Abstract
Background
Fibrinogen concentrate treatment can improve coagulation during massive traumatic bleeding. The aim of this in vitro study was to determine whether fibrinogen concentrate, or a combination of factor XIII and fibrinogen concentrates, could reverse a haemodilution-induced coagulopathy during hypothermia.
Methods
Citrated venous blood from 10 healthy volunteers was diluted in vitro by 33% with 130/0.42 hydroxyethyl starch (HES) or Ringer’s acetate (RAc). The effects of fibrinogen concentrate corresponding to 4 gram per 70 kg, or a combination of the same dose of fibrinogen with factor XIII (20 IU per kg), were measured using rotational thromboelastometry (ROTEM). The blood was analysed at 33°C or 37°C with ROTEM EXTEM and FIBTEM reagents. Clotting time (CT), clot formation time (CFT), alpha angle (AA) and maximal clot formation (MCF) were recorded.
Results
Fibrinogen with or without factor XIII improved all ROTEM parameters in either solution irrespective of temperature, with the exception of EXTEM-AA and EXTEM-CFT in HES haemodilution. Fibrinogen increased FIBTEM-MCF more in the samples diluted with RAc than HES, particularly in presence of factor XIII.
Conclusions
Fibrinogen improved in vitro haemodilution-induced coagulopathy at both 33°C and 37°C, though more efficiently after crystalloid than HES haemodilution. Factor XIII had an additional effect on FIBTEM-MCF, but only after crystalloid dilution.
Keywords: Factor XIII, Fibrinogen, Hemodilution, Hypothermia, Hemostasis, Thrombelastography
Introduction
Haemodilution and hypothermia both contribute to coagulopathy and aggravate acute traumatic coagulopathy, which has been recognized as a significant cause of death in patients with traumatic injuries [1]. We therefore studied the efficacy of concentrates of fibrinogen and factor XIII (FXIII) in improving in vitro coagulopathy induced by haemodilution and hypothermia.
Hypothermia impairs coagulation mainly by platelet inhibition [2], whereas haemodilution principally impairs plasma coagulation [3]. In addition to the dilutional effects seen with crystalloids, synthetic colloid solutions impair fibrinogen polymerization and platelet function [3]. Hypothermia and haemodilution induced coagulopathy has been studied extensively with whole blood viscoelastic haemostatic assays (VHA) such as thromboelastometry (ROTEM), but there are few studies of simultaneous hypothermia and haemodilution [4,5]. In addition, in vitro correction of haemodilution-induced coagulopathy with fibrinogen concentrate has been studied extensively [6,7], but not during hypothermia. Previous in vitro studies indicate an additional effect of high doses of FXIII together with fibrinogen to correct haemodilution-induced coagulopathy, but this needs to be studied at clinically relevant dosages [7-9]. Therefore, the purpose of this study was to evaluate the in vitro effects of mild hypothermia in the context of coagulopathy induced by haemodilution with crystalloid or hydroxyethyl starch solutions; and the effects of fibrinogen concentrate, alone or in combination with factor XIII concentrate using ROTEM, a well-known VHA. The primary hypothesis was that fibrinogen concentrate, with or without factor XIII, reduce dilutional coagulopathy and secondly that hypothermia attenuates their corrective effects.
Material and Methods
Volunteers
Ten healthy individuals (one woman and nine men, age 27–65 years) gave their written informed consent to participate in this study. None of the subjects received any medication in the preceding 7 days and none had a history of coagulopathy. The Regional Ethical Review Board in Lund, Sweden, approved the study (DNR 2008:484).
Sampling
Blood was sampled into citrated plastic vacuum tubes (BD Vacutainer® Coagulation Tube, PET, 0.3 ml 0.109 M citrate). Venepuncture with minimal stasis was conducted, using a 21 Gauge needle, and the first tube was discarded. The samples were incubated at 33°C or 37°C for 30 minutes to ensure temperature equilibration and analysed within 4 hours of sampling.
Hypothermia and haemodilution
A digital thermometer submerged into a fluid-filled reference tube was used to check all blood sample temperatures. The ROTEM instruments were set at either 33°C or 37°C. The blood samples were diluted 2:1 volume by volume with either Ringer’s acetated solution (RAc; Fresenius Kabi, Bad Homburg, Germany(G)) or 6% hydroxyethyl starch in saline (HES; MW 130 kDa, substitution ratio 0.42, Venofundin®, B.Braun, Melsungen, G) that is 33% haemodilution.
