ABSTRACT
Background:
The reasons for the chronic viral persistence of hepatitis B virus infection (HBV) are unknown, but are probably related to host immune factors. Cytokines play a significant role in immune defense. Interleukin-1 (IL-1) is a proinflammatory cytokine and some studies have demonstrated that IL-1 production was impaired in patients with chronic infections of hepatitis B virus, implying that IL-1 may play a role in viral clearance, progression of fibrosis and in malignant potential of HBV. In this study, along with routine laboratory tests, has been performed the analysis of serum levels of proinflammatory cytokine IL-1 α in order of better understanding and monitoring of chronic hepatitis B.
Objective:
The aim of this study was to analyze the usefulness of laboratory tests, which are routinely used in the assessment of liver disease with specified immunological parameters in patients with chronic hepatitis B.
Patients and methods:
Total of 60 subjects was divided into two groups: HBV- PCR positive and negative group. The control group of 30 healthy participants was included. Apart from standard laboratory tests, the analysis included serum levels of cytokine IL-1 α.
Results and discussion:
IL-1α had the highest mean concentration in group 1–viral hepatitis C, with PCR positive test (5.73 pg / ml), and then in group 2- viral hepatitis B, PCR negative test (5.39 pg / ml). ANOVA test proves that IL-1α in the healthy group (3) was different from other groups as follows: in relation to group 1 statistical significance level was p <0.001 (F = 32 75 5); in relation to group 2 was also statistically significant at p <0.001 (F = 182 361); Cytokine IL-1 was statistically analyzed separately and compared by group 1 and 2 using Student t-test for independent samples. Statistical significance was observed at p = 0.026. IL-1 α was positively correlated with the duration of the illness (p <0.01) and with serum ALT activity (p <0.01) and serum AST activity (p <0.01). Using multivariate analysis model “Factor Analysis”, was made significant stratification predictive parameters in relation to the cytokine IL-1α, stratified significance is indicated as follows: 1. Age, 2. history of receiving transfusions, 3. ALT, 4. AST, 5. MELD score (negative), 6. Child-Pugh score (Negative).
Conclusion:
IL-1α was significantly elevated in inflammatory conditions of pronounced activity (PCR positive hepatitis). IL-1α may have important role as marker of both inflammation and hepatic injury, particularly in the course of hepatitis B. Results suggest that inflammatory and immune parameters, analyzed together can significantly contribute to the understanding and predicting of chronic liver damage. IL-1 can be used as important parameter of inflammatory activity and fibrosis evaluation and eventually prediction of malignant transformation in chronic liver damage.
Keywords: chronic hepatitis B, parameters of inflammation, IL- 1α
1. INTRODUCTION
The immune response is a key component in the activation and maintenance of antiviral immunity, through induction of cytokines and initiation of the adaptive immune response. Immunoregulatory cytokines influence the persistence of hepatitis B virus (HBV) chronic infection and the extent of liver damage. Human hepatitis B virus (HBV) can cause acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma (1). Reasons for persistent HBV infection are unknown, but they are probably related to host immune factors. Interleukin-1 (IL-1) plays an important role in inflammation and regulation of immune response, and membrane form of trimeric IL-1/IL-1 receptor/mIL-1RAcP complex and demonstrates that HBeAg can trigger host IL-1 response by binding to mIL-1RAcP. HBV e antigen (HBeAg), a secreted protein and not required for viral replication, is thought to play an immunoregulatory role during viral infection. However, the functional involvement of HBeAg in host immune response has not been fully elucidated. HBeAg can bind to interleukin-1 receptor accessory protein (IL-1RAcP) (2).
The pro-inflammatory cytokines are involved in viral clearance and in metabolic and viral hepatic diseases. Th1 cytokines positively correlate with hepatic inflammation in HBV infection. Functional impairment, suppression or deletion of antigen-specific T cells appears to be a key determinant of progression to chronicity and malignant progression. The importance of the cytokine milieu in determining viral clearance has been emphasized by recent studies (3-7). Cytokines are low-molecular-weight mediators of cellular communication produced by multiple cell types in the liver, with the Kupffer cell critically important. Proinflammatory cytokines such as interleukin-1, tumor necrosis factor and interleukin-8 are acute-phase cytokines and play a role in the liver injury of acute and chronic liver diseases (8).
