Abstract
Somatoform pain is a highly prevalent, debilitating condition and a tremendous public health problem. Effective treatments for somatoform pain are urgently needed. The etiology of this condition is, however, still unknown. On the basis of a review of recent basic and clinical research, we propose one potential mechanisms of symptom formation in somatoform pain and a developmental theory of its pathogenesis. The emerging evidence from animal and human studies in developmental neurobiology, cognitive-affective neuroscience, psychoneuroimmunology, genetics, epigenetics, and clinical and treatment studies of somatoform pain all point to the existence of a shared physical and social pain neural system. Research findings also show that non-optimal early experiences interact with genetic predispositions to influence the development of this shared system and ability to regulate it in an effective way. Interpersonal affect regulation between infant and caregiver is crucial for the optimal development of these brain circuits. The aberrant development of this shared neural system during infancy, childhood and adolescence, therefore, may ultimately lead to an increased sensitivity to physical and social pain and to problems with their regulation in adulthood. The authors critically review translational research findings that support this theory and discuss its clinical and research implications. Specifically, the proposed theory and reviewed research suggest that psychotherapeutic and/or pharmacologic interventions that foster the development of affect regulation capacities in an interpersonal context will also serve to more effectively modulate aberrantly activated neural pain circuits and thus be of particular benefit in the treatment of somatoform pain.
Keywords: Somatoform Pain, Pain Disorder, Somatization, Developmental Neuroscience, Interpersonal Affect Regulation, Translational Research
Introduction
Somatoform Pain (SP) is one of the primary symptoms of somatization spectrum disorders (SSD)(1) - ‘a tendency to experience and communicate somatic distress in response to psychosocial stress.’ These disorders are highly prevalent, debilitating, and challenging to treat. SSD prevalence rates vary depending on the number of medically unexplained symptoms included in the diagnosis, reaching up to 17% of the general population and accounting for nearly 25% of all visits to primary care clinics (1-5). SSD leads to high levels of disability (6) and excessive and ineffective use of health care (7). SSD costs an estimated $256 billion annually in the U.S., an amount nearly double the annual $132 billion cost of diabetes (8). The extant treatments are only moderately effective and/or not well validated. The quest for development of effective treatments and prevention would be most efficient if it were based on a comprehensive understanding of the causes of this condition. Its etiology, however, is still unknown. The purpose of this review is to present a developmental theory of pathogenesis of somatoform pain based on an integration of research findings from clinical and basic sciences.
The nosology of SP is complex and evolving, which reflects continuous debate on diagnostic classification of SSDs in general. In the DSM-IV, three disorders include SP: Somatization disorder, Pain disorder, and Undifferentiated somatoform disorder. This classification is, however, widely challenged (9-12) and in the DSM-5 proposal SP would move into a new more comprehensive diagnostic category “Complex Somatic Symptom Disorder,” with the subtype modifier “with predominant pain.” SP is the most common symptom in the research categories of Abridged Somatoform disorder, Multisomatoform disorder, and Medically Unexplained Symptoms (9, 10). In non-psychiatric medical offices many SP patients are diagnosed with “functional syndromes” (e.g. Irritable Bowel Syndrome, Fibromyalgia). SP may overlap significantly with these functional syndromes as both include pain symptoms. However, unlike SP, these syndromes do not require the criterion that “psychological factors play a major role in the onset or maintenance of pain.” Therefore, a subgroup of patients with functional syndromes may suffer from SP. Because the focus of this review is SP, we will only review studies of functional syndromes (e.g. fibromyalgia) that address psychological factors, therefore making those studies specifically relevant for SP. SP can also exacerbate an existing medical condition or be comorbid with other psychiatric disorders (e.g., depression, anxiety, and hypochondriasis). The validity of different diagnostic nosologies for SSDs is, however, beyond the scope of this review. Herein we focus specifically on somatoform pain and the potential mechanism of formation of this symptom.
Developmental Theory of Somatoform Pain
Several basic assumptions are at the foundation of this proposed developmental theory. First, Cartesian mind-body dualism is replaced by a postulation that ‘mind’ and ‘body’ are not distinct entities, but rather different levels of inquiry about the human condition. Going beyond the notion of ‘mind-body connection,’ we assume that: (a) any psychological process is biological (e.g., the subjective feeling of being in love involves changes in the brain and body), and (b) any biological process is experienced by a person subjectively, with or without that person's conscious awareness, and can be influenced by this subjective experience (e.g., a patient with depression may have longer recovery after a routine surgery due to an altered post-surgical immune response modulated by the patient's depressed state). It is by the integration of multiple levels of inquiry that a more comprehensive understanding of the etiology of a clinical problem is established (13). Second, somatization is not itself a disorder. Rather, it is one of many natural ways people experience and communicate distress. Moreover, somatization is a developmentally appropriate response to stress in infants and children that diminishes with maturation as more mature capacities for distress- and affect-regulation are developed. The extent to which somatic reactions become chronic and distressing varies on a continuum. Herein we focus on somatic reactions to stress that become overwhelming or severely impair a person's functioning, warranting clinical intervention. Third, the same presenting symptoms can have various etiological mechanisms; therefore, the proposed theory may be applicable to some but not all manifestations of somatoform pain.