Fibrinogen and factor XIII
Human fibrinogen concentrate (Riastap®, CSL Behring Marburg, G) and factor XIII (FXIII) concentrate (Fibrogammin®; now registered as Cluvo®, CSL Behring) were dissolved according to the manufacturer’s instructions, giving concentrations of 20 mg/ml and 62.5 IU/ml respectively. 120 μl of fibrinogen concentrate or 120 μl of fibrinogen +15 μl of FXIII were added to the respective blood sample, to a total sample volume of 3000 μl. These dosages correspond to 4 g of fibrinogen and 1550 IU of FXIII to a 70-kg man or 55 mg fibrinogen and 22 IU FXIII per kg of body weight. The FXIII dosage followed recommendations from the manufacturer on how to treat haemorrhage in congenital FXIII-deficient patients (10–20 IU/kg of body weight). The 4 g fibrinogen dosage is in line with current guidelines on treatment of massive bleeding.
ROTEM
Rotational thromboelastometry (ROTEM®; Pentapharm, Munich, Germany), a viscoelastic coagulation analysis instrument, was used according to the manufacturer’s instructions. Two ROTEM assays, EXTEM and FIBTEM, were used. Coagulation was stimulated with tissue factor in the EXTEM test and the following parameters were recorded: clotting time (CT), clot formation time (CFT), alpha-angle (AA) and maximal clot formation (MCF). The following EXTEM parameters measure the clot velocity: CT shows how long clot initiation takes while CFT and AA reflect the clot amplification and propagation. EXTEM-MCF measures the maximal clot strength and is dependent on platelet count and function, as well as fibrin formation and polymerisation. The FIBTEM test is identical to the EXTEM test except for that cytochalacin D, a platelet inhibitor is added to the test reagent. This results in FIBTEM-MCF representing clot strength dependent on fibrin formation and polymerization alone. The only FIBTEM parameter recorded was FIBTEM-MCF. The last parameter EX-FIBTEM-MCF is a surrogate measure of platelet activity, which is calculated as FIBTEM-MCF subtracted from EXTEM-MCF.
Statistical analysis
For all statistical calculations the software package R, version 3.0.0, was used [10]. Repeated measures were analysed using univariate mixed models, using the package nlme ver. 3.1-109 [11]. Heterogeneous variances were evaluated with weighting. Post-hoc comparisons were made using the Multcomp package ver. 1.2-17 [12] using false discovery rate adjustment of p-values [13]. Data were analysed in two steps. The first step with haemodilution (control, RAc, HES) and two different temperatures (33°C, 37°C) sought to evaluate the potentially different effects of haemodilution with different solutions on normo- and hypothermic blood respectively. The second step used haemodilution with two different solutions (RAc, HES), two temperatures (33°C, 37°C) and the addition of coagulation factors (control, fibrinogen, fibrinogen + FXIII); this analysis evaluated the potential interaction between coagulation factors and the different solutions or different temperatures.
Results
Baseline values
In the undiluted samples, all EXTEM parameters and FIBTEM-MCF were within the normal range at 37°C [14].
Hypothermia and haemodilution
All clot velocity parameters were impaired by hypothermia of 33°C: in both undiluted and diluted blood CT and CFT were prolonged and AA was decreased (Figure 1). EXTEM-MCF decreased to a small extent during hypothermia, but only during concurrent haemodilution with either solution (Figure 2). Despite these hypothermic effects, the average values in undiluted samples were still mainly within the normal range at 33°C: a few samples’ EXTEM-CFT exceeded the reference range and EXTEM-AA and FIBTEM-MCF were below the reference range in a few of the samples.
Figure 1.
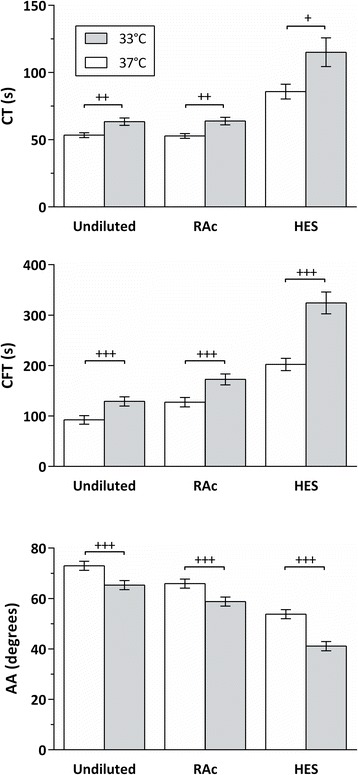
Effects of hypothermia (33° vs. 37 C) and haemodilution on the ROTEM EXTEM parameters clotting time (CT), clot formation time CFT) and alpha angle (AA). RAc: Ringer’s acetate, HES: hydroxyethyl starch. All differences between haemodilution groups (Undiluted vs. RAc, Undiluted vs. HES, and RAc vs. HES) within each temperature were significant (P < 0.001), except the differences of CT between Undiluted and RAc at both temperatures, which were not significant. Brackets show significant differences between temperatures. (+: P < 0.05, ++: P < 0.01, +++: P < 0.001). Data are presented as mean values, error bars are 95% simultaneous confidence intervals. N = 10.
