ABSTRACT
Introduction:
High intensity cutaneous stimulus transiently suppresses tonic voluntary muscle activity resulting in cutaneous silent period (CSP).
Aim:
The aim of our study was to evaluate the normal values of an onset latency L1, a late latency L2 and a duration of CSP after stimulating sensory fibres of the median nerve.
Material and Methods:
This prospective study was performed at the Neurology Department, Clinical Center of Sarajevo University in period from January 1st 2013 to December 1st 2013. In our study we examined 61 subjects. The group included our relatives, coworkers and friends. The informed consent from testing subjects was obtained.
Results:
The origin of silent period is stimulation of small A-delta nerve fibres. The pre-synaptic or post-synaptic interruption of the electrical volley to motor neurons is discussed. Median values of muscle activity suppression in healthy female is 55.0 ms (45.0-74.0) and 59.0 ms (52.0-67) male subjects. There is a correlation between the onset latency L1and the late L2 latency (p‹0.03). In the on-going study it seems that delay of L1 and shorter muscle activity suppression might provide a sign of small nerve fibres involvement.
Conclusion:
The use of CSP improves the value of neurophysiology examination.
Keywords: small nerve fibres, cutaneous silent period
1. INTRODUCTION
Routine neurophysiology nerve conduction study and needle electromyography (EMG) reflect the function of fastest, large-diameter conducting motor and sensory nerve fibres (1). EMG examination is insufficient assessing the integrity of the whole nerve, to distinguish between axonotmesis and neurotmesis. To rule out the possible interruption of nerve, an additional neurophysiology method is needed. Cutaneous silent period (CSP) is a non- invasive technique which provides the insight to the function of the small-diameter nerve fibres and completes the neurophysiology nerve examination (2).
A sufficient nociceptive cutaneous stimulus exites A-delta nerve fibres and evokes a transient inhibition of voluntary muscle activity occurring in muscles ipsilateral and contralateral to the electrical stimulus (3, 4). The CSP is often used to assess the pathophysiology of ocular (5), oromandibular (5), cervical (6) and brachial dystonia (7). The abnormal CSP was also observed in Parkinson’s disease (8) and intramedullary lesion of the spinal cord (9). Quantitative sensorymetry is performed in many laboratories to evaluate the small nerve fibres (10). Recently to evaluate nerve integrity also ultrasound examination is recommended (11).
2. AIM
The aim of our study was to evaluate the normal values of an onset latency L1, a late latency L2 and a duration of CSP after stimulating sensory fibres of the median nerve. This procedure might be helpful in assessing the function of small diameter nerve fibres.
3. MATERIALS AND METHODS
This prospective study was performed at the Neurology Department, Clinical Center of Sarajevo University in period from January 1st 2013 to December 1st 2013. In our study we examined 61 subjects. The group included our relatives, coworkers and friends. The informed consent from testing subjects was obtained. The subjects with diabetes, alcoholism, polyneuropathy, kidney dysfunction, systemic inflammatory and malignant diseases and the subjects receiving psychotropic drugs respectively, were excluded. The study was approved by the local ethics committee.
All subjects sat in a comfortable chair in a calm room. Using a Synergy EMG machine, the CSP by single electrical stimulation (0.5 ms duration and 80-100 mA intensity, sweeps 250 ms, filters 30 and 10 kH) at the tip of digit II by bipolar electrode placed on the palmar part of the digit was elicited. Correct placement of the bipolar stimulating electrode on the palmar side of the digit is very important to avoid a possible activation of radial sensory fibres. The superficial electrodes (Care Fusion, Middletonn, WI, USA) on the muscle belly of abductor pollicis brevis were placed (Figure 1). During near-maximum activated APB muscle electrical stimulus was delivered. At least 4 individual responses were recorded and superimposed. The onset latency (L1) was recorded at the beginning of muscle activity suppression and the second–late latency (L2) at the start of new muscle activity. The difference between two latencies indicates the duration of CSP.
Figure 1.
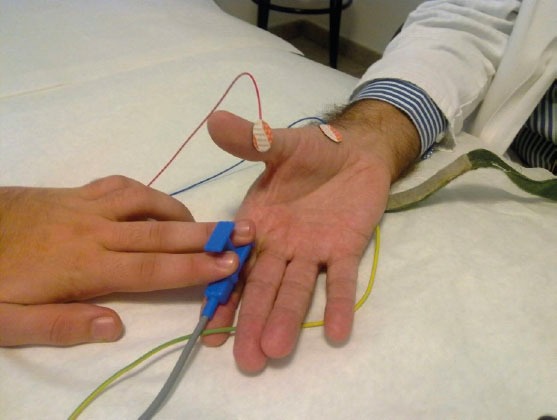
The technical procedure by detection of cutaneous silent period
The data is evaluated by descriptive statistics and determination of Spearman’s correlation coefficient, Mann-Whitney U and Wilcoxon W tests. The onset latencies L1, the late latencies L2 and the duration of CSP–inhibition of muscle activity–were statistically analyzed.