Interleukin-1 (IL-1) has central role in inflammatory process especially acute inflammation. It is an indicator of the intensity of inflammation activity (9). Some studies have demonstrated that IL-1 production was impaired in patients with chronic hepatitis B, implying that IL-1 may play a role in viral clearance, progression of fibrosis and in malignant potential of HBV (10-16).
The objective of this study was to determine and analyze serum level of IL-1α in patients with chronic hepatitis B in correlation of the presence of viral genetic replication and functional liver status.
2. PATIENTS AND METHODS
The study was conducted as an open, one year, comparative clinical trial. Before entering the study, each patient reviewed and signed an informed consent. All research described in study, involving human subjects and material derived from human subjects complied with ethical principles. Standards of Good Clinical Practice, Good Laboratory Practice and The declaration of Helsinki were followed. The study was conducted at the Department of gastroenterology and Hepatology, Clinical Centre University of Sarajevo.
Patients
Total of 90 patients of either sex, 18–80 years of age, were recruited. Inclusion criteria were patients with positive serum HBV antibodies, polymerase chain reaction test was performed. Previous liver biopsy diagnosis of hepatitis B was needed, no more than 6 months before study. Exclusion criteria were: presence of liver disease caused by a hereditary condition, cardiac liver cirrhosis, and liver disease occurred during pregnancy, vascular disease of the liver, primary biliary cirrhosis, as and subjects with liver transplant, acute hepatitis, evidence of acute or chronic inflammatory syndrome of other known origin, immunodeficiency states.
Respondents with diagnosis of chronic hepatitis B – 60 patients were divided in two groups:
Chronic hepatitis B, HBV-RNA-PCR positive test,
Chronic hepatitis B, HBV-RNA-PCR negative test.
A control group of 30 healthy subjects was included as well as 3 groups.
Methods
A physical examination was carried out and medical history was taken during the pre-study visit. The following data were recorded from all patients: age, gender, BMI, history of narcotics consumption or transfusion receiving, history of liver disease–liver biopsy, comorbidity. Biochemical parameters were recorded: full blood count, international normalized ratio (INR), active partial thromboplastin time (APTT) and routine liver function tests including bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase and gamma-glutamyl transferase, proteinogram. Polymerase chain reaction for detecting viral RNA in serum was used.
Serum levels of cytokine IL-1α where determined and measured by quantitative Sandwich Enzyme Immunoassay technique. Monoclonal antibodies specific for IL-1α were coated on microparticules, which come into contact with the sample, and is based on the occurrence of a specific color, measuring the intensity, extent and concentration of cytokine in the sample.
Analysis was made from frozen serum samples that were collected in serum separator tube. Values were expressed as pg/ml. Functional status of the liver was determined by the modified Child Pugh and MELD scores (24, 25).
Statistical analysis
For the processing of data computer program “Statistica for Windows 5.0” was used. Categorical data were expressed as proportions (%), and continuous data as means ± standard deviation (SD). Statistical methods used in this study where: analysis of variance test (ANOVA test), multivariate analysis model “Factor Analysis”, and Student’s t-test for independent samples. The level of significance was p <0.05.
3. RESULTS
A total of 90 subjects, divided into two groups of 30 patients, also with a group of 30 healthy subjects treated for comparison. The Table 1 shows the basic demographic and anthropological parameters of subjects per group, which were not significantly different by group.
Table 1.
The basic demographic and anthropological parameters of subjects per group.
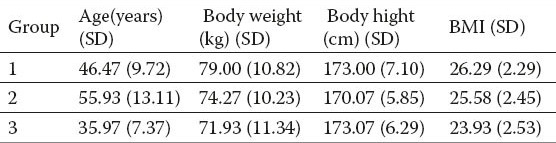
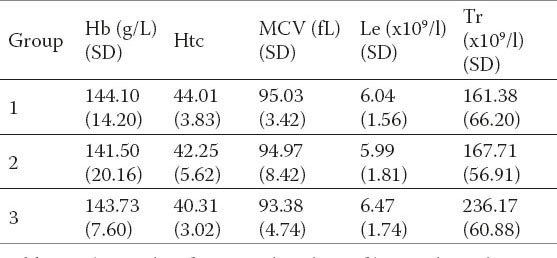
Increased enzyme activity was noted in groups of patients with liver disease compared to the control group of healthy subjects. INR and APTT did not significantly differ by group (Table 2). The values of total protein were not significantly different (Table 3). There were no statistically significant differences in the analysis of hematological parameters (Table 4).
