Abstract
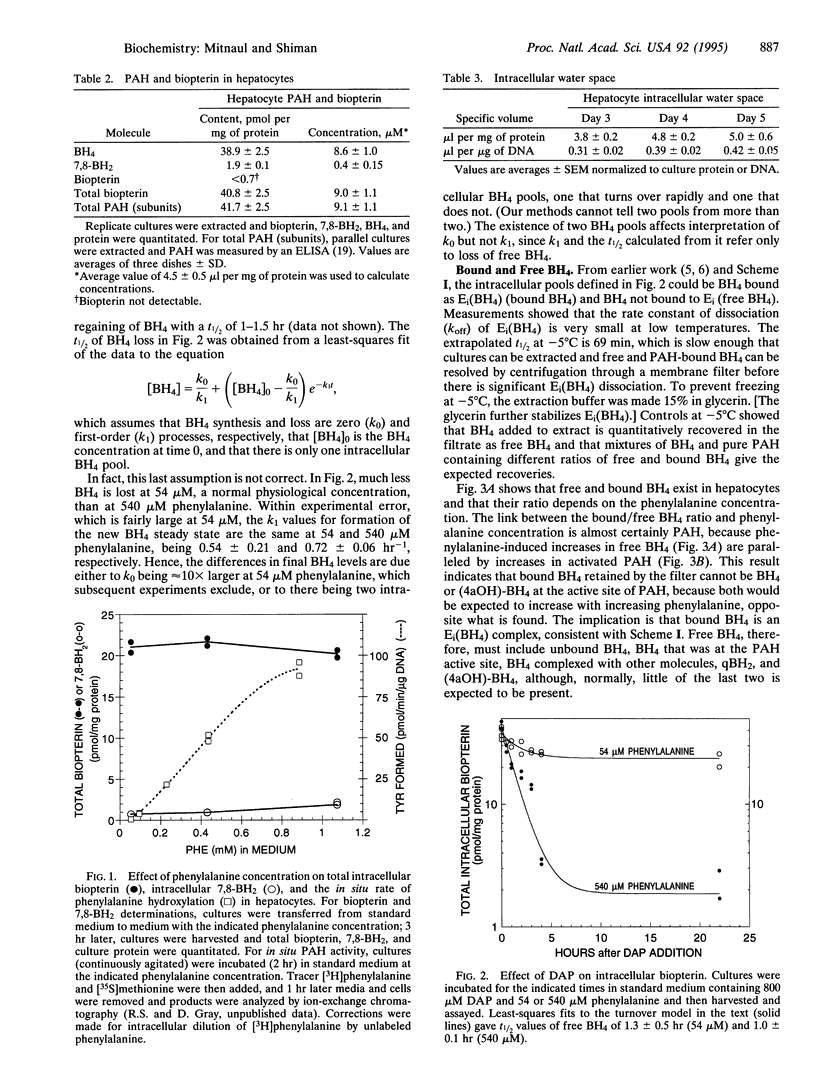
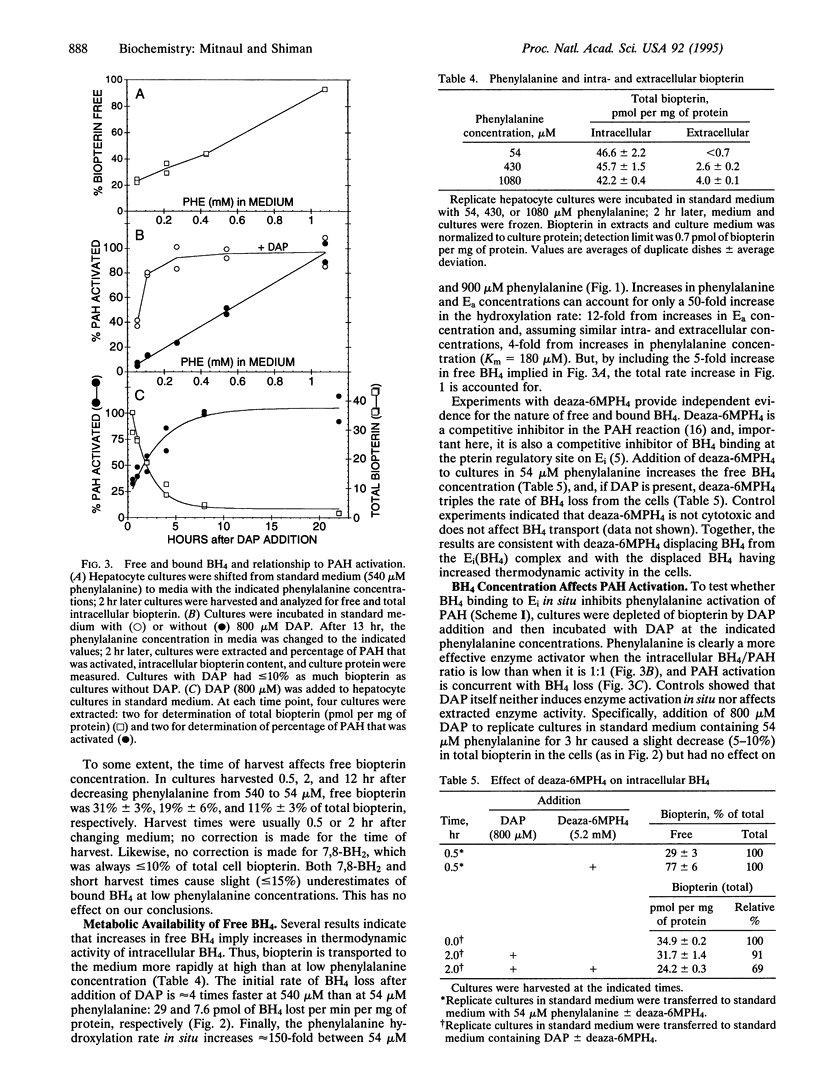
This work had two purposes: (i) to determine in vivo whether liver phenylalanine hydroxylase (PAH) is regulated by its substrates phenylalanine and tetrahydrobiopterin (BH4) as studies with purified enzyme suggest and (ii) to investigate in vivo the relationship between PAH activity and BH4 turnover. We found there are two BH4 pools in hepatocytes, one that is metabolically available (free BH4) and one that is not (bound BH4). Bound BH4 appears bound to PAH; the PAH-BH4 complex has much less catalytic activity and is less readily phenylalanine activated than uncomplexed enzyme. Interconversion of activated and unactivated PAH and bound and free BH4 is driven by phenylalanine; and free BH4 concentration is determined by the state of activation and activity of PAH. In hepatocytes, BH4 and PAH (subunit) concentrations are equal, all intracellular BH4 appears to be available to PAH, and free BH4 turns over rapidly (t1/2 approximately 1 hr). There is no evidence for feedback inhibition of BH4 synthesis; the BH4 synthetic rate appears high when free BH4 concentration is high and low when free BH4 is low. The data provide support in vivo that phenylalanine and BH4 are positive and negative regulators of the activity and activation state of PAH in the proposed manner; they also imply that regulation of BH4 turnover and PAH activity are linked processes, which are both controlled by phenylalanine concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dhondt J. L., Ardouin P., Hayte J. M., Farriaux J. P. Developmental aspects of pteridine metabolism and relationships with phenylalanine metabolism. Clin Chim Acta. 1981 Oct 26;116(2):143–152. doi: 10.1016/0009-8981(81)90017-6. [DOI] [PubMed] [Google Scholar]
- Fukushima T., Nixon J. C. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980 Feb;102(1):176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- Harada T., Kagamiyama H., Hatakeyama K. Feedback regulation mechanisms for the control of GTP cyclohydrolase I activity. Science. 1993 Jun 4;260(5113):1507–1510. doi: 10.1126/science.8502995. [DOI] [PubMed] [Google Scholar]
