Abstract
High-altitude natives are challenged by hypoxia and a potential compensation could be reduced blood P50, as seen in several high-altitude mammalian species. In 21 Qinghai Tibetan males and 9 Han Chinese, all resident at 4200 m, standard P50 was calculated from measurements of arterial PO2 and forehead oximeter oxygen saturation (SpO2), which was validated in a separate examination of 13 healthy sea-level subjects. In both Tibetans and Han Chinese, standard P50 was 24.5 (± 1.4 and 2.0 mmHg, respectively) and was lower than in the sea-level subjects (26.2 ± 0.6 mm Hg, p < 0.01). There was no relationship between P50 and [Hb] (the latter ranging from 15.2 and 22.9 g/dl in Tibetans). During peak exercise, P50 was not associated with alveolar-arterial PO2 difference or VO2/kg. There appears to be no apparent benefit of a lower P50 in this adult high-altitude Tibetan population.
Keywords: P50, hypoxia, altitude
1. Introduction
Hemoglobin (Hb) P50 is the PO2 at which Hb is 50% saturated with O2, and is commonly used to describe the position of the Hb-O2 dissociation curve. For a given hemoglobin type, blood P50 depends on pH, PCO2, temperature and the concentration of 2,3-diphosphoglycerate (DPG). This has led to definition of the standard P50 (about 27 mmHg in healthy subjects at sea level), as the P50 when pH is 7.40, the PCO2 is 40 mmHg, and temperature is 37 degrees centigrade (Lenfant et al., 1968).
Acute hypoxic exposure, as occurs during rapid ascent to altitude, causes a reduction in blood-O2 binding affinity (i.e. an increase in P50) due to elevated 2,3-DPG levels that occur over several hours and persist at least several weeks (Wagner et al., 2007). However, greater blood-O2 affinities have been documented in several vertebrate species that are native to high altitude (Monge & Leon-Velarde, 1991), and in some cases, this effect is due to genetically based increases in Hb-O2 affinity (i.e. deer mice (Storz et al., 2009; Storz et al., 2010; Natarajan et al., 2013) and hummingbirds (Projecto-Garcia et al., 2013)).
Human highland populations exhibit a wide range of standard P50. Sherpa P50 values (Samaja et al., 1979) have been reported as comparable to those of non-native acclimatized visitors of European descent (~28 ± mmHg) (Samaja et al., 1979; Wagner et al., 2007) with the exception of one report (22.6 ± 0.5 mmHg (Morpurgo et al., 1976)). Tibetan subjects examined at intermediate and high altitude exhibit considerable variation in standard P50 (Tashi et al., 2014) that is, on average, less than lowlander and Andean (Lenfant et al., 1968) values at high altitude. Considering previous studies have shown that average P50 differs among populations from the same continental region, it is important to note that such disparities may result from differences in equipment, environmental factors, techniques used for analysis (Winslow et al., 1985), or sub-population structure (Wuren et al., 2014). To our knowledge, variance in P50 has not been compared to variance in [Hb], nor has P50 been examined in relation to exercise capacity, or oxygen transport, in Tibetan inhabitants residing in the northeast region of the Qinghai-Tibetan Plateau. Some Tibetans in this region have adaptive genetic factors associated with reduced [Hb] (Beall et al., 2010; Simonson et al., 2010; Yi et al., 2010) in addition to several genetic regions yet to be examined in a physiological context. One such region contains the beta-hemoglobin gene cluster on chromosome 11, which exhibits an adaptive signal in this population (Simonson et al., 2010) and other Tibetan groups (Yi et al., 2010). These findings suggest adaptive genetic associations could underlie important changes related to Hb structure/function and/or expression at this locus that may affect P50.
A reduction in P50 has usually been regarded as beneficial to the high-altitude organism by enhancing the rate of diffusive equilibration of O2 in the pulmonary capillary with that in the alveolar gas in a hypoxic environment that is challenging to the pulmonary uptake of O2. A left-shifted blood-O2 dissociation curve better maintains the alveolar to capillary diffusion gradient as the red cell progress along the pulmonary capillary and capillary PO2 increases towards the alveolar value. It is therefore possible that Tibetan blood-O2 binding affinity has also adapted (increased) to compensate for reduced PO2 in the Tibetan native environment.
Therefore, the purpose of this study was to determine a) whether average P50 in a Tibetan population resident at 4200 m above sea level is lower than values reported in native lowlanders studied both at sea level and after acclimatization at altitude; and b) if so, is P50 reduced more in those Tibetans who also have lower [Hb]? We hypothesized that in Tibetans who have adapted with low [Hb], standard blood P50 would be lower, and thus blood-O2 affinity would indeed be higher, than in those with high [Hb].
Considering only a pulse oximeter was available for measuring O2 saturation in the remote Tibetan site, we were limited to these measurements of arterial saturation. Therefore, in sea-level subjects, we determined whether the same pulse oximeter was accurate in measuring arterial saturation compared to direct measurements with co-oximeter and also whether the range of arterial saturations observed in the Tibetan and Han Chinese subjects encompassed values low enough to accurately estimate P50.
2. Methods
2.1 Overview
The project was partly conducted in the remote Tibetan village of Maduo (altitude 4200 m). Twenty-one Tibetan males and nine Han Chinese males resident at 4200 m for at least two years were studied at this altitude (Simonson et al., 2012b) and analyzed to determine if [Hb] is related to P50. As a part of this study, arterial blood samples were collected at rest and during exercise. Hemoglobin P50 was determined using the approach described previously (Wagner et al., 2007) whereby blood gas values from all samples from any one subject were combined into one data set in which the P50 that best fit paired saturation and PO2 values of all samples in the set was determined by least squares minimization. In this approach, all PO2 values, which were measured at 37 degrees centigrade, were first corrected to pH 7.40 and PCO2 40 mmHg using equations contained within the Kelman subroutines that describe the O2 dissociation curve (Kelman, 1966, 1967), so that the outcome was the standard P50. The usual technique for measuring PO2, PCO2 and pH uses a blood gas electrode system, and this was available at the study site in Maduo (iSTAT Portable Clinical Analyzer, Abbott, USA). The usual technique for measuring O2 saturation in a blood sample, be it arterial or venous, uses a co-oximeter. Because P50 is often in the range of venous PO2, it has always been customary to include venous samples in the data set. However, a co-oximeter was unavailable in Maduo, and therefore only pulse oximetry could be used for measuring saturation, limiting the data to arterial saturation (during exercise, at altitude). Saturation was measured using a forehead sensor pulse oximeter (Nellcor N-395 Pulse Oximeter).