Figure 2.
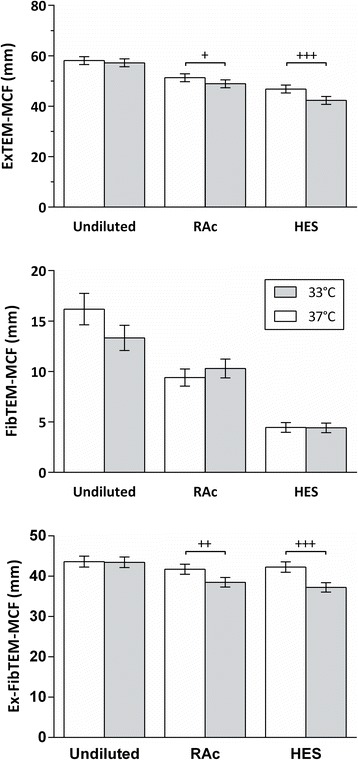
Effects of hypothermia (33° vs. 37 C) and haemodilution on the ROTEM parameters. EXTEM-MCF, FIBTEM-MCF and EX-FIBTEM-MCF; the latter calculated as FIBTEM-MCF subtracted from EXTEM-MCF. RAc: Ringer’s acetat, HES: hydroxyethyl starch. All differences of EXTEM-MCF and FIBTEM-MCF between haemodilution groups (Undiluted vs. RAc, Undiluted vs. HES, and RAc vs. HES) within each temperature were significant (P < 0.001), except the difference of FIBTEM-MCF between Undiluted and RAc at 33°C. Differences of EX-FIBTEM-MCF were only significant (P < 0.001) between Undiluted and haemodilution with either RAc or HES, at 33°C. Brackets show significant differences between temperatures. (+: P < 0.05, ++: P < 0.01, +++: P < 0.001). Data are presented as mean values, error bars are 95% simultaneous confidence intervals. N = 10.
At normothermia, haemodilution with HES significantly impaired all measured parameters, whereas RAc significantly impaired all parameters except CT. In addition, HES impaired ROTEM parameters significantly more than RAc (Figures 1 and 2). Moreover, the combination of hypothermia and HES haemodilution interacted to impair CFT and AA in relation to undiluted samples (CFT: P < 0.001 and AA: P < 0.05), as well as in relation to RAc haemodiluted samples (CFT: P < 0.001 and AA: P < 0.01).
Addition of fibrinogen with or without factor XIII
Addition of fibrinogen concentrate to haemodiluted samples enhanced coagulation in general, except for CFT and AA in HES-haemodiluted samples. With FIBTEM-MCF and EXTEM-AA, there were significant interactions between fibrinogen concentrate and RAc haemodilution as compared to HES haemodilution, that is FIBTEM-MCF and AA were more effectively increased during RAc haemodilution than during HES haemodilution (P < 0.05 at both temperatures with FIBTEM-MCF and P < 0.05 at 37°C with AA) (Figures 3 and 4).
Figure 3.
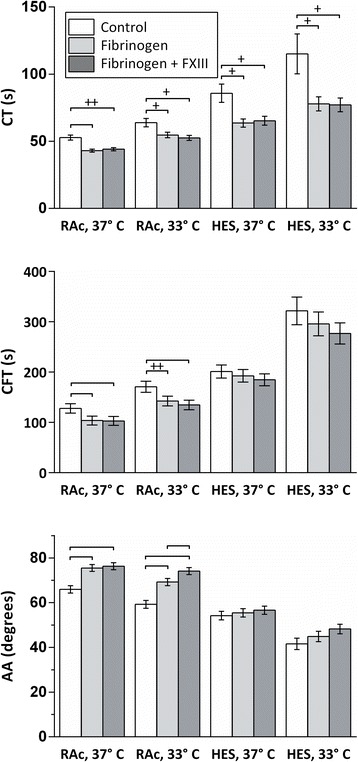
Effects of coagulation factor concentrate (Fibrinogen or Fibrinogen with factor XIII (FXIII)) at two different temperatures (33° vs. 37 C) during haemodilution with Ringer’s acetate (RAc) or hydroxyethyl starch (HES). ROTEM EXTEM parameters shown are clotting time (CT), clot formation time CFT) and alpha angle (AA). Statistically significant differences are marked with brackets. All significances are P < 0.001, except where elsewise stated; +: P < 0.05, ++: P < 0.01. Data are presented as mean values, error bars are 95% simultaneous confidence intervals. N = 10.
Figure 4.