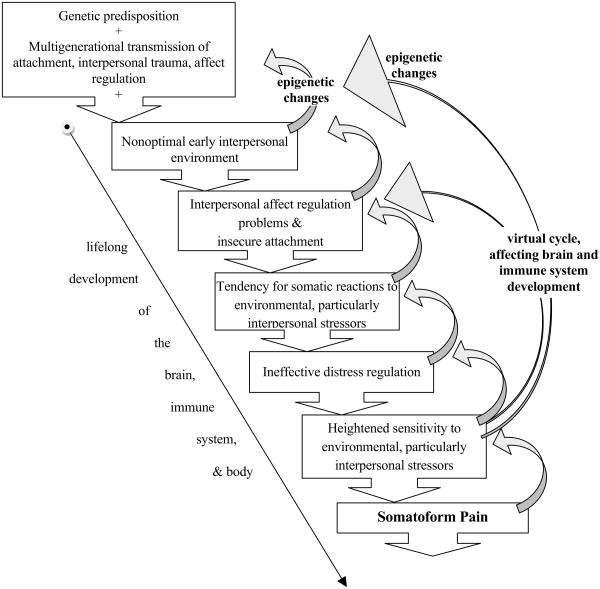
The essence of this developmental theory of SP is that suboptimal early interpersonal experiences with caregivers may interact with one's genetic predisposition, leading to disrupted maturation of neural circuits involved in affect regulation and interpersonal functioning, yielding the persistence into adulthood of developmentally earlier tendencies to experience distress somatically. Recent research has shown that affects associated with interpersonal distress and attachment share neurological substrates with somatic pain (14). Therefore, the interplay of adverse early experience and a genetic predisposition may influence the development of this shared neural system, resulting in increased susceptibility to SP in adulthood due to altered neural dynamics of somatic distress, interpersonal distress, and affect regulation (Figure 1).
Figure 1.
Developmental theory of somatoform pain.
In prenatal and early postnatal life, distress and excitement are experienced primarily somatically, as higher-order affect regulation and cognition are not yet developed. The mother (or primary caregiver) reduces the infant's distress by attending to the infant's needs, either eliminating the source of distress (e.g., feeding) or helping the infant regulate the distress (e.g., holding and caressing a baby whose stomach hurts). The distress at this stage of development is regulated primarily via interpersonal interactions. These early interactions lay the foundation for the ways in which the infant will regulate its distress in the future.
During optimal early infant development, the mother/caregiver is sensitively attuned to the baby's physical and emotional needs. As the infant develops, its mother gradually increases the separation, allowing the maturing infant to learn to regulate its own distress while providing the needed care so that the infant is not overwhelmed with unmet needs or excessive stimulation. The infant's affective reactions gradually evolve from the primarily somatic to more complex experiences of emotion and their regulation, including cross-modal symbolization of affect (e.g., responding to a soothing song), developing an internal representation of the caregiver (e.g., a memory of mom when she is not in sight), developing a notion of object constancy (e.g., the anticipation that the caregiver will attend to the baby's needs), using a toy or a transitional object for comfort (e.g., “security blanket”), and, later in development, expressing feelings in words, play, or fantasy, and making more appropriate attributions regarding inner emotional states. Caregivers support these growing capacities in the infant in various ways, including the sharing and mirroring of affect, which contributes to the development of self- and other- representations -- the building blocks of affect regulatory capacities.
However, if the infant is overwhelmed by unmet needs or excessive stimulation, if the caregiver does not effectively help the infant learn to regulate its affect, or if constitutional predispositions interfere with the learning of self-regulatory strategies, the child may continue to experience and express emotional distress somatically. Similarly, overprotection or problems with proper caregiver-infant separation (e.g., due to caregiver's own needs for closeness or dependency) may compromise the development of the baby's capacities to self-regulate affect.
Moreover, a child-parent interaction is a mutually regulated system. Unable to express emotions to others in a non-somatic way (through play, gestures, or linguistic expression of affect), a child may often fail to elicit emotional support, attention, and understanding from caregivers that support the further development of more mature ways of self-regulating emotional distress. An absence of the desired response from others, in turn, may be perceived by the child as evidence that others are not able to respond in the desired way and/or do not sufficiently care about him or her, which may diminish the child's openness to expressing affect, thus creating a vicious cycle of unexpressed, unregulated affect, increased emotional distress, increased likelihood of somatic expression of that distress, further emotional withdrawal of caregivers, all leading to an even greater emotional distress in the child—a learning experience, which inevitably leads the child to be less and less likely to express emotions in non-somatic ways.