These limitations posed two questions: First, was the pulse oximeter sufficiently accurate in measuring arterial saturation, and second, was the degree of arterial desaturation observed in each subject sufficient to allow accurate determination of P50? To resolve these uncertainties, additional exercise studies were performed in San Diego (SD), California, USA, on return from Maduo using seven sea-level residents. Additionally, previously unused arterial blood gas data (unpublished data) from an exercise study conducted for other purposes in Athens, Greece, using six healthy cyclists were included with permission. Data from all sea-level subjects were collected and analyzed for the primary purpose of validating the use of arterial O2 saturation measurements from a forehead oximeter (rather than co-oximeter venous and arterial O2 saturation) to estimate P50.
2.2 Methods in subjects at 4200 m altitude in Tibet
Native highland Tibetan and Han Chinese male subjects residing in the village at 4200 m completed a health history questionnaire and physical examination; heart/lung disease, diabetes, anemia, or hypertension were used as exclusion criteria from the study. A total of 21 healthy Tibetan and nine Han males (23 ± 6 and 26 ± 9 years of age, respectively) agreed to volunteer and were included, and all subjects provided informed written consent per Qinghai Medical College and University of California San Diego (UCSD) ethical guidelines and studies were approved by both review boards. First, [Hb] was measured from peripheral venous blood (2 ml) with a Mindray Hematology Analyzer (BC-2300, Shenzhen, People’s Republic of China). EKG leads and a Nellcor forehead oximetry sensor were attached to measure heart rate and arterial O2 saturation. After locally anesthetizing the skin over the intended placement site, a 20-gauge catheter was placed into the radial artery of one wrist using sterile technique and secured to assure no bleeding and to minimize risk of infection.
Subjects were then seated on a cycle ergometer, a 2ml resting heparinized blood sample was drawn from the arterial catheter for blood gas analysis, and data from the forehead oximeter were recorded. Subjects were then asked to warm up by pedaling at light exercise breathing room air (at between 45 and 90 watts, according to individual subject preference). After two minutes, subjects were asked to pedal at a moderate level and when VO2 stabilized, an arterial blood sample (2ml) was collected while forehead saturation measurements were recorded. After two minutes at this level of exercise, subjects pedaled at maximal effort. Towards the end of this period, another 2ml arterial blood sample was drawn and forehead saturation recorded. Blood gas analysis measurements, which included PO2, PCO2, pH, and base deficit, were therefore recorded during rest, submaximal, and maximal exercise using an i-Stat Abbott blood gas analyzer at 37C. All data collected from rest to peak VO2 were used for analyses.
2.3 Methods in sea-level subjects
While the following technical experiments were secondary to the primary objective of assessing P50 in Tibetan and Han Chinese, they are now described in some detail because of the importance of method validation in Maduo.
We conducted analyses in lowlanders at sea level in order to determine: 1) whether arterial saturation measurements from the forehead oximeter used in Maduo correspond to those simultaneously collected from a radial arterial catheter and analyzed by a co-oximeter, and 2) whether arterial saturation measurements alone are low enough to accurately estimate P50 during exercise under hypoxic conditions, or whether both arterial and venous data are required for an accurate P50 estimate. Since arterial saturation was never lower than 70% in the Maduo Tibetans at maximal exercise, and previous validation of forehead oximetry fell within a comparable range (73-100%) (Yamaya et al., 2002), we used only those data in the saturation range of 70-100% for the forehead pulse oximeter comparison with co-oximetry in Caucasians at sea level.
The 13 subjects were divided in two cohorts. Group A was a group of six Greek cyclists (studied for other purposes in Athens, Greece), from whom samples of arterial and peripheral venous blood were available (and used with permission) during exercise in normoxia and hypoxia. The samples from these cyclists were collected following 5 minutes of rest, then 5, 3, and 2 minutes at 30%, 80%, and 100% maximal effort, each at FIO2s of 0.21, 0.15, and 0.12 O2, respectively, (with one hour rest between each FIO2). Using those samples, PO2, PCO2, pH, were determined by blood gas electrodes and saturation by co-oximetry. Group B was a group of seven American cyclists, studied in San Diego only for the purposes of the present project, in whom similar measurements including arterial blood gases were made both at rest and during exercise in normoxia and hypoxia (FIO2=0.12), with the addition of the very same Nellcor forehead pulse oximeters used in Maduo. Studies conducted in both Group A and B subjects were used for comparison of P50 estimates based on arterial and arterial plus venous O2 saturation measurements; cooximeter vs oximeter comparisons were conducted based on O2 saturation data collected in Group B. All studies were approved by the University of Athens and UCSD review boards.
Prior to the study, all subjects provided written informed consent, and were then screened to determine their maximal exercise capacity, which was used to set an appropriate exercise regime for light (20-30% of max) and moderate (50%-75% of max) exercise under varying inspired O2 conditions (12%, 15%, 21%) lasting no more than 15 minutes for the entire period of rest and exercise (1-3 minutes at each level of exercise). Catheters (20 gauge, 25 mm) were placed in one radial artery and one peripheral, superficial arm vein using local anesthetic and sterile technique as described above for Tibetan subjects. Once seated on the cycle ergometer and after four minutes of rest, 2 ml of blood was drawn from arterial and venous catheters. During each blood sample taken in group B subjects, data from two the Nellcor forehead oximeters (designated as A and B) were recorded.
2.4 Data analysis in sea-level subjects and estimated P50 in all groups
Correspondence between O2 saturation measurements from the two Nellcor forehead oximeters (labeled A and B) and from the paired blood samples analyzed by co-oximeter (IL model 682) was examined using Bland-Altman plots (Bland & Altman 1986) for the seven SD subjects (Figure 1). Adequacy of using only arterial saturation for P50 determination was determined in the 13 sea-level subjects by testing whether P50 derived from arterial saturation measurements alone (reflecting only saturations greater than 70%) differed from estimates derived from data sets containing both arterial and venous blood, which provided information both about the upper and lower regions of the curve (Figure 2).
Figure 1.
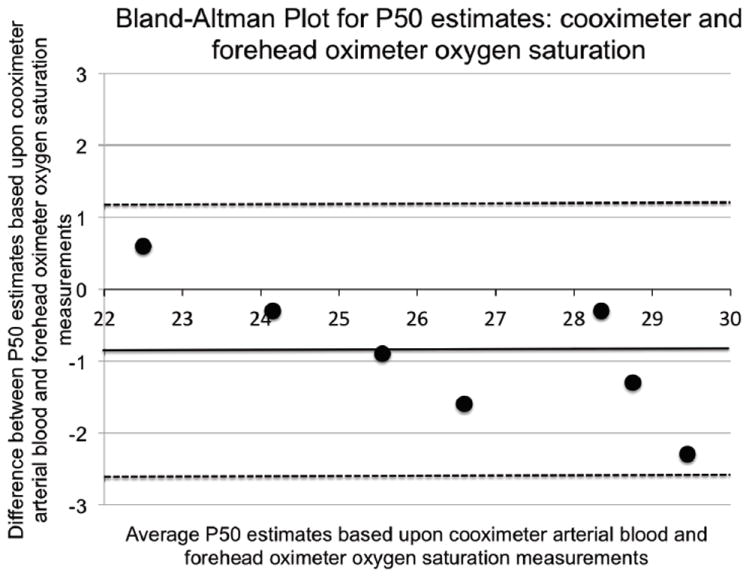
Bland-Altman plot comparing P50 estimates based on arterial blood cooximeter to forehead oximeter saturation measurements in seven sea level subjects (70-100% values, which are within the range examined in Tibetan and Han Chinese subjects examined at 4200 m, are included).Mean difference, standard deviation: (-0.87, 0.96); 95% confidence limits (-2.76, 1.01) are shown as dashed lines; a solid line indicates identity.