4. RESULTS
In our study 61 subjects were enrolled. The demographic data and CSPs with the onset, the late latencies – L1, L2 and the duration of muscle activity suppression are depicted in Table 1. In our group of healthy subjects significantly more female (74%) than male subjects (26%) were included but the sex didn’t affect the onset (L1), late latencies (L2) and the duration of silent period, respectively. The median onset L1 latency and late latency L2 of both sex groups were similar. The duration of muscle activity suppression didn’t show statistical difference between male and female. The age of both groups was close together and median values of CSP duration were comparable (Table 1). The onset latency (L1) showed a mild correlation with the late latency (L2). By a longer L1 a longer L2 is expected. There is a mild negative correlation between L2 latency and age. The greater age of subjects makes L2 latency slightly shorter (Table 2). In a patient with a severe carpal tunnel syndrome a routine EMG revealed a prolonged distal motor latency and a smaller amplitude of compound muscle action potential – M wave (Figure 2). The sensory nerve action potential of the median nerve on the ring finger was absent, while sensory nerve action potential of the ulnar nerve was clearly recorded (Figure 3). In a case with median nerve entrapment neuropathy a shorter suppression of voluntary muscle activity (31 ms) was recorded comparing to the suppression duration of a healthy subject (60 ms). The delay of L1 latency (27 ms longer) was observed in patient with the median nerve entrapment than in a healthy subject (Figure 4). A prolonged L1 latency in patients with carpal tunnel syndrome was described (12).
Table 1.
Demographic data and the values of cutaneous silent period
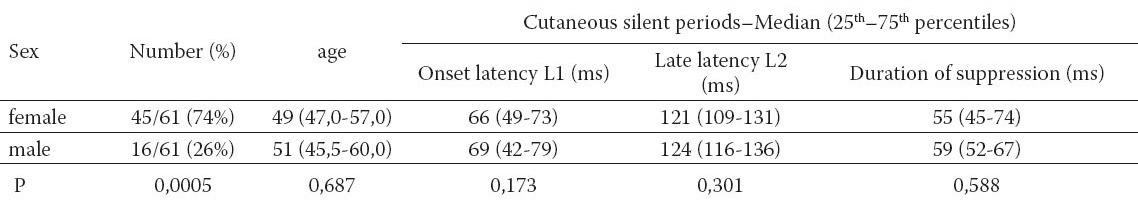
Table 2.
Correlation between the onset (L1), the late latencies (L2), age and sex. CSP (L1) – the onset latency L1 of cutaneous silent period. CSP (L2) – the late latency L2 of cutaneous silent period
Figure 2.
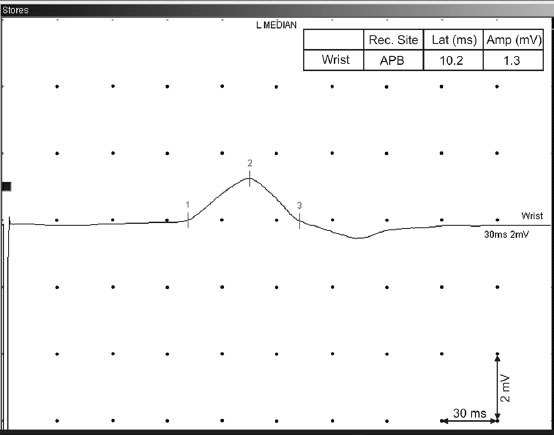
Motor conduction study of median nerve. Rec. Site – recording site; Lat (ms) – latency in miliseconds; Amp (mV) – amplitude in milivolts
Figure 3.