Table 2.
The results of average activity values of enzymes, bilirubin, INR and APTT in groups.
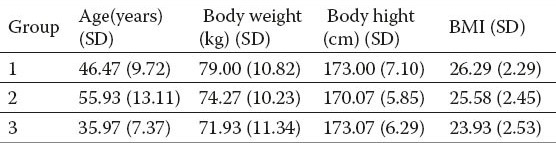
Table 3.
The results of statistical analysis of protein parameters in groups.
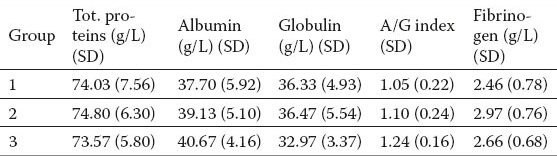
Table 4.
Tthe results of statistical analysis of hematological parameters in groups.
The histogram 1 presents average values of interleukin 1 (IL-1α) in groups. ANOVA analysis of variance test proves that IL-1α in the healthy group (3) was different from other groups as follows: in relation to group 1 statistical significance level was p <0.001 (F = 32 755); in relation to group 2 was also statistically significant at p <0.001 (F = 182 361); IL-1α had the highest mean concentration in group 1–viral hepatitis B, with PCR positive test (5.73 pg / ml), and then in group 2- viral hepatitis B, PCR negative test (5.39 pg / ml).
Figure 1.
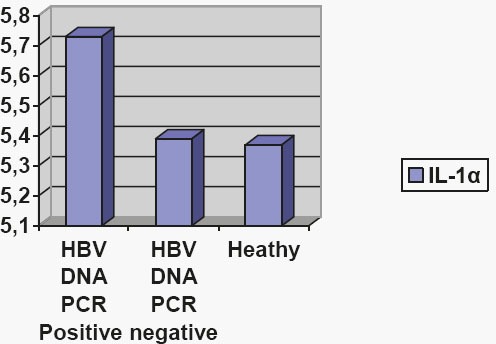
The average values of interleukin 1 (IL-1α) in groups.
Cytokine IL-1α was statistically analyzed separately and compared by groups using Student t-test for independent samples. Statistical significance was observed between groups 1 and 2 at p = 0.026. IL-1 α was positively correlated with the duration of the illness (p <0.01) and with serum ALT activity (p <0.01) and serum AST activity (p <0.01). Using multivariate analysis model “Factor Analysis”, was made significant stratification predictive parameters in relation to the cytokine IL-1α, stratified significance is indicated as follows: 1. Age, 2. history of receiving transfusions, 3. ALT, 4. AST, 5. MELD score, 6. Child-Pugh score.
4. DISCUSSION
In clinical practice, for determining the degree of functional status of liver Child Turcotte Pugh and MELD scoring systems are used. In this study, along with routine laboratory tests, has been performed the analysis of serum levels of proinflammatory cytokine IL-1α in order of better understanding and monitoring of chronic hepatitis B.
Measurements of average values of enzyme activity (AST, ALT, γGT and AP) and bilirubin in groups showed increased activity of these enzymes in groups of patients with chronic hepatitis compared to controls, which as expected due to chronic liver process. The values of total protein and hematological parameters were not significantly different by groups. Functional indicators of liver damage (Child Pugh and MELD scores) showed a correlation in both groups, but not as good assessment system the intensity of inflammation and involvement of the liver fibrosis process. What explains their primary purpose in evaluating the terminal stages of liver damage.
Statistical analysis of the cytokines analyzed showed very impressive results. An analysis of serum level of Interleukin-1α showed a high degree of correlation with active replication of genetic material (group 1), resulting in a high degree of statistical significance. The most active inflammatory process occurred in this group, while in other group inflammatory reaction has subsided, and has been better controlled, which is consistent with the results. The increase of IL-1α diverts an inflammatory reaction of the predominantly exudative–cellular responses, under the influence of IL-1α, to fibroblast–granulation response. Histologically speaking, at this stage to expect significant activity of fibroblasts, fibrous components of reproduction in inflammatory region. If this process is extremely intense generated significant predisposition to replace functional liver tissue with fibrosis, which may have long-term pathological changes in liver structure, and then the functional repercussions. At this stage it is necessary to evaluate how favorable is “repair inflammatory reaction”. If it is too intense, the stabilization of the formed fibrous tissue, in the long term, could functionally suppress liver tissue. It follows that monitoring the changes in concentrations of IL-1 and other cytokines (TGF-β1) might have a use value “predictor of cirrhosis”.