Figure 2.
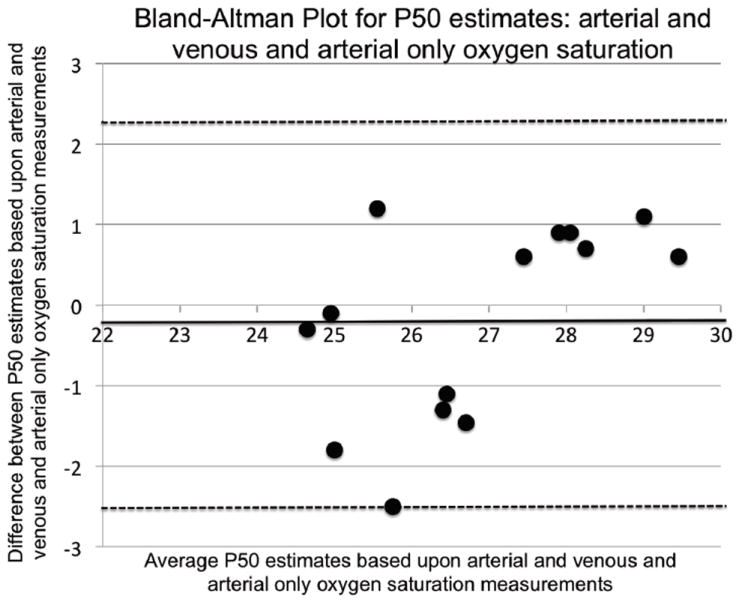
Bland-Altman plot comparing standard P50 calculated in 13 sea-level subjects based upon 1) the use of arterial plus venous (AV) saturation data to arterial (A) saturation data alone. Mean difference, standard deviation: (-0.2, 1.23). 95% confidence limits (-2.59, 2.23) are shown as dashed lines; a solid line indicates identity.
In order to determine the standard P50, we first computed “virtual PO2” from actual (measured) PO2 values, using computer algorithms published by Kelman (Kelman, 1966, 1967). Virtual PO2 is the PO2 that would exist after correcting for temperature, PCO2, and pH when any of these variables differed from standard conditions (37° C, 40 mmHg, and 7.4, respectively), as previously reported (Wagner et al., 2007). Temperature required no correction since the samples were measured in an analyzer at 37 degrees. Using a range of trial P50 values from 10 to 40 mmHg in 0.1 mmHg increments, each sample’s O2 saturation was then calculated from the virtual PO2 for each trial P50. The squared difference between calculated and measured saturations (from the co-oximeter for sea-level and forehead oximeter for highland resident subjects, respectively) was summed for each subject, and the trial P50 that resulted in the lowest sum of squares was determined as the standard P50 for that subject.
In vivo P50 during peak exercise was also estimated in Tibetans and Han Chinese by using Kelman routines with correction for PCO2 and pH from arterial blood gases; core temperature could not be measured, and was estimated at 37° C for all subjects as the short duration and limited power output during peak exercise (<2 minutes) would not be expected to affect core temperature beyond an average of 0.6° C (data from (Wagner et al., 1986; Wagner et al., 1987)).
Using all data points from the set of arterial and venous samples spanning from rest to maximal exercise and FIO2values of 0.21, 0.15 and 0.12 resulted in a set of data for each subject in which some points lay on the flat part of the blood-O2 dissociation curve (i.e., resting arterial data at FIO2=0.21) while others lay on the steep portion of the dissociation curve (venous data during hypoxic exercise). The non-linear nature of the blood-O2 dissociation curve means that a given error in measured saturation for a point on the flat part of the curve would result in a large error in estimated P50, while the same error in saturation for a point on the steep portion of the curve would give rise to a much smaller error in P50. We therefore excluded saturation limits greater than 95% when estimating P50.
2.5 Data analysis of subjects in Tibet
We also performed regression analysis of data from the 21 Tibetan subjects at maximal exercise to determine whether a relationship was present between a) P50 and [Hb], b) P50 and the alveolar-arterial PO2 difference (AaPO2), c) P50 and systemic O2 extraction, and d) P50 and peak VO2/kg in the 21 Tibetan subjects at maximal exercise. AaP O2 and systemic O2 extraction were determined using computer algorithms that model diffusive O2 uptake in the lungs and tissues, respectively, using a forward integration procedure (Wagner & West, 1972; Wagner, 1996). Mixed venous O2 content was determined by solving for this unknown variable in the Fick equation: VO2, cardiac output (PhysioFlow Enduro™, Paris, France, based on Signal-Morphology Impedance Cardiology), and arterial oxygen saturation/[Hb] were measured; the only remaining variables, venous SO2 and CvO2, were calculated, and PvO2 was determined from the dissociation curve for that SO2/CvO2 (base excess and O2-CO2 interactions taken into account by the same Kelman computer routines used to compute P50). The first comparison tests the hypothesis that as [Hb] is reduced in adapted Tibetans, P50 is also lower, as seen in many high-altitude native mammals. The second comparison tests the hypothesis that, if all other factors affecting pulmonary gas exchange were equal, AaPO2 should be less when P50 is lower, as explained in the introduction. The third comparison tests the hypothesis that, if all other factors affecting systemic O2 extraction were equal, extraction should be less when P50 is lower.
3. Results
The results will be presented in the following order: First, correspondence between the arterial saturation measured by pulse oximeter and that measured directly in arterial blood by co-oximeter will be shown for the seven sea-level subjects studied in San Diego. Second, the P50 values estimated from arterial data alone will be compared to those determined from both arterial and venous samples for the 13 sea-level subjects providing such data. Next, the values of the standard P50 will be shown for all three-subject groups: 13 sea-level subjects; 21 Tibetan natives at 4200 m; nine Han residents at 4200 m. Finally, in the cohort of 21 Tibetan and nine Han Chinese males at 4200 m, the relationships between P50 and [Hb], SaO2, AaPO2, systemic O2 extraction, and peak VO2/kg will be shown.