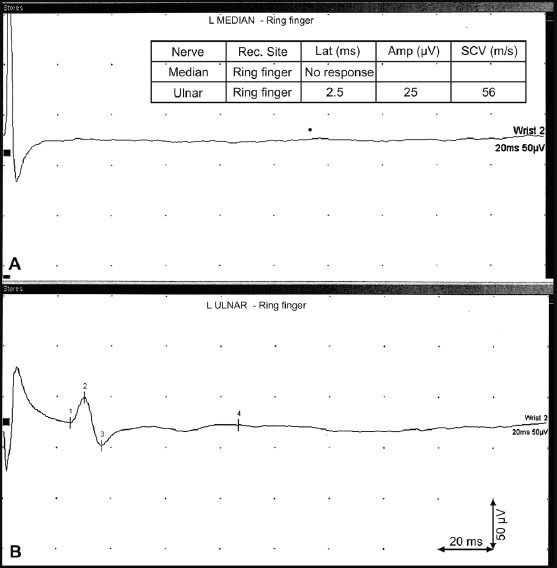
Sensory conduction study of median nerve. A – absent median sensory nerve action potential – detection on ring finger; B – ulnar sensory nerve action potential – ring finger
Figure 4.
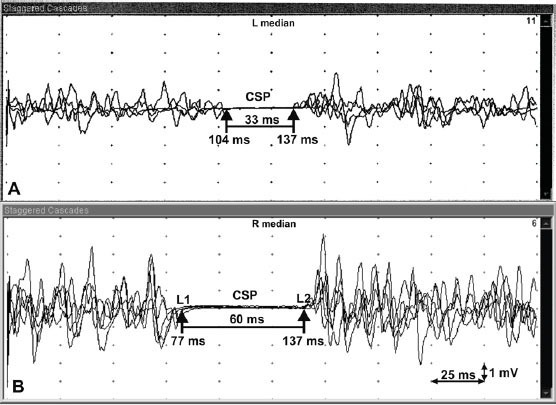
Cutaneous silent period (CSP). A – CSP of patient with carpal tunnel syndrome; B – CSP of a healthy subject; L1 – the onset latency; L2 – the late latency; L2–L1 – duration of muscle activity suppression
5. DISCUSSION
The current study demonstrated a method of CSP measurement. CSP was recorded stimulating the sensory fibres of the median nerve. The decision to evaluate CSP of the median nerve was based on the fact that median nerve entrapment is the most common entrapment neuropathy in humans (13). The focal demyelination of larger – diameter fibres is the primary mechanism of entrapment neuropathy. The second step of nerve entrapment leading to nerve ischemia affects predominantly smaller-diameter fibres producing paraesthesias and pain (14,15). The routine EMG cannot assess the function of small-diameter fiber function which can be evaluated by CSP. Different studies demonstrated the role of CSP in evaluation of corticospinal impairment (16), spinal cord lesion (9), syringomyelia (17) and rigidity in Parkinson’s disease (18).
The sex of subjects didn’t influence the onset (L1) and late (L2) latencies as well as the duration of muscle activity suppression (Table 1). To obtain an optimal CSP it is very important to achieve near maximal contraction of the thenar muscles. At least 4 recordings of CSPs are recommended. The superimposed CSP recordings improve the exact measurement of latencies and duration of muscle activity suppression. The correct position of the stimulating electrode on the palmar side of the second finger enables avoiding the stimulation of radial sensory fibres (Figure 1). An optimal CSP by higher single electrical pulses was delivered. The importance of considerable stimulus intensity more than voluntary muscle contraction is reported (19). There is a mild correlation between L1 and L2 (Table 2). The shorter duration of CSP and delayed L1 latency indicate the involvement of smaller-diameter median nerve fibres but preserved median nerve integrity (Figure 3). Additional involvement of small nerve fibres might influence the improvement of entrapment neuropathy after surgery.