- Hsieh M. C., Berry H. K. Distribution of phenylalanine hydroxylase (EC 1.14.3.1) in liver and kidney of vertebrates. J Exp Zool. 1979 May;208(2):161–167. doi: 10.1002/jez.1402080204. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Stinson-Fisher C., Shiman R., Jefferson L. S. Regulation of albumin synthesis by hormones and amino acids in primary cultures of rat hepatocytes. Am J Physiol. 1987 Mar;252(3 Pt 1):E291–E298. doi: 10.1152/ajpendo.1987.252.3.E291. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Imai Y. Affinity purification of alkylglycerol monooxygenase from rat liver microsomes by chimyl alcohol-Sepharose 4B column chromatography. J Lipid Res. 1985 Mar;26(3):393–395. [PubMed] [Google Scholar]
- Kapatos G. Tetrahydrobiopterin synthesis rate and turnover time in neuronal cultures from embryonic rat mesencephalon and hypothalamus. J Neurochem. 1990 Jul;55(1):129–136. doi: 10.1111/j.1471-4159.1990.tb08830.x. [DOI] [PubMed] [Google Scholar]
- Kaufman S. The phenylalanine hydroxylating system from mammalian liver. Adv Enzymol Relat Areas Mol Biol. 1971;35:245–319. doi: 10.1002/9780470122808.ch6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lazarus R. A., Dietrich R. F., Wallick D. E., Benkovic S. J. On the mechanism of action of phenylalanine hydroxylase. Biochemistry. 1981 Nov 24;20(24):6834–6841. doi: 10.1021/bi00527a015. [DOI] [PubMed] [Google Scholar]
- Marota J. J., Shiman R. Stoichiometric reduction of phenylalanine hydroxylase by its cofactor: a requirement for enzymatic activity. Biochemistry. 1984 Mar 13;23(6):1303–1311. doi: 10.1021/bi00301a044. [DOI] [PubMed] [Google Scholar]
- Miller M. R., McClure D., Shiman R. p-Chlorphenylalanine effect on phenylalanine hydroxylase in hepatoma cells in culture. J Biol Chem. 1975 Feb 10;250(3):1132–1140. [PubMed] [Google Scholar]
- Shiman R., Gray D. W., Hill M. A. Regulation of rat liver phenylalanine hydroxylase. I. Kinetic properties of the enzyme's iron and enzyme reduction site. J Biol Chem. 1994 Oct 7;269(40):24637–24646. [PubMed] [Google Scholar]