3.1 Results in sea-level subjects and estimated P50 in all groups
Overall, agreement is excellent for heart rate, which indicates appropriate acquisition of pulse-rate signal, and generally excellent for saturation as measurements below 70% were excluded from analysis (low saturations are commonly known to be less accurate) (Table 1). The two pulse oximeters agreed with each other more than either agreed with the co-oximeter, suggesting that pulse oximeter values were most different from those of the co-oximeter. Table 1 gives the mean values for saturation, heart rate, and P50 estimated from O2 saturation measurements for the three devices. Mean saturation values are significantly if slightly (< 1 %) higher for two pulse oximeters compared to the co-oximeter (p < 0.03, 0.59), and average heart rate measured by the two pulse oximeters was slightly lower (one beat/minute) than that indicated by EKG. Estimates of P50 using O2 saturation measurements from the cooximeter and each pulse oximeter were not different (p > 0.8 and 0.5 for co-oximeter versus oximeters A and B, respectively; Table 1) and fall within the range of acceptable limits based on Bland-Altman analysis (Figure 1). The standard P50 in sea-level (26.6 ± 2.2; n=13), Tibetan (24.5 ± 1.4; n=21), and Han Chinese (24.5 ± 2.0; n=9) subjects, the latter two groups both living and studied at 4200 m, were all estimates based on saturation measurements between 70% and 95%. Both Han and Tibetan values were significantly lower than those of the sea-level subjects, whose values correspond well with generally accepted sea-level norms.
Table 1.
Means and standard deviations of pulse oximeter and co-oximeter saturation measurements of percent SaO2, standard P50 based on each method for measuring O2 saturation, and pulse oximeter and EKG heart rate (HR) measurements in Caucasian subjects.
| Pulse Oximeter A | Pulse Oximeter B | Co-oximeter/EKG | |
|---|---|---|---|
| SaO2 (percent) | 91.5±8.5 | 91.0±9.1 | 90.8±8.2 |
| P50 (standard, mmHg) | 26.0±2.2 | 26.9±3.0 | 26.2±0.6 |
| Heart Rate (beats/minute) | 125.9±38.5 | 125.9±38.4 | 127.0±37.7 |
Figure 2 shows how different methods (with and without inclusion of venous saturation data) are reflected in P50 values obtained. As explained in the methods section, we compared estimates obtained from arterial (A) samples alone with those from both arterial and venous (AV) samples. The motivation for comparing AV and A data in Caucasian subjects was to determine whether just arterial saturation measurements during exercise under hypoxic conditions could be used to estimate P50 in contrast to needing both arterial and venous data, as the latter provide points at the lower end of the O2 dissociation curve. Figure 2 shows that either approach falls within acceptable limits based on Bland-Altman analysis. While the arterial values appear systematically greater than the arterial and venous combined values, the insignificant difference is less than 0.32 mmHg.
3.2 Results in subjects at high altitude
Figure 3 shows, over a wide range of Hb concentration, the lack of relationship between P50 and [Hb] (A) but a significant relationship between P50 and SaO2 (B) in the 21 Tibetans and nine Han Chinese. Figure 4 shows the relationships between in vivo P50 and gas exchange in the lungs as reflected by the alveolar-arterial PO2 difference (panel A), in the tissues as reflected by % O2 extraction (panel B), and in overall peak VO2/kg (panel C). There was no significant relationship between P50 and these variables at maximal exercise.
Figure 3.
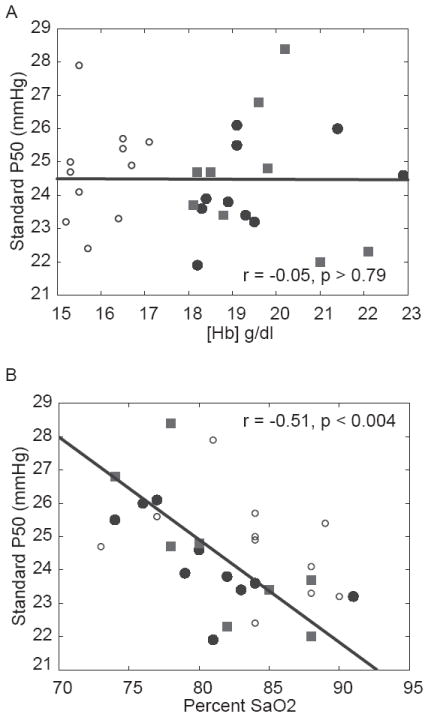
Data showing the relationship between P50 and [Hb] (A) and SaO2 during peak exercise (B). A) No relationship was found between [Hb] and P50. (B) Exercise SaO2 and P50 in Tibetan and Han Chinese subjects are significantly correlated. Han data are indicated by squares; low and high [Hb] in Tibetans indicated by open and closed circles, respectively.
Figure 4.
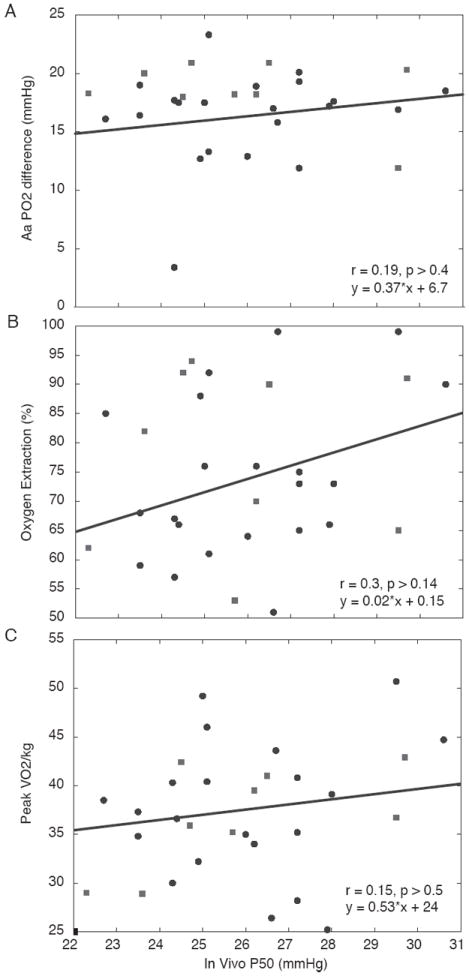
Data showing the relationship between P50 and Alveolar-arterial PO2 difference (A), O2 extraction (B), and peak VO2/kg (C). Han data are indicated by squares; Tibetan data are indicated by circles. A low in vivo P50 did not affect these variables over the P50 range encountered.