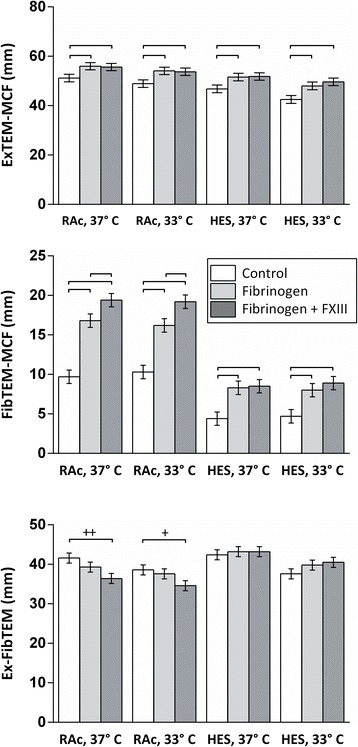
Effects of coagulation factor concentrate (Fibrinogen or Fibrinogen with factor XIII (FXIII)) at two different temperatures (33° vs. 37 C) during haemodilution with Ringer’s acetate (RAc) or hydroxyethyl starch (HES). ROTEM parameters shown are EXTEM-MCF, FIBTEM-MCF and EX-FIBTEM-MCF; the latter calculated as FIBTEM-MCF subtracted from EXTEM-MCF. Statistically significant differences are marked with brackets. All significances are P < 0.001, except where elsewise stated; +: P < 0.05, ++: P < 0.01. Data are presented as mean values, error bars are 95% simultaneous confidence intervals. N = 10.
Almost all the parameters were improved to the same degree by fibrinogen combined with FXIII, as by fibrinogen alone. However, FXIII had additional effects to the fibrinogen effects during RAc haemodilution; AA increased more at 33°C and FIBTEM-MCF increased more at both 33° and 37°C after addition of fibrinogen + FXIII as compared with fibrinogen alone (Figures 3 and 4). The better effect of fibrinogen combined with FXIII on FIBTEM-MCF is also demonstrated by significant synergies between RAc haemodilution and fibrinogen + FXIII as compared to control (P < 0.001 at both temperatures) as well as compared to fibrinogen alone (P < 0.05 at both temperatures).
Platelet dependent clot strength (EX-FIBTEM-MCF)
The platelet dependent clot strength (EX-FIBTEM-MCF) was 72% and 76% of the EXTEM-MCF, at 37°C and 33°C respectively. Hypothermia at 33°C and haemodilution in combination decreased EX-FIBTEM-MCF, but hypothermia or haemodilution alone had no effect on EX-FIBTEM-MCF. HES and RAc affected EX-FIBTEM-MCF to the same extent (Figure 2). Addition of fibrinogen or fibrinogen + FXIII did not significantly change EX-FIBTEM-MCF with HES haemodilution, whereas EX-FIBTEM-MCF significantly decreased after adding fibrinogen + FXIII during RAc haemodilution (Figure 4).
Discussion
Principal findings
Our principal finding is that fibrinogen concentrate, with or without factor XIII concentrate, reduces dilutional coagulopathy irrespective of temperature (33° or 37°C) and that this effect was more pronounced with RAc than HES haemodilution. We also found that hypothermia affects coagulation more during haemodilution with HES than with RAc.
Hypothermia and haemodilution
The effects of cooling undiluted blood to 33°C on ROTEM parameters were small. Although significant impairments of clot initiation (CT) and propagation (CFT, AA) were seen, measures of clot strength (EXTEM-MCF and FIBTEM-MCF) were unaffected. These results are in line with results from other in vitro studies with VHA’s, which demonstrate mild to moderate hypothermia to decrease the rate of clot formation but not the clot strength [15,16], even though more severe hypothermia (<28°C) may decrease clot strength [17]. An in vitro study on the relative effects of hypothermia on platelets and plasma coagulation indicated an impaired platelet adhesion as the primary cause of coagulopathy during mild hypothermia [2]. This may explain our previous results with free oscillation rheometry (FOR), which measured a decreased clot velocity as well as clot strength during hypothermia [9]. ROTEM is possibly better at detecting plasma coagulation than platelet function whereas FOR is more sensitive to platelet function.
The observations that haemodilution impaired parameters reflecting both the rate of clot formation and clot strength, and that HES attenuated these parameters more than RAc, is in line with previous research [18,19]. Numerous studies with VHA’s have shown a progressive decrease of initiation, amplification and propagation of coagulation as well as clot strength, where clot strength was more affected by synthetic colloids than crystalloids [9,20,21]. Synthetic colloids, such as gelatine and HES, interfere with fibrin network structure and consequently decrease clot strength more than explained by dilution of plasma proteins alone [22]. In contrast, some investigations have shown that haemodilution up to 50% with isotonic saline may induce a hypercoagulable state [23]. This has been suggested to be caused by decreased plasma antithrombin levels [24]. However, these studies used native non-activated blood, which may explain the difference from our results [25].