6. CONCLUSION
CSP is an inhibitory spinal reflex produced by small A-delta nerve fibres and provides an important information of nerve fibre continuity. To obtain reliable CSP a proper muscle contraction and particularly suitable strong stimulus intensity of the target sensory nerve fibres is required. This neurophysiology method is useful in assessing severe entrapment neuropathy, traumatic nerve injury or different polyneuropathies. The measurement of CSP enables a new information of nerve function and increases the sensitivity of routine EMG.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Serrao M, Parisi L, Pierelli F, Rossi P. Cutaneous afferents mediating the cutaneous silent period in the upper limbs: evidences for a role of low-threshold sensory fibres. Clinical Neurophysiology. 2001;112:2007–2014. doi: 10.1016/s1388-2457(01)00675-7. [DOI] [PubMed] [Google Scholar]
- 2.Logigian EL, Plotkin GM, Shefner JM. The cutaneous silent period is mediated by spinal inhibitory reflex. Muscle Nerve. 1999 Apr;22:467–472. doi: 10.1002/(sici)1097-4598(199904)22:4<467::aid-mus7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Inghilleri M, Cruccu G, Argenta M, Polidori, et al. Silent period in upper limb muscles after noxius cutaneous stimulation in man. Electroenecephalography and Clin Neurophysiology. 1997;105:109–115. doi: 10.1016/s0924-980x(97)96579-6. [DOI] [PubMed] [Google Scholar]
- 4.Leis AA, Kofler M, Ross M. The silent period in pure sensory neuronopathy. Muscle and Nerve. 1992;15:1345–1348. doi: 10.1002/mus.880151209. [DOI] [PubMed] [Google Scholar]
- 5.Berardelli A, Rothwell JC, Day BL, et al. Pathophysiology of blephasospasm and oromandibular dystonia. Brain. 1985;108:593–608. doi: 10.1093/brain/108.3.593. [DOI] [PubMed] [Google Scholar]
- 6.Carella F, Ciano C, Musicco M, Scaioli V. Exteroceptive reflexes in dystonia: a study of the recovery cycle of the R2 component of the blink reflex and of the exteroceptive suppression of the contracting sternocleidomastoid muscle in blepharospasm and torticollis. Mov Disord. 1994;9:183–187. doi: 10.1002/mds.870090210. [DOI] [PubMed] [Google Scholar]
- 7.Pullman SL, Ford B, Elibol B, Uncini A, Su P.C, Fahn S. Cutaneous electromyographic silent period findings in brachial dystonia. Neurology. 1996;46:503–508. doi: 10.1212/wnl.46.2.503. [DOI] [PubMed] [Google Scholar]
- 8.Fuhr P, Zeffiro T, Hallet M. Cutanous reflexes in Parkinson's disease. Muscle and nerve. 1992;15:733–739. doi: 10.1002/mus.880150618. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg DH, Logigian EL, Kelly JJ., Jr Cervical astrocytoma with arm rigidity: clinical and electrophysiologic features. Neurology. 1988 Oct;38(10):1635–1637. doi: 10.1212/wnl.38.10.1635. [DOI] [PubMed] [Google Scholar]
- 10.Denislic M, Meh D, Popovic M, Kos-Golja M. Small nerve fibre dysfunction in a patient with Sjögren's syndrome. Neurophysiology and morphological confirmation. Scand J Rheumatol. 1995;24(4):257–259. doi: 10.3109/03009749509100886. [DOI] [PubMed] [Google Scholar]
- 11.Tajika T, Kobayashi T, Yamamoto A, Kaneko T, Takagishi K. Diagnostic utility of sonography and correlation between sonographic and clinical findings in patients with carpal tunnel syndrome. J Ultrasound Med. 2013;32:1987–1993. doi: 10.7863/ultra.32.11.1987. [DOI] [PubMed] [Google Scholar]
- 12.Koo YS, Park HR, Joo BE, et al. Utility of the cutaneous silent period in the evaluation of carpal tunnel syndrome. Clin Neurophysiol. 2010;121:1584–1588. doi: 10.1016/j.clinph.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Aurora SK, Ahmad BK, Aurora TK. Silent period abnormalities in carpal tunnel syndrome. Muscle Nerve. 1998;21:1213–1215. doi: 10.1002/(sici)1097-4598(199809)21:9<1213::aid-mus16>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Fullerton PM. The effect of ischemia on nerve conduction in the carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 1963;26:385–397. doi: 10.1136/jnnp.26.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner RA, Andary M. Carpal tunnel syndrome: patholphysiology and clinical neurophysiology. Clin Neurophysiol. 2002;113:1373–1381. doi: 10.1016/s1388-2457(02)00169-4. [DOI] [PubMed] [Google Scholar]
- 16.Gilio F, Bettolo CM, Conte A, et al. Influence of corticospinal tract on the cutaneous silent period: A study in patients with pyramidal syndrome. Neuroscience letters. 2008;433:109–113. doi: 10.1016/j.neulet.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 17.Štetkárová I, Kofler M, Leis AA. Cutaneous and mixed silent period in syringomyelia. Clin Neurophysiol. 2001;112:78–85. doi: 10.1016/s1388-2457(00)00486-7. [DOI] [PubMed] [Google Scholar]
- 18.Fuhr P, Zeffiro T, Hallett M. Cutaneous reflexes in Parkinson's disease. Muscle Nerve. 1992;15:733–739. doi: 10.1002/mus.880150618. [DOI] [PubMed] [Google Scholar]
- 19.Rodi Z, Springer C. Influence of muscle contraction and intensity of stimulation on the cutaneous silent period. Muscle Nerve. 2010;43:324–328. doi: 10.1002/mus.21868. [DOI] [PubMed] [Google Scholar]