4. Discussion
4.1 Summary of main findings
There were four main observations made in the present study: First, standard P50 in native Qinghai Tibetans at 4200 m was 2 mmHg lower than normal sea level values, at 24.5 ± 1.4 mmHg; also, standard P50 in sea-level Han Chinese relocated to this altitude (resident approximately two years) was not different from Tibetan values at 24.5 ± 2.0 mmHg, while P50 in sea- level subjects, using the same technique applied in the altitude studies, was found to be 26.6 ± 2.2 mm Hg, well within the normal range. Second, arterial O2 saturation in Tibetans was higher when P50 was lower, but P50 was unrelated to [Hb], AaPO2, O2 extraction or exercise capacity. Third, determining P50 from arterial saturation values alone measured at rest and during exercise at altitude, spanning the saturation range down to 70% was found to yield a P50 value not different from that measured using both arterial and venous data (which bracket P50). Fourth, the very same pulse oximeters used in Maduo yielded arterial saturation values in San Diego that were very close to those measured simultaneously in radial arterial blood by co-oximetry.
4.2 Findings in Tibetans and Han Chinese
P50 in Han Chinese and Tibetans at 4200 m were similar and significantly lower than those in sea-level subjects. No difference in peak VO2/kg was observed between groups, consistent with some (Brutsaert 2008; Faoro et al., 2014) but not other studies (Ge et al., 1995; Sun et al., 1990) regarding aerobic capacity in different ethnic groups. The former findings do not exclude the possibility that individual P50 and VO2 values may be correlated. Additionally, while no group signal was detected in our data, this may be limited by a modest sample size.
At least two of the following criteria for peak VO2 were met for participants of the study: 1) heart rate ≥ age predicted (220 minus age) maximum (this is conservative, as maximal heart rate is generally reduced in chronic hypoxia); 2) respiratory exchange ratio > 1.10; 3) with increasing workload, there was no further increase (or decrease) in VO2; and 4) despite an increase in workload, there was no further increase in heart rate; 5) rest to peak exercise increase in base deficit of 6 mmol/liter or more. Overall, at exhaustion, average heart rate was 172/min; RER was 1.19; and base deficit was 13 mmol/l (rising from 4 mmol/l at rest). This was similar in both Tibetans and Han Chinese groups.
It is recognized that P50 increases to values in excess of 30 mmHg as sea-level residents acclimatize to high altitude, due to elevated 2,3-DPG levels (Wagner et al., 2007). The Han and Tibet data we report are thus not just considerably different (by 6 mmHg or more), but actually opposite in direction, from the acute and sub-acute responses seen in acclimatizing lowlanders (Wagner et al., 2007). Interestingly, it appears that native high-altitude Andeans at or above 3700m have increased P50 values, with values similar to those in acclimatized lowlanders (30.6 ± 0.7 mmHg) (Lenfant et al., 1968); Andeans also have not evolved a sea-level [Hb] phenotype like many Tibetans. This further underscores likely different evolutionary hypoxia-response pathways between Andeans and Tibetans.
The possible effect(s) of 2,3-DPG, ATP, intracellular Cl and Hb isoforms were not examined in this study, but warrant future investigation based on the findings presented here. In addition, the calculated SO2 values at higher PO2 may be slightly underestimated based on the O2 dissociation curve (Severinghaus, 1979) used in the Kelman subroutines thereby overestimating P50; in such case, the estimates of P50 shown here might be even lower than reported. That the sea-level subjects had normal standard P50 values of 26.6 mm Hg suggests this may be of minor significance in the present study.
These observations raise a number of questions. First, if Han and Tibetans have similar, lower P50 values and yet only Tibetans have adapted by reduced [Hb], does this suggest that the reduced P50 in Tibetans is not genetically related to the [Hb] adaptation? Second, why do Han and Tibetans exhibit a reduced P50 (compared to sea- level subjects) while high-altitude natives in South America have a P50 higher than that of sea-level subjects? Third, what is the functional importance of the P50 changes in any of these three groups compared to sea-level subjects? The latter is discussed in some detail in the next section.
Similarly reduced P50 in Tibetan and Han could be a result of convergent alteration(s) to this physiological outcome or shared common ancestry, although the reliability of P50 in nine Han Chinese will need to be explored in future studies with more subjects of this ethnicity. The human beta-globin gene cluster on chromosome 11 is a candidate gene identified in more than one genomic study of adaptation in Tibetans (reviewed in (Simonson et al., 2012a)). It is therefore plausible that genetic changes in protein-coding variants, such as those reported in high-altitude deer mice (Natarajan et al., 2013), and/or alterations in regulatory regions, which control Hb isoform levels that naturally vary among humans (Thein et al., 2009), may be associated with P50 in Tibetans. Whether this will account for the reduced P50, and whether similar genetic changes will be found in Han Chinese, both remain to be determined.
4.3 Significance: A) Relationship between P50 and SaO2 but not between P50 and [Hb]
Greater blood-O2 binding affinity is a very common, putatively adaptive mechanism well documented in native high-altitude species with genetic variants that underlie Hb-O2 binding affinity such as deer mice (Storz et al., 2009; Storz et al., 2010; Natarajan et al., 2013) and hummingbird (Projecto-Garcia et al., 2013), in addition to reports of increased affinity in Andean llama, vicuña (Hall et al., 1936), chinchillas and guinea pigs (Velarde et al., 1991), yak and pika on the Tibetan Plateau (Adams et al., 1975; Ge et al., 1998). Previous work indicates how low P50 at altitude may be beneficial at high altitude (Eaton et al., 1974; Hebbel et al., 1978). Considering P50 is, on average, lower in the Tibetan subjects examined here, it is conceivable that a reduction in P50 would be associated with reduced [Hb], a trait shown to be previously associated with adaptive genetic factors in this population. There was, however, no correlation between P50 and [Hb] in our Tibetan subjects or when Tibetan and Han Chinese data are analyzed together as a single group (Figure 3A). Since lower [Hb] was previously found to be associated with improved exercise capacity in the Tibetan subjects examined here (Simonson et al., 2012b), it is possible that in vivo P50, which is not associated with [Hb] nor peak VO2/kg as shown (Figure 4), has minor if any influence in the adult Tibetan males examined here; however, whether lower P50 results from genetically based changes in Tibetans remains undetermined.
P50 in Tibetan and Han Chinese subjects is, however, associated with SaO2 (Figure 3B). We considered three possible physiological explanations for the SaO2-P50 relationship. One is that subjects with low P50 ventilate more, but this was not the case, as ventilation did not correlate with P50 (data not shown). A second is that gas exchange is more efficient in subjects with a low P50, as assessed by the Alveolar-arterial (A-a) PO2 difference. This too was not found to be the case. The higher saturation at lower P50 is therefore likely the direct result of a left shift in the blood-O2 dissociation curve with similar PO2 values.