Hypothermia in combination with haemodilution mainly affected ROTEM parameters measuring clot velocity. Thus, HES haemodilution interacted with hypothermia to further impair CFT and AA. This is in line with our previous study with free oscillation rheometry (FOR) [9], but few other studies have addressed the combined effect of haemodilution and hypothermia. A study from 1994 measuring activated partial thromboplastin time found additional but not synergistic effects of hypothermia and haemodilution with a crystalloid [5]. Possible explanations for the observed synergy between hypothermia and HES, may be that both hypothermia [2,26] and HES [27,28] have direct effects on platelets, and that also plasma coagulation activity is decreased during both hypothermia [2] and haemodilution [29]. Platelet activation is very important for the thrombin burst associated with propagation of coagulation, which may explain why the ROTEM parameters CFT and AA were particularly affected by the combination of HES-haemodilution and hypothermia.
Addition of fibrinogen with or without factor XIII
Our results imply that fibrinogen concentrate can be used to improve coagulation at 33°C. This was also suggested, albeit not statistically significantly, in our previous study with FOR [9]. The results from the present study also imply that a clot weakened by RAc is easier to improve than a clot weakened by HES, which corroborates results from previous studies [8,30,31]. Although fibrinogen also improved fibrinogen-dependent clot strength (FIBTEM-MCF) irrespective of fluid or temperature, this improvement was greater during RAc than HES haemodilution. The general view that hypothermia-induced coagulopathy is refractory to treatment with coagulation factor concentrates [32] is contradicted by our results, as well as by a previous study where fibrinogen concentrate increased whole blood coagulation at 32°C [33]. It is a principal finding of our study, that the clot stabilizing effect of fibrinogen concentrate on coagulopathy induced by RAc is as effective at 33°C as it is during normothermia. This implies that correction of hypothermia before substitution with fibrinogen concentrate is clinically not necessary.
Factor XIII improved fibrinogen’s effect on FIBTEM-MCF during RAc-haemodilution but not during HES haemodilution. In line with our study, several previous in vitro haemodilution studies on healthy volunteers have shown that although FXIII alone does not improve dilutional coagulopathy, it can enhance fibrinogen’s corrective effect [7-9,34]. These results contradict an in vitro study on blood from intensive care patients where supraphysiological doses of FXIII were used [35]. In this study, a high pre-existing concentration of fibrinogen in plasma may explain the corrective effect of FXIII alone. It is probably only worthwhile to administer factor XIII to correct a coagulopathy if normalizing plasma fibrinogen concentrations is cared for. Consequently, it may be of value to combine fibrinogen with factor XIII in order to improve clot stability during RAc-haemodilution.
Platelet dependent clot strength (EX-FIBTEM-MCF)
The derived parameter EX-FIBTEM-MCF is considered to reflect platelet dependent clot strength. EX-FIBTEM-MCF decreased both after haemodilution and mild hypothermia. This is in line with studies using the cone and platelet analyser, where haemodilution [18] and mild hypothermia [2] showed reduced platelet aggregation. In contrast, some studies have found hypothermia to increase platelet aggregation, as measured with impedance aggregometry [36,37]. Aggregation is a process where fibrinogen stick platelets to each other through the platelet receptor glycoprotein IIb/IIIa, and fibrinogen concentrate has previously been shown to counteract glycoprotein IIb/IIIa–directed platelet inhibition [38]. We therefore expected the hypothermia induced reduction of platelet dependent clot strenght to be reversed by fibrinogen concentrate, but instead, EX-FIBTEM-MCF decreased significantly after the addition of fibrinogen + FXIII concentrates to RAc diluted blood. A recent study found platelet aggregation to be reduced or unaffected by in vitro supplementation with fibrinogen concentrate, depending on which platelet activator being used [39]. However, we believe that the reduction of EX-FIBTEM-MCF should not be interpreted as a decreased platelet function but that the addition of fibrinogen + FXIII increased FIBTEM-MCF values more than EXTEM-MCF, and hence the so called platelet dependent clot strength (EX-FIBTEM-MCF) decreased regardless of actual platelet function. There are principally two possible explanations of this shortcoming of ROTEM: 1) the ROTEM instrument may be more sensitive to changes at low levels of clot strength (MCF) than at normal levels; or 2) platelets are not fully inhibited by the FIBTEM assay. ROTEM uses forced oscillation and an arbitrary clot strength scale with a theoretical maximum of 100 mm, which makes a limited sensitivity at the upper scale possible. Platelet inhibition with cytochalacin D has been shown to be improved if combined with abciximab, a fibrinogen receptor inhibitor [40]. In conclusion, we and others question the reliability of EX-FIBTEM-MCF as a platelet function parameter [41] and suggest that other methods may be more suitable.