- Shiman R., Gray D. W., Pater A. A simple purification of phenylalanine hydroxylase by substrate-induced hydrophobic chromatography. J Biol Chem. 1979 Nov 25;254(22):11300–11306. [PubMed] [Google Scholar]
- Shiman R., Gray D. W. Substrate activation of phenylalanine hydroxylase. A kinetic characterization. J Biol Chem. 1980 May 25;255(10):4793–4800. [PubMed] [Google Scholar]
- Shiman R., Jones S. H., Gray D. W. Mechanism of phenylalanine regulation of phenylalanine hydroxylase. J Biol Chem. 1990 Jul 15;265(20):11633–11642. [PubMed] [Google Scholar]
- Shiman R., Mortimore G. E., Schworer C. M., Gray D. W. Regulation of phenylalanine hydroxylase activity by phenylalanine in vivo, in vitro, and in perfused rat liver. J Biol Chem. 1982 Oct 10;257(19):11213–11216. [PubMed] [Google Scholar]
- Shiman R. Purification and assay of rat liver phenylalanine 4-monooxygenase. Methods Enzymol. 1987;142:17–27. doi: 10.1016/s0076-6879(87)42004-1. [DOI] [PubMed] [Google Scholar]
- Shiman R. Relationship between the substrate activation site and catalytic site of phenylalanine hydroxylase. J Biol Chem. 1980 Nov 10;255(21):10029–10032. [PubMed] [Google Scholar]
- Shiman R., Xia T., Hill M. A., Gray D. W. Regulation of rat liver phenylalanine hydroxylase. II. Substrate binding and the role of activation in the control of enzymatic activity. J Biol Chem. 1994 Oct 7;269(40):24647–24656. [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Watanabe Y., Tsubokura S., Kagamiyama H., Hayaishi O. Decrease in tetrahydrobiopterin content and neurotransmitter amine biosynthesis in rat brain by an inhibitor of guanosine triphosphate cyclohydrolase. Brain Res. 1988 Apr 12;446(1):1–10. doi: 10.1016/0006-8993(88)91290-5. [DOI] [PubMed] [Google Scholar]
- Xia T., Gray D. W., Shiman R. Regulation of rat liver phenylalanine hydroxylase. III. Control of catalysis by (6R)-tetrahydrobiopterin and phenylalanine. J Biol Chem. 1994 Oct 7;269(40):24657–24665. [PubMed] [Google Scholar]