Primary liver cancer is an important cause of cancer death, and hepatocellular carcinoma (HCC) accounts for 70%-85% of total liver cancer worldwide. Chronic hepatitis B virus (HBV) infection contributes to > 75% of HCC cases. High serum viral load is the most reliable indicator of viral replication in predicting development of HCC. HBV genotype C is closely associated with HCC in cirrhotic patients aged > 50 years, whereas genotype B is associated with development of HCC in non-cirrhotic young patients and postoperative relapse of HCC. HBV load, genotype C, viral mutations and expression of inflammatory molecules in HBV-related HCC tissues are significantly associated with poor prognosis. Imbalance between intratumoral CD8(+) T cells and regulatory T cells or Th1 and Th2 cytokines in peritumoral tissues can predict prognosis of HBV-related HCC. These factors are important for developing active prevention and surveillance of HBV-infected subjects who are more likely to develop HCC, or for tailoring suitable treatment to improve survival or postpone postoperative recurrence of HCC. Earlier studies suggests that elevated levels of IL-1 in samples of liver tissue indicate the pronounced activity of hepatitis B and persistence of viral activity (16, 17) and it is to expect increased serum levels of this cytokine in this conditions, which results from this research.
In chronic inflammated hepatic tissues, high HBV DNA, HBV mutations, high densities of macrophages, activated stellates and mast cells, high expression of macrophage colony-stimulating factor/its receptor and placental growth factor, Th1/Th2-like cytokine shift, inflammation-related signature and activation of carcinogenesis-related pathways predict late recurrence of HCC (18-23).
Further studies should be focused on the development of a robust strategy by integrating the viral factors, inflammatory factors and clinical factors of complementary prognostic value to ensure high validity of the assessment for HCC occurrence prediction.
5. CONCLUSION
Analysis of proinflammatory cytokine IL-1α and functional status of the liver revealed detailed information about the conditions of chronic hepatitis B. IL-1α was significantly elevated in inflammatory conditions of pronounced activity (PCR positive hepatitis) and can be used as important parameter of inflammatory activity and fibrosis evaluation and eventually malignant transformation in chronic liver damage.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Cheong JY, Cho SW, Hwang IL, Yoon SK, Lee JH, Park CS, Lee JE, Hahm KB, Kim JH. Association between chronic hepatitis B virus infection and interleukin-10, tumor necrosis factor-αgene promoter polymorphisms. Journal of Gastroenterology and Hepatology. 2006;21:1163–1169. doi: 10.1111/j.1440-1746.2006.04304.x. [DOI] [PubMed] [Google Scholar]
- 2.Yang CY, Kuo TH, Ting LP. Human hepatitis B viral e antigen interacts with cellular interleukin-1 receptor accessory protein and triggers interleukin-1 response. J Biol Chem. 2006;281(45):34525–34536. doi: 10.1074/jbc.M510981200. [DOI] [PubMed] [Google Scholar]
- 3.Kim SS, Cheong JY, Lee D, Lee SK, Kim MH, Kwack K, Yang SJ, Lee HY, Cho SW. Interleukin-1ß and interleukin-1 receptor accessory protein gene polymorphisms are associated with persistent hepatitis B virus infection. Hepatogastroenterology. 2012;59(113):190–197. doi: 10.5754/hge10375. [DOI] [PubMed] [Google Scholar]
- 4.Wang FS. Current status and prospects of studies on human genetic alleles associated with hepatitis B virus infection. World J Gastroenterol. 2003;9(4):641–644. doi: 10.3748/wjg.v9.i4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramezani A, Banifazl M, Mamishi S, Sofian M, Eslamifar A, Aghakhani A. The influence of human leukocyte antigen and IL-10 gene polymorphisms on hepatitis B virus outcome. Hepat Mon. 2012;12(5):320–325. doi: 10.5812/hepatmon.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramezani A, Hasanjani Roshan MR, Kalantar E, Eslamifar A, Banifazl M, Taeb J, Aghakhani A, Gachkar L, Velayati AA. Association of human leukocyte antigen polymorphism with outcomes of hepatitis B virus infection. J Gastroenterol Hepatol. 2008;23(11):1716–1721. doi: 10.1111/j.1440-1746.2008.05482.x. [DOI] [PubMed] [Google Scholar]