Limitations
There are several methodological concerns when performing in vitro coagulation tests. For example, there is no activation of procoagulative or fibrinolytic responses secondary to tissue trauma [1]. Furthermore, shear strains applied with viscoelastic haemostatic assays such as ROTEM are substantially lower than those found in the human circulation, which may modify clot structure during measurements [42]. There are also concerns with in vitro haemodilution: acid–base regulation is not physiological and the decreased haematocrit may increase fibrin thread formation in the reaction chamber [43]. Therefore, we restricted dilution to 33%. Finally, there is much debate regarding the use of native whole blood versus citrated whole blood in VHAs. We used citrated blood, since it can be incubated and stored for up to four hours whereas native blood must be analysed within 4 minutes. However, incubation of citrated blood over 30 minutes has been shown to decrease thrombelastographic differences between native and citrate blood analyses [25].
Conclusion
In conclusion, our results show that fibrinogen with or without FXIII corrected in vitro dilutional coagulopathy also during hypothermia and that the corrective effects were weaker during haemodilution with HES, as compared to RAc. Factor XIII enhanced the corrective effects of fibrinogen, but only during RAc haemodilution. Finally, HES haemodilution interacted with hypothermia to impair coagulation.
Funding
Grants from Region Skåne (grant numbers: 194251, 356111) supported this work. CSL Behring supported with drugs for the in vitro study.
Abbreviations
- HES
Hydroxyethyl starch
- RAc
Ringer’s acetate
- ROTEM
Rotational thromboelastometry
- CT
Clotting time
- CFT
Clot formation time
- AA
Alpha angle
- MCF
Maximal clot formation
- VHA
Viscoelastic haemostatic assays
- FXIII
Factor XIII
- FOR
Free oscillation rheometry
Footnotes
Competing interests
U.S. received research grants from CSL Behring 2010 and lecture fees from CSL Behring 2014.
U.S. is a member of Guideline Committee for Critical Bleeding, Swedish Society of Thrombosis and Haemostasis, www.ssth.se.
None of the other authors has any competing interests to declare.
Authors’ contributions
DW: study design, volunteer recruitment, laboratory work, main responsible for manuscript preparation; ODT: manuscript preparation and linguistic correction; FN: statistical analysis: KO: manuscript preparation; US: study design, manuscript preparation and providing laboratory facilities. All the authors have approved the final manuscript for submission.
Contributor Information
Dag Winstedt, Email: dag.winstedt@med.lu.se.
Owain D Thomas, Email: odt@cantab.net.
Fredrik Nilsson, Email: fredrik.x.nilsson@skane.se.
Knut Olanders, Email: knut.olanders@skane.se.
Ulf Schött, Email: ulf.schott@skane.se.
References
- 1.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 2.Wolberg AS, Meng ZH, Monroe DM, 3rd, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221–1228. doi: 10.1097/01.TA.0000064328.97941.FC. [DOI] [PubMed] [Google Scholar]
- 3.Bolliger D, Gorlinger K, Tanaka KA. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology. 2010;113:1205–1219. doi: 10.1097/ALN.0b013e3181f22b5a. [DOI] [PubMed] [Google Scholar]
- 4.Darlington DN, Kremenevskiy I, Pusateri AE, Scherer MR, Fedyk CG, Kheirabaldi BS, Delgado AV, Dubick MA. Effects of In vitro hemodilution, hypothermia and rFVIIa addition on coagulation in human blood. Int J Burns Trauma. 2012;2:42–50. [PMC free article] [PubMed] [Google Scholar]
- 5.Gubler KD, Gentilello LM, Hassantash SA, Maier RV. The impact of hypothermia on dilutional coagulopathy. J Trauma. 1994;36:847–851. doi: 10.1097/00005373-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Fries D, Innerhofer P, Reif C, Streif W, Klingler A, Schobersberger W, Velik-Salchner C, Friesenecker B. The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg. 2006;102:347–351. doi: 10.1213/01.ane.0000194359.06286.d4. [DOI] [PubMed] [Google Scholar]
- 7.Haas T, Fries D, Velik-Salchner C, Reif C, Klingler A, Innerhofer P. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg. 2008;106:1360–1365. doi: 10.1213/01.ane.0b013e3181684339. [DOI] [PubMed] [Google Scholar]