These compromised interpersonal interactions are usually not isolated events, but ongoing characteristics of a child's environment, affecting the development of brain circuits, the immune system, and other bodily systems (Figure 1). Difficulties in coping with interpersonal stressors in infancy, childhood, and adolescence therefore likely alter development of the brain (15, 16) and may perpetuate and accentuate the tendency to experience emotional distress somatically. As adults, people with predominantly somatic ways of expressing distress may have problems establishing and maintaining relationships, further exacerbating interpersonal affect regulation problems, poor social support, and loneliness. Ironically, SP patients often seek help for somatic symptoms from their medical doctors, who are unable to provide pain relief using current medical treatments. Often doctor's failure to help is perceived by SP patients as yet another interpersonal rejection, further perpetuating the cycle of distress and pain. This theory of pathogenesis and symptom formation suggests that treatment of SP should focus on helping patients develop more mature ways of regulating affect within an interpersonal environment. The developmental origins of SP have been suggested by many clinicians (17). Here we present recent translational research findings that provide evidence in support of this theory and offer a neurocircuitry based understanding of one of the potential mechanisms of symptom formation mechanisms in SP.
Multidisciplinary Research Findings
Clinical Research
Evidence from clinical research suggests that SP patients tend to have a high prevalence of insecure attachment, interpersonal problems, and affect regulation difficulties; that many of them grow up in a non-optimal interpersonal environment; and that their somatic symptoms are often activated and maintained by interpersonal distress.
Early Childhood Experiences
A history of non-optimal early childhood care or trauma is common among SSD patients: early childhood caregivers of SSD patients were reported to be unavailable or providing less maternal care (18), were more likely to have long-term disability (19), or to be more punitive and rejecting (20) than caregivers of control subjects. SP and fibromyalgia patients report more sexual and physical abuse, emotional misattunement, lack of physical affection, separations and substance abuse in parents than medically explained pain patients (21). SSD inpatients reported high rates of loss of a parent or a caretaker before age 17 (22). SSD outpatients had more stress factors associated with changes in interpersonal relationships and the death or disease of close relatives compared to non-SSD patients (23). In a study of 515 traumatized people, those who survived interpersonal traumas (e.g., loss or abuse) had significantly more somatic symptoms than did the victims of disasters (e.g., earthquake or fire) (24). Recent studies suggest that the parental style (e.g., rejection, hostility, emotional unavailability) rather than abuse per se is associated with SSD (25, 26).
Affect Regulation
Somatization is conceptualized by many as a disorder of affect regulation largely related to patients' marked difficulties with awareness and expression of emotions (27). A strong association between somatization and alexithymia was reported in numerous studies of both clinical and nonclinical populations (28, 29), and among patients with SP specifically (30-32).
Alexithymia, in turn, is strongly associated with interpersonal difficulties, insecure attachment, and problems trusting others (33-36). A longitudinal study of 42 infants using the strange situation paradigm (an observational method to assess attachment in infancy) showed that insecurely attached infants were more likely to have a failure or significant delay in the acquisition of verbal expression of internal states and verbal expression of emotions later in childhood (37). Among 149 inpatients, alexithymic patients presented with a more avoidant social interaction style and more insecure attachment than did non-alexithymic patients (38). In nonclinical samples, alexithymia was associated with a fearful and preoccupied attachment style and with the number of reported somatic symptoms (39) and with mistrust, discomfort with closeness, and need for acceptance by others (36). Alexithymia is also associated with a history of interpersonal trauma, such as a history of childhood emotional, physical, and sexual abuse (40), or a history of maternal abuse and paternal indifference (34) among patients with chronic pain syndromes. In a sample of 3733 children, SP was strongly associated with affect-regulation related psychopathology (e.g. depression, or anxiety) (41).
Deficits in mentalization - or theory-of mind functioning- may also contribute to problems regulating one's own affect and interpersonal relationships. Results of a recent study among psychiatric inpatients which examined both deficits of emotional awareness in an interpersonal context and deficits in mentalization suggested that measures of the levels of emotional awareness (assessed from blindly rated patient narratives) combined with measures of theory-of-mind functioning allow correct diagnostic classification of 80% of SSD patients (42).