4.4 Significance: B) P50 and pulmonary gas exchange
Increased blood-O2 affinity has long been considered to enhance diffusive O2 loading in the pulmonary capillary, which is challenged in normal subjects at altitude because gas exchange takes place entirely on the steep part of the Hb-O2 curve (West, 2012). A left-shifted Hb-O2 dissociation curve allows more O2 molecules to move from alveolar gas to pulmonary capillary blood without as much increase in capillary PO2 as would occur with a right-shifted curve. This preserves the alveolar to capillary diffusion gradient for O2, and explains enhanced diffusive equilibration. Thus, a left shift should be of value in augmenting O2 transport at the reduced PIO2 at altitude, increasing arterial PO2 and reducing the alveolar-arterial PO2 difference (AaPO2).
However, in the present study, no relationship was found between either arterial PO2 or AaPO2 and P50 in the Tibetans, which was unexpected given the preceding logic. Prior findings in healthy humans during exercise at altitude have shown that the majority of the AaPO2 is due to failure of diffusion equilibration (Wagner et al., 1986; Wagner et al., 1987). The results of this study show that AaPO2 is unrelated to in vivo P50 (Figure 4A), implying that reduced P50 in Tibetans is of little significance in pulmonary gas exchange, whether in the context of VA/Q inequality or diffusion limitation.
We considered whether the benefit of a reduced P50 might not be seen over the relatively narrow range of the standard P50 (21.9-27.9 mmHg) and in vivo (22.3-30.6 mmHg) P50 values encountered. Note that in vivo P50 was greater than standard P50 because, during exercise, the effect of acidosis outweighs the influence of lowered PCO2, resulting in an overall right-shift of the blood-O2 dissociation curve. To evaluate the possibility of reduced P50 in either case, we used algorithms for computing the rise in PO2 along the pulmonary capillary (Wagner & West, 1972; West & Wagner, 1980). Using the average blood gas data measured at peak exercise in the Tibetans (Simonson et al., 2012b; Simonson et al., 2012c), we calculated the alveolar-arterial PO2 difference that would be expected as a function of P50 over a wide range, from 15 to 32 mmHg. Figure 5, upper panel, shows the outcome. In essence, the benefit of a reduced P50 at the intermediate altitude of the Tibetan subjects (4200 m), as reflected by a reduction in alveolar-arterial PO2 difference, is not seen across the range of both standard and in vivo P50 we encountered. It is only when P50 falls below about 20 mmHg that conventional logic applies and the alveolar-arterial PO2 difference starts to fall. In sum, the lower P50 appears to have little or no functional significance for pulmonary gas exchange during exercise in this adult population at this altitude.
Figure 5.
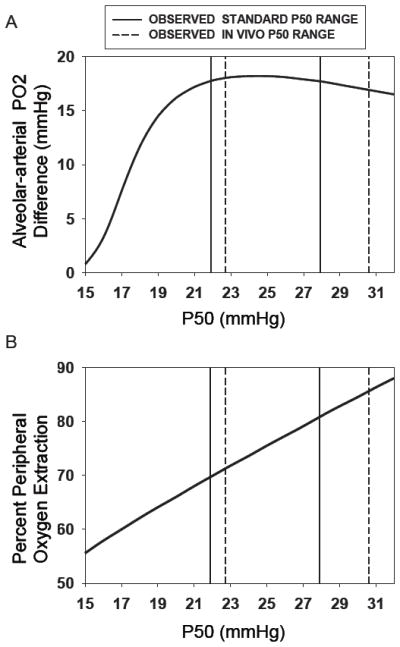
Theoretical calculations of the Alveolar-arterial PO2 difference (AaPO2) (upper panel) and peripheral O2 extraction (lower panel) during peak exercise as a function of standard and in vivo P50 (ranges indicated by solid and dashed lines, respectively). While extraction increases essentially linearly with P50 over a wide range, AaPO2 falls with P50 only when the latter is lower than about 20 mmHg. These outcomes help explain the observations in Figure 4.
4.5 Significance: C) P50 and systemic O2 extraction
Variation in in vivo P50 among Tibetan subjects appears not to be associated with systemic O2 extraction. However, reduction in P50 should interfere with O2 extraction, the standard explanation being as for the lungs (described above), but opposite in direction: Just as a lower P50 should enhance diffusive equilibration in the lungs, a higher P50 should enhance diffusive unloading of O2 in the muscles. This is because with a right-shifted blood-O2 dissociation curve, there can be greater movement of O2 across the muscle capillaries while maintaining a higher capillary PO2 and thus capillary to mitochondrial O2 diffusion gradient (compared to a left-shifted curve). Because of the findings in the upper panel of Figure 5, showing that P50 would not affect pulmonary gas exchange in the actual range encountered, we performed similar modeling of peripheral O2 extraction by diffusion again using the same algorithms and actual average blood gas data from the Tibetans at peak exercise. In this case (Figure 5, lower panel) the relationship between % O2 extraction and P50 was essentially linear, and similar to the findings shown in Figure 4B. Over the range of standard and in vivo P50 values (21.9-27.9 and 22.3-30.6 mmHg, respectively), the calculations in Figure 5 suggest extraction increasing by 10 percentage points from about 70% to about 80%.
The high variance and lack of relationship between P50 and extraction in Figure 4B are likely due to influences of additional factors important to extraction, such as blood flow rate and muscle O2 diffusional conductance. Feedback mechanisms, including tissue-specific changes in metabolism and pH, are also plausible contributors based on findings in other species (Storz & Moriyama, 2008) but have not been examined here. Other research in this population, focused specifically on high and low [Hb] in Tibetans and relationships to O2 transport ((Simonson et al., 2012b), unpublished data), indicates no significant difference in ventilation or blood gas measurements. The consequent effects, if any, on the overall transport and utilization of O2 would require analysis of several additional variables, which is beyond the scope of the work reported here. However, we determined there is no significant association between peak VO2/kg and in vivo P50 in high-altitude subjects examined (Figure 4C).
The wide range of P50 observed in Han Chinese and Tibetans may be attributed to various experimental and/or biological factors. We suspect that the amount of time participants spent at altitude did not contribute to this variation as Tibetan subjects were permanent residents and Han Chinese had been resident above 4000m for at least two years. Considering many adaptive factors have been reported in Tibetans, it is likely that different genetic loci and/or variants within the Hb genetic cluster may, at least in part, underlie this variation, perhaps “pulling” P50 across the wide range observed. Furthermore, [Hb] varies across a wide range, even though P50 and [Hb] are not correlated.
While the lower P50 does not play a major role during exercise at altitude, the context in which these adult subjects were examined, we speculate that it may be important at other stages such as fetal development, when O2-binding affinity is greatest (~19mmHg at sea level) to ensure in utero survival, or pertinent in neonatal development. In such cases, increased affinity may persist without rigid constraint into adulthood. It therefore remains to be determined if adaptive variants underlie the lower P50 observed in this population, whether such changes may be beneficial under different conditions (e.g. development) and/or are related to other adaptive changes that serve to compensate or coordinate hypoxia tolerance in these populations.