- 8.Schlimp CJ, Cadamuro J, Solomon C, Redl H, Schochl H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, gelatine, hydroxyethyl starch or saline in vitro. Blood Transfus. 2013;11:510–517. doi: 10.2450/2012.0171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winstedt D, Tynngard N, Olanders K, Schott U: Free oscillation rheometry monitoring of haemodilution and hypothermia and correction with fibrinogen and factor XIII concentrates.Scand J Trauma Resusc Emerg Med 2013, 21:20. [DOI] [PMC free article] [PubMed]
- 10.The R Project for Statistical Computing [www.r-project.org]
- 11.Pinheiro J, Bates D, DebRoy S, Sarkar D: Linear and Nonlinear Mixed Effects Models.R Package 2013, Version 3.1
- 12.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 14.Lang T, Bauters A, Braun SL, Potzsch B, von Pape KW, Kolde HJ, Lakner M. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301–310. doi: 10.1097/01.mbc.0000169225.31173.19. [DOI] [PubMed] [Google Scholar]
- 15.Dirkmann D, Hanke AA, Gorlinger K, Peters J. Hypothermia and acidosis synergistically impair coagulation in human whole blood. Anesth Analg. 2008;106:1627–1632. doi: 10.1213/ane.0b013e31817340ad. [DOI] [PubMed] [Google Scholar]
- 16.Kettner SC, Sitzwohl C, Zimpfer M, Kozek SA, Holzer A, Spiss CK, Illievich UM. The effect of graded hypothermia (36 degrees C-32 degrees C) on hemostasis in anesthetized patients without surgical trauma. Anesth Analg. 2003;96:1772–1776. doi: 10.1213/01.ANE.0000062520.65192.C9. [DOI] [PubMed] [Google Scholar]
- 17.Rundgren M, Engstrom M. A thromboelastometric evaluation of the effects of hypothermia on the coagulation system. Anesth Analg. 2008;107:1465–1468. doi: 10.1213/ane.0b013e31817ee955. [DOI] [PubMed] [Google Scholar]
- 18.Caballo C, Escolar G, Diaz-Ricart M, Lopez-Vilchez I, Lozano M, Cid J, Pino M, Beltran J, Basora M, Pereira A, Galan AM. Impact of experimental haemodilution on platelet function, thrombin generation and clot firmness: effects of different coagulation factor concentrates. Blood Transfus. 2013;11:391–399. doi: 10.2450/2012.0034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen VG. Colloids decrease clot propagation and strength: role of factor XIII-fibrin polymer and thrombin-fibrinogen interactions. Acta Anaesthesiol Scand. 2005;49:1163–1171. doi: 10.1111/j.1399-6576.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 20.Casutt M, Kristoffy A, Schuepfer G, Spahn DR, Konrad C. Effects on coagulation of balanced (130/0.42) and non-balanced (130/0.4) hydroxyethyl starch or gelatin compared with balanced Ringer’s solution: an in vitro study using two different viscoelastic coagulation tests ROTEM and SONOCLOT. Br J Anaesth. 2010;105:273–281. doi: 10.1093/bja/aeq173. [DOI] [PubMed] [Google Scholar]
- 21.Hartog CS, Reuter D, Loesche W, Hofmann M, Reinhart K. Influence of hydroxyethyl starch (HES) 130/0.4 on hemostasis as measured by viscoelastic device analysis: a systematic review. Intensive Care Med. 2011;37:1725–1737. doi: 10.1007/s00134-011-2385-z. [DOI] [PubMed] [Google Scholar]
- 22.Weiss G, Lison S, Spannagl M, Heindl B. Expressiveness of global coagulation parameters in dilutional coagulopathy. Br J Anaesth. 2010;105:429–436. doi: 10.1093/bja/aeq199. [DOI] [PubMed] [Google Scholar]
- 23.Ruttmann TG, James MF, Viljoen JF. Haemodilution induces a hypercoagulable state. Br J Anaesth. 1996;76:412–414. doi: 10.1093/bja/76.3.412. [DOI] [PubMed] [Google Scholar]
- 24.Ruttmann TG, Jamest MF, Lombard EH. Haemodilution-induced enhancement of coagulation is attenuated in vitro by restoring antithrombin III to pre-dilution concentrations. Anaesth Intensive Care. 2001;29:489–493. doi: 10.1177/0310057X0102900507. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald SG, Luddington RJ. Critical factors contributing to the thromboelastography trace. Semin Thromb Hemost. 2010;36:712–722. doi: 10.1055/s-0030-1265288. [DOI] [PubMed] [Google Scholar]
- 26.Ng KF, Cheung CW, Lee Y, Leung SW. Low-dose desmopressin improves hypothermia-induced impairment of primary haemostasis in healthy volunteers. Anaesthesia. 2011;66:999–1005. doi: 10.1111/j.1365-2044.2011.06821.x. [DOI] [PubMed] [Google Scholar]