- 7.Yan ZH, Fan Y, Wang XH, Mao Q, Deng GH, Wang YM. Relationship between HLA-DR gene polymorphisms and outcomes of hepatitis B viral infections: a meta-analysis. World J Gastroenterol. 2012;18(24):3119–3128. doi: 10.3748/wjg.v18.i24.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA. Biologic basis role of interleukin 1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 9.Koulentaki M, Notas G, Petinaki E, Valatas V, Mouzas IA, Castanas E, Kouroumalis EA. Nitric oxide and pro-inflammatory cytokines in acute hepatitis B. Eur J Intern Med. 2004 Feb;15(1):35–38. doi: 10.1016/j.ejim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Akpolat N, Yahsi S, Godekmerdan A, Demirbag K, Yalniz M. Relationship between serum cytokine levels and histopathological changes of liver in patients with hepatitis B. World J Gastroenterol. 2005;11(21):3260–3263. doi: 10.3748/wjg.v11.i21.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105–117. doi: 10.4137/BMI.S10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veidal SS, Bay-Jensen AC, Tougas G, Karsdal MA, Vainer B. Serum markers of liver fibrosis: combining the BIPED classification and the neo-epitope approach in the development of new biomarkers. Dis Markers. 2010;28(1):15–28. doi: 10.3233/DMA-2010-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leeming DJ, Karsdal MA, Byrjalsen I, Bendtsen F, Trebicka J, Nielsen MJ, Christiansen C, Moller S, Krag A. Novel serological neo-epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment Pharmacol Ther. 2013;38(9):1086–1096. doi: 10.1111/apt.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grigorescu M. Noninvasive biochemical markers of liver fibrosis. J Gastrointestin Liver Dis. 2006;15(2):149–159. [PubMed] [Google Scholar]
- 15.Saxena R, Chawla YK, Verma I, Kaur J. Interleukin-1 polymorphism and expression in hepatitis B. virus-mediated disease outcome in India. J Interferon Cytokine Res. 2013;33(2):80–89. doi: 10.1089/jir.2012.0093. [DOI] [PubMed] [Google Scholar]
- 16.Kao JH, Chen PJ, Chen DS. Recent advances in the research of hepatitis B virus-related hepatocellular carcinoma: epidemiologic and molecular biological aspects. Adv Cancer Res. 2010;108:21–72. doi: 10.1016/B978-0-12-380888-2.00002-9. [DOI] [PubMed] [Google Scholar]
- 17.Han YF, Zhao J, Ma LY, Yin JH, Chang WJ, Zhang HW, Cao GW. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J Gastroenterol. 2011;17(38):4258–4270. doi: 10.3748/wjg.v17.i38.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bréchot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127(5):56–61. doi: 10.1053/j.gastro.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Chemin I, Zoulim F. Hepatitis B, virus induced hepatocellular carcinoma. Cancer Lett. 2009;286(1):52–59. doi: 10.1016/j.canlet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Liao R, Wu H, Yi Y, Wang JX, Cai XY, He HW, Cheng YF, Zhou J, Fan J, Sun J, Qiu SJ. Clinical significance and gene expression study of human hepatic stellate cells in HBV related-hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:22. doi: 10.1186/1756-9966-32-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Zhang Q, Chang W, Du Y, Zhang H, Cao G. Viral and host inflammation-related factors that can predict the prognosis of hepatocellular carcinoma. Eur J Cancer. 2012;48(13):1977–1987. doi: 10.1016/j.ejca.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Celikbilek M, Dogan S, Gursoy S, Zararsız G, Yurci A, Ozbakır O, Guven K, Yucesoy M. Noninvasive assessment of liver damage in chronic hepatitis B. World J Hepatol. 2013;5(8):439–445. doi: 10.4254/wjh.v5.i8.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LP, Zhao J, Du Y, Han YF, Su T, Zhang HW, Cao GW. Antiviral treatment to prevent chronic hepatitis B or C-related hepatocellular carcinoma. World J Virol. 2012;1(6):174–183. doi: 10.5501/wjv.v1.i6.174. [DOI] [PMC free article] [PubMed] [Google Scholar]