4.6 Methodological issues
Taken together, the above findings indicate that valid measurements of blood-P50 can be obtained at altitude in the absence of standard co-oximetry and in the absence of data at a low (<70%) saturation, using only arterial blood gases and pulse oximetry. We used a pulse oximeter that employs optodes laid against the forehead rather than the more common digital pulse oximeters. During peak exercise, our experience has been that fingertip oximeters may not always be reliable when subjects grip cycle handlebars tightly. This risk is compounded by exercise in a cold environment (the rooms in which we performed the studies in Maduo were unheated and at relatively low temperatures), potentially comprising digital perfusion and thus oximeter signals. Whether the methods we used would have produced similar results using more common fingertip oximeters was not studied and is thus unclear.
Because we did not have blood temperature, we modeled the effects of a 0.6° C increase in blood temperature, which is the average increase reported in previous studies under comparable conditions (Wagner et al., 1986; Wagner et al., 1987) (i.e., several minutes of exercise at 150-180 watts, which was the range observed in high-altitude subjects examined here). We found that a) AaPO2 was estimated to fall by just 0.1 mm Hg (from 18.6° C to 18.5° C as temperature was raised from 37° C to 37.6° C), and b) that O2 extraction was estimated to increase from 75.4 to 77.0% for the same 0.6 degree temperature increase. These are small effects, and indicate that the uncertainty in core temperature, which would affect in vivo but not standard P50 numbers, would not have changed the overall outcome or interpretation of the study.
4.7 Conclusions
High-altitude native Tibetans living at 4200 m have a standard P50 that is about 2 mmHg lower than that of sea-level natives, and perhaps as much as 6 mmHg lower than that of both Andean high-altitude natives and Caucasian sea-level natives acclimatized to high altitude. The reduction in P50 was found to be unrelated to [Hb], suggesting the absence of a link between [Hb] itself and blood P50. To our surprise, P50 in Han Chinese living at the same altitude was similarly reduced, further casting doubt that genetic adaptations associated with [Hb] are also related to P50. During maximal exercise at 4200 m, reduction in in vivo P50 does not appear to enhance pulmonary gas exchange, impair systemic O2 extraction, or influence peak VO2/kg. Thus, the low P50 was not found to confer any physiological advantage. Why Tibetans appear to be adapting to altitude by reducing [Hb] and not improving pulmonary gas exchange while Andeans appear to have evolved with distinct pulmonary adaptations (increased lung volumes and diffusing capacity) but remain polycythemic remains to be elucidated.
Table 2.
Hemoglobin concentration, percent oxygen saturation, blood gas variables (PaO2, PaCO2, and pH), and standard and in vivo P50 during peak exercise at 4200m in Tibetan and Han Chinese males. Hemoglobin concentration is significantly different in Tibetan and Han Chinese subjects (p < 0.05).
| Group Ethnicity | [Hb] (g/dl) | Arterial PO2 (mmHg) | Arterial PCO2 (mmHg) | Arterial pH | % SaO2 | Standard P50 (mmHg) | In vivo P50 (mmHg) |
|---|---|---|---|---|---|---|---|
| All subjects: Mean, Standard deviation | 18.2 ±2.1 | 45.7 ±4.6 | 26.0 ±2.6 | 7.31 ±0.05 | 82.0 ±5.0 | 24.5 ±1.6 | 26.0 ±2.1 |
| Tibetan: Mean, Standard deviation | 17.7 ±2.1 | 46.3 ±4.9 | 26.0 ±2.9 | 7.31 ±0.06 | 82.3 ±5.2 | 24.5 ±1.4 | 26.0 ±2.0 |
| Han Chinese: Mean, Standard deviation | 19.6 ±1.4 | 44.3 ±3.7 | 26.0 ±1.8 | 7.30 ±0.04 | 81.2 ±4.9 | 24.5 ±2.0 | 25.9 ±2.5 |
New Findings.
What is the central question of this study?
Is Tibetan and Chinese highlanders’ blood oxygen-binding affinity (P50) different from that of other populations (at altitude or sea level) and does Tibetan P50 relate to hemoglobin concentration and/or exercise capacity at altitude?
What is the main finding and its importance?
Tibetans and Chinese at 4200m have slightly lower P50 than sea-level residents.
During maximal exercise at 4200m, reduced P50 does not enhance pulmonary gas exchange, impair systemic oxygen extraction, or affect peak exercise capacity.
Oxygen saturation measurements based upon forehead oximetry are sufficiently reduced during exercise at altitude (and accurate compared to those obtained from arterial blood by co-oximetry) to reliably determine P50.
Acknowledgments
We thank all participants involved in the studies and Drs. Zhaxi Cairang, Zhou Maocu, Sangri Jiancuo, and Drorjii from Maduo Hospital.
Funding
This research is funded by NIH P01 HL0981830, and T32 HL098062, NIH K99 HL118215, and Parker B. Francis Fellowship to TSS, the National Basic Research Program of China 2012CB518200, Program of International S&T Cooperation of China 0S2012GR0195, National Natural Science Foundation of China 30393133.