- 27.Franz A, Braunlich P, Gamsjager T, Felfernig M, Gustorff B, Kozek-Langenecker SA. The effects of hydroxyethyl starches of varying molecular weights on platelet function. Anesth Analg. 2001;92:1402–1407. doi: 10.1097/00000539-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Liu FC, Liao CH, Chang YW, Liou JT, Day YJ. Hydroxyethyl starch interferes with human blood ex vivo coagulation, platelet function and sedimentation. Acta Anaesthesiol Taiwan. 2009;47:71–78. doi: 10.1016/S1875-4597(09)60027-8. [DOI] [PubMed] [Google Scholar]
- 29.Petroianu GA, Maleck WH, Koetter KP, Liu J, Schmitt A. Effect of in vitro hemodilution with hydroxyethyl starch and dextran on the activity of plasma clotting factors. Crit Care Med. 2003;31:250–254. doi: 10.1097/00003246-200301000-00039. [DOI] [PubMed] [Google Scholar]
- 30.De Lorenzo C, Calatzis A, Welsch U, Heindl B. Fibrinogen concentrate reverses dilutional coagulopathy induced in vitro by saline but not by hydroxyethyl starch 6% Anesth Analg. 2006;102:1194–1200. doi: 10.1213/01.ane.0000200297.98089.ce. [DOI] [PubMed] [Google Scholar]
- 31.Winstedt D, Hanna J, Schott U. Albumin-induced coagulopathy is less severe and more effectively reversed with fibrinogen concentrate than is synthetic colloid-induced coagulopathy. Scand J Clin Lab Invest. 2013;73:161–169. doi: 10.3109/00365513.2012.762114. [DOI] [PubMed] [Google Scholar]
- 32.Krause KR, Howells GA, Buhs CL, Hernandez DA, Bair H, Schuster M, Bendick PJ. Hypothermia-induced coagulopathy during hemorrhagic shock. Am Surg. 2000;66:348–354. [PubMed] [Google Scholar]
- 33.Hanke AA, Dellweg C, Schochl H, Weber CF, Juttner B, Johanning K, Gorlinger K, Rahe-Meyer N, Kienbaum P. Potential of whole blood coagulation reconstitution by desmopressin and fibrinogen under conditions of hypothermia and acidosis–an in vitro study using rotation thrombelastometry. Scand J Clin Lab Invest. 2011;71:292–298. doi: 10.3109/00365513.2011.561870. [DOI] [PubMed] [Google Scholar]
- 34.Schramko AA, Kuitunen AH, Suojaranta-Ylinen RT, Niemi TT. Role of fibrinogen-, factor VIII- and XIII-mediated clot propagation in gelatin haemodilution. Acta Anaesthesiol Scand. 2009;53:731–735. doi: 10.1111/j.1399-6576.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 35.Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmness in Rotation Thromboelastometry (ROTEM) Thromb Haemost. 2010;104:385–391. doi: 10.1160/TH09-12-0858. [DOI] [PubMed] [Google Scholar]
- 36.Faraday N, Rosenfeld BA. In vitro hypothermia enhances platelet GPIIb-IIIa activation and P-selectin expression. Anesthesiology. 1998;88:1579–1585. doi: 10.1097/00000542-199806000-00022. [DOI] [PubMed] [Google Scholar]
- 37.Scharbert G, Kalb ML, Essmeister R, Kozek-Langenecker SA. Mild and moderate hypothermia increases platelet aggregation induced by various agonists: a whole blood in vitro study. Platelets. 2010;21:44–48. doi: 10.3109/09537100903420269. [DOI] [PubMed] [Google Scholar]
- 38.Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa receptor-directed platelet inhibition. Am Heart J. 2002;143:725–732. doi: 10.1067/mhj.2002.120299. [DOI] [PubMed] [Google Scholar]
- 39.Shams Hakimi C, Fagerberg Blixter I, Hansson EC, Hesse C, Wallen H, Jeppsson A. Effects of fibrinogen and platelet supplementation on clot formation and platelet aggregation in blood samples from cardiac surgery patients. Thromb Res. 2014;134:895–900. doi: 10.1016/j.thromres.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Lang T, Toller W, Gutl M, Mahla E, Metzler H, Rehak P, Marz W, Halwachs-Baumann G. Different effects of abciximab and cytochalasin D on clot strength in thrombelastography. J Thromb Haemost. 2004;2:147–153. doi: 10.1111/j.1538-7836.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 41.Thomas O, Gustafsson A, Schott U: Rotational thromboelastometry and multiple electrode platelet aggregometry in four patients with abnormal routine coagulation studies before removal of epidural catheters after major surgery: a case series and research study.J Med Case Reports 2013, 7:282. [DOI] [PMC free article] [PubMed]
- 42.Evans PA, Hawkins K, Lawrence M, Williams RL, Barrow MS, Thirumalai N, Williams PR. Rheometry and associated techniques for blood coagulation studies. Med Eng Phys. 2008;30:671–679. doi: 10.1016/j.medengphy.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa S, Szlam F, Bolliger D, Nishimura T, Chen EP, Tanaka KA. The impact of hematocrit on fibrin clot formation assessed by rotational thromboelastometry. Anesth Analg. 2012;115:16–21. doi: 10.1213/ANE.0b013e31824d523b. [DOI] [PubMed] [Google Scholar]


