for patients with grade III glioma and 7 months for patients with grade IV glioma).
Our results add to the growing body of evidence that carboplatin/cysplatin, alone or in combination with cytotoxic agent had a moderate activity in unselected patients with recurrent malignant glioma.
It has to be considered that all above studies (see Table 2) have very heterogeneous populations with a high variation patients, who had already received various therapeutic modalities and were not only treated for the first but also for a second or fourth relapse of the disease. Thus caution in making comparisons between their results is required. However, carboplatin tents to have a minor response rate (lower than 15%) and in a half of patients no activity. Despite these non enthusiastic response rates, the median time to progression is about 11 weeks (+/- 3 weeks) and median survival ranging from 20 to 36 weeks.
In our cohort of patients, 28 % had at least a minor response to carboplatin/cysplatin which is higher to that reported in above studies; the rate of SD was also higher at 49% and the rate of disease progression lower at 23%. This discrepancy can in part be explained by the problem of pseudoprogression in malignant glioma. This phenomenon describes progressive and enhancing lesions on MRIs, which are not related to the tumor progression, but to treatment effects (16) and has been originally described after concomitant radio-chemotherapy with temozolomide. Probably, it can be assumed that these changes might be in some cases responsible for treatment discontinuation in the above studies and not a true objective tumor progression. However, pseudoprogression after platinum therapy has not been described in the literature to date and therefore we would suggest to perform a radiological follow-up examination in patients with radiological signs of tumor progression to rule out pseudoprogression and a misleadingly termination of therapy.
There has been reported a potential cross-resistance between temozolomide and carboplatin. Since no differences in response rates according to the previous response status toward nitrosoureas or temozolomide were noted, our results did not support this hypothesis.
Interestingly, despite increased rates of partial responses and lower rates of progressive disease, our progression and survival times are consistent with previous trials of carboplatin in recurrent high grade glioma. One possible explanation is that the patients in this cohort have been pre-treated with nitrosoureas or temozolomide with a median time to progression of 10.2 months suggesting that duration time of response to carboplatin depends on the time interval from diagnosis. As would be expected, patients with grade III gliomas had better survival times as opposed to grade IV glioma patients.
In addition to tumor grading, patient age, the extent of surgery and performance status is recognized as prognostic factors. However, none of these variables was found to significantly influence the outcome of our patients.
Taken together, carboplatin/cysplatin does have modest impact in survival of patients with recurrent high-grade glioma.
Currently, in the recurrence setting of high grade glioma, it exists many of phase II trial with heterogenous patients cohort, different treatment modalities (Temozolomide and non-temozolomide containing regimen) and different endpoints. More recently, there has been a growing interest in the potential role of bevacizumab, alone or in combination with irinotecan in patients with high-grade glioma (17). Though all of them may not be compared directly with each other, all reported OS are more or less similar (8). Nevertheless, these values should be regarded as rough estimates and may not be used for a final judgment as they might be due to selection effects.
5. CONCLUSION
Single-agent carboplatin or cysplatin has modest activity in patients with recurrent HGG previously treated with first line of chemotherapy, nitrosoureas or temozolomide. Despite the improvement of median survival of patients achieving stable disease or a partial response to treatment, more effective regimens are required for this patient population. New molecular targeted therapies are of a special interest. Prospective and randomized studies are needed to compare the efficacy of each “old” agents against newer targeted drugs (alone or in combination) to prove their role in the relapse situation.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998;40:51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 3.Huncharek M, Kupelnik B, Bishop D. Platinum analogues in the treatment of recurrent high grade astrocytoma. Cancer Treat Reviews. 1998;24:307e316. doi: 10.1016/s0305-7372(98)90054-8. [DOI] [PubMed] [Google Scholar]
- 4.Huncharek M, Muscat J. Treatment of recurrent high grade astrocytoma; results of a systematic review of 1,415 patients. Anticancer Res. 1998;18:1303–1311. [PubMed] [Google Scholar]
- 5.Yung WKA, Mechtler L, Gleason MJ, et al. Intravenous carboplatin for recurrent malignant glioma: A phase II study. J Clin Oncol. 1991;9:860–864. doi: 10.1200/JCO.1991.9.5.860. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Setlow RB. Inactivation of O-6-alkylguanine-DNA Alkyltransferase in HeLa cells by cisplatin. Carcinogenesis. 1989;10:1681–1684. doi: 10.1093/carcin/10.9.1681. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 8.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 9.Yung WK, Mechtler L, Gleason MJ. Intravenous carboplatin for recurrent malignant glioma: a phase II study. J Clin Oncol. 1991;9:860e864. doi: 10.1200/JCO.1991.9.5.860. [DOI] [PubMed] [Google Scholar]
- 10.Warnick RE, Prados MD, Mack EE. et al. A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neuro-Oncol. 1994;19:69e74. doi: 10.1007/BF01051050. [DOI] [PubMed] [Google Scholar]
- 11.Twelves CJ, Ash CM, Miles DW, et al. Activity and toxicity of carboplatin and iproplatin in relapsed high grade glioma. Cancer Chemother Pharmacol. 1991;27:481e483. doi: 10.1007/BF00685164. [DOI] [PubMed] [Google Scholar]
- 12.Poisson M, Pereon Y, Chiras J, Delattre JY. Treatment of recurrent malignant supra-tentorial glioma with carboplatin. J Neuro-Oncol. 1991;10:139e144. doi: 10.1007/BF00146875. [DOI] [PubMed] [Google Scholar]
- 13.Prados MD, Warnick RE, Ronald E, et al. Intravenous carboplatin for recurrent gliomas: a dose escalating phase II trial. Am J Clin Oncol. 1996;19:609e612. doi: 10.1097/00000421-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Prados MD, Schold SC, Fine HA, et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combinationwith carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro-Oncol. 2003;5:96e103. doi: 10.1093/neuonc/5.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray LJ, Bridgewater CH, Levy D. Carboplatin chemotherapy in patients with recurrent high-grade glioma. Clin Oncol (R Coll Radiol) 2011;23(1):55–61. doi: 10.1016/j.clon.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Brandsma D, Stalpers L, Taal W, Sminia P, Bent van den MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 17.Poulson HS, Grunnet K, Sorenson M, et al. Bevacizumab plus irinotecan in the treatment of patients with progressive recurrent malignant brain tumours. Acta Oncol. 2009;48:52e58. doi: 10.1080/02841860802537924. [DOI] [PubMed] [Google Scholar]