References
- Adams WH, Graves IL, Pyakural S. Hematologic observations on the yak. Proc Soc Exp Biol Med. 1975;148:701–705. doi: 10.3181/00379727-148-38613. [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 2010;107:11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- Brutsaert TD. Do high-altitude natives have enhanced exercise performance at altitude? Appl Physiol Nutr Metab. 2008;33:582–592. doi: 10.1139/H08-009. [DOI] [PubMed] [Google Scholar]
- Eaton JW, Skelton TD, Berger E. Survival at extreme altitude: protective effect of increased hemoglobin-oxygen affinity. Science. 1974;183:743–744. doi: 10.1126/science.183.4126.743. [DOI] [PubMed] [Google Scholar]
- Faoro V, Huez S, Vanderpool R, Groepenhoff H, de Bisschop C, Martinot JB, Lamotte M, Pavelescu A, Guénard H, Naeije R. Pulmonary circulation and gas exchange at exercise in Sherpas at high altitude. J Appl Physiol. 2014;116(7):919–926. doi: 10.1152/japplphysiol.00236.2013. [DOI] [PubMed] [Google Scholar]
- Ge RL, He Lun GW, Chen QH, Li HL, Gen D, Kubo K, Matsuzawa Y, Fujimoto K, Yoshimura K, Takeoka M, Kobayashi T. Wilderness Environ Med. 1995;6:391–400. [Google Scholar]
- Ge RL, Kubo K, Kobayashi T, Sekiguchi M, Honda T. Blunted hypoxic pulmonary vasoconstrictive response in the rodent Ochotona curzoniae (pika) at high altitude. Am J Physiol. 1998;274:H1792–1799. doi: 10.1152/ajpheart.1998.274.5.H1792. [DOI] [PubMed] [Google Scholar]
- Hall FG, Dill DB, Guzman Barron ES. Comparative physiology in high altitudes. Journal of Cellular and Comparative Physiology. 1936;8:301–313. [Google Scholar]
- Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG, Berger EM. Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest. 1978;62:593–600. doi: 10.1172/JCI109165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol. 1966;21:1375–1376. doi: 10.1152/jappl.1966.21.4.1375. [DOI] [PubMed] [Google Scholar]
- Kelman GR. Digital computer procedure for the conversion of PCO2 into blood CO2 content. Respir Physiol. 1967;3:111–115. doi: 10.1016/0034-5687(67)90028-x. [DOI] [PubMed] [Google Scholar]
- Lenfant C, Torrance J, English E, Finch CA, Reynafarje C, Ramos J, Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest. 1968;47:2652–2656. doi: 10.1172/JCI105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge C, Leon-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev. 1991;71:1135–1172. doi: 10.1152/physrev.1991.71.4.1135. [DOI] [PubMed] [Google Scholar]
- Morpurgo G, Arese P, Bosia A, Pescarmona GP, Luzzana M, Modiano G, Krishna ranjit S. Sherpas living permanently at high altitutde: a new pattern of adaptation. Proc Natl Acad Sci U S A. 1976;73:747–751. doi: 10.1073/pnas.73.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF. Epistasis among adaptive mutations in deer mouse hemoglobin. Science. 2013;340:1324–1327. doi: 10.1126/science.1236862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projecto-Garcia J, Natarajan C, Moriyama H, Weber RE, Fago A, Cheviron ZA, Dudley R, McGuire JA, Witt CC, Storz JF. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc Natl Acad Sci U S A. 2013;110:20669–20674. doi: 10.1073/pnas.1315456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaja M, Veicsteinas A, Cerretelli P. Oxygen affinity of blood in altitude Sherpas. J Appl Physiol. 1979;47:337–341. doi: 10.1152/jappl.1979.47.2.337. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- Simonson TS, McClain DA, Jorde LB, Prchal JT. Genetic determinants of Tibetan high-altitude adaptation. Hum Genet. 2012a;131:527–533. doi: 10.1007/s00439-011-1109-3. [DOI] [PubMed] [Google Scholar]
- Simonson TS, Wei G, Wagner H, Wuren T, Yan M, Qin G, Ge RL, Wagner PD. Experimental Biology. San Diego, California, USA: 2012b. Exercise capacity and oxygen transport in native Tibetan highlanders with high-compared to low-hemoglobin concentration. [Google Scholar]
- Simonson TS, Wei G, Wagner H, Wuren T, Yan M, Qin G, Wagner PD, Ge RL. Experimental Biology. San Diego, California, USA: 2012c. Similar peak VO2 at altitude in native lowland (Han Chinese) and Tibetan highland inhabitants despite different hemoglobin concentration. [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- Storz JF, Moriyama H. Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt Med Biol. 2008;9:148–157. doi: 10.1089/ham.2007.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 2010;213:2565–2574. doi: 10.1242/jeb.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci U S A. 2009;106:14450–14455. doi: 10.1073/pnas.0905224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SF, Droma TS, Zhang JG, Tao JX, Huang SY, McCullough RG, McCullough RE, Reeves CV, Reeves JT, Moore LG. Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Respir Physiol. 1990;79(2):151–161. doi: 10.1016/0034-5687(90)90015-q. [DOI] [PubMed] [Google Scholar]
- Tashi T, Feng T, Koul P, Amaru R, Hussey D, Lorenzo FR, Ge RL, Prchal JT. High altitude genetic adaptation in Tibetans: No role of increased hemoglobin-oxygen affinity. Blood Cells Mol Dis. 2014;53:27–29. doi: 10.1016/j.bcmd.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18:216–223. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde FL, Espinoza D, Monge C, de Muizon C. A genetic response to high altitude hypoxia: high hemoglobin-oxygen affinity in chicken (Gallus gallus) from the Peruvian Andes. C R Acad Sci III. 1991;313:401–406. [PubMed] [Google Scholar]
- Wagner PD. A theoretical analysis of factors determining VO2 MAX at sea level and altitude. Respir Physiol. 1996;106:329–343. doi: 10.1016/s0034-5687(96)00086-2. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Gale GE, Moon RE, Torrebueno JR, Stolp BW, Saltzman HA. Pulmonary Gas-Exchange in Humans Exercising at Sea-Level and Simulated Altitude. J Appl Physiol. 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Sutton JR, Reeves JT, Cymerman A, Groves BM, Malconian MK. Operation Everest II: pulmonary gas exchange during a simulated ascent of Mt. Everest. J Appl Physiol. 1987;63:2348–2359. doi: 10.1152/jappl.1987.63.6.2348. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Wagner HE, Groves BM, Cymerman A, Houston CS. Hemoglobin P(50) during a simulated ascent of Mt. Everest, Operation Everest II. High Alt Med Biol. 2007;8:32–42. doi: 10.1089/ham.2006.1049. [DOI] [PubMed] [Google Scholar]
- Wagner PD, West JB. Effects of diffusion impairment on O 2 and CO 2 time courses in pulmonary capillaries. J Appl Physiol. 1972;33:62–71. doi: 10.1152/jappl.1972.33.1.62. [DOI] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology The Essentials. Lippincott Williams & Wilkins, a Wolters Kluwer business; Baltimore, MD: 2012. [Google Scholar]
- West JB, Wagner PD. Predicted gas exchange on the summit of Mt. Everest. Respir Physiol. 1980;42:1–16. doi: 10.1016/0034-5687(80)90100-0. [DOI] [PubMed] [Google Scholar]
- Winslow RM, Monge C, Winslow NJ, Gibson CG, Whittembury J. Normal whole blood Bohr effect in Peruvian natives of high altitude. Respir Physiol. 1985;61:197–208. doi: 10.1016/0034-5687(85)90126-4. [DOI] [PubMed] [Google Scholar]
- Wuren T, Simonson TS, Qin G, Xing J, Huff CD, Witherspoon DJ, Jorde LB, Ge RL. Shared and unique signals of high-altitude adaptation in geographically distinct Tibetan populations. PLoS One. 2014;9:e88252. doi: 10.1371/journal.pone.0088252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya Y, Bogaard HJ, Wagner PD, Niizeki K, Hopkins SR. Validity of pulse oximetry during maximal exercise in normoxia, hypoxia, and hyperoxia. J Appl Physiol. 2002;92:162–168. doi: 10.1152/japplphysiol.00409.2001. [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J, Wang J. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]