Attachment and Interpersonal Functioning in SP
A review of human and animal research from 1966 to 2000 on the association of attachment and interpersonal problems with somatic distress or disease suggested that insecure attachment contributes to maladaptive regulation of stress and affect, which, in turn, leads to somatic expression of the distress (43). Additionally, in several large studies of primary care patients, preoccupied and fearful attachment styles were significantly positively correlated with increased reporting of somatic symptoms (44), and patients with medically unexplained symptoms were significantly more likely to have insecure attachment than were participants with medically explained symptoms (45). In a community sample of 1,997 adults, anxious attachment style (especially distrust and a fear of loss) was associated with the highest levels of reported somatic symptoms (46). Similarly, in a community sample of 101 couples, insecure attachment style and childhood traumas were strongly associated with somatization (47). Insecure attachment style may actually exacerbate somatization in that patients who anticipate that other people will be rejecting and hurtful, may eventually elicit such behavior from others which, in turn, confirms fears of rejection and perpetuates the vicious cycle (48).
Well-validated interviews and observer-rated measures that bypass the limitations inherent in self-report instruments provide important evidence for these observations. For example, the Adult Attachment Interview revealed a higher prevalence of insecure attachment in SSD patients compared with healthy controls (49). Especially important for the developmental theory proposed here, are the results of a longitudinal study of 87 children observed between the ages of 11 months and 9 years, showing that attachment style (assessed using the “Strange Situation Paradigm” at 15 months of age) was a strong predictor of somatic complaints such as headaches, stomachaches, and eating problems in middle childhood (50). Attachment representations assessed using blind scorings of the Rorschach test revealed predominantly an avoidant interaction pattern in 85% of somatoform patients compared with 1% of psychotic patients; this avoidant interaction pattern is characteristic of people who recall their parents as rejecting their affective expressions of a desire for closeness (51). A study of the internal representations of relationships revealed that 90% of SP patients compared with only 10% of healthy controls had an unfulfilled desire for interpersonal closeness combined with a fear of being rejected, hurt or abandoned as their primary representation of relationships (32). Of particular note is a longitudinal study of SSD patients which showed that their somatic distress correlated highly with weekly exacerbations of relational problems (52). A study of 127 primary care patients with medically unexplained symptoms and their significant others demonstrated an association between interpersonal context and somatic distress (53).
Developmental Neuroscience-Animal Models
While human studies suggest a link between disruptions in early caretaking and the development of insecure attachment and SP, animal studies provide the opportunity to test these hypotheses directly by experimentally manipulating early life experiences to examine their effects on later development (54). Beginning with Harlow's demonstration that interactions with a caregiver (and not just protection and nutrient supply) are essential for survival of an infant monkey (55), numerous animal studies confirm that disruptions in early care lead to physiological changes that affect subsequent development (14, 56-58).
Of particular relevance to the developmental theory of SP are studies which demonstrate that early maternal separation in rats changes nociception and analgesia (59-61), decreases the number of brain opioid receptors (61) and reduces opioid effects during pain (62); increases susceptibility to infection (63), to stress-related gastric ulcers (64) and to high blood pressure (65); increases reactivity to stress (66, 67), reduces GABA-A receptor levels in the medial prefrontal cortex and in noradrenergic cell body regions of the locus coeruleus and the nucleus tractus solitarius (68), and decreases growth hormone factor (69). Studies show that maternal separation causes acute changes in an infant rat's physiology and behavior which are not simply expressions of an infant's stress response but rather reflect the loss of specific physiological and behavioral mother-infant regulatory interactions. Subsequent research on these ‘maternal regulators’ revealed an extended system, linking brain, autonomic, and endocrine pathways, as well as behavioral and sleep-wake state organization, including specific thermal, nutrient, and sensory-motor interactions between mother and pup (54, 58). Other animal studies have also linked naturally occurring poor maternal care (70) or experimentally simulated early abuse (e.g., by associating maternal care with shock) with aberrant neural development (71). The discovery of these maternal regulatory interactions provided a new understanding of how different patterns and qualities of maternal interaction shape the course of infant development, including effects on pain and interpersonal stress neural substrates.
Moreover, animal models point specifically to the existence of a shared neural system involved in the regulation of somatic pain and social distress. The opioid system, for example, plays a role in both analgesia and the reaction to social separation. The administration of opioids decreases separation cries among dog pups (72), rat pups (73) and nonhuman primates (74). Oxytocin is also involved in both nociception and affiliative behaviors, mating and infant-caregiver attachment (75-77). The anterior cingulate cortex (ACC), which is involved in the processing of somatic pain, is also involved in animals in the production of separation vocalizations and the maintenance of affiliative behaviors (14, 56). Thus, animal research supports the hypothesis that somatic pain and early attachment share neural systems and that the development of these systems can be compromised by non-optimal maternal-infant regulation.
Cognitive-Affective Neuroscience
Cognitive-affective neuroscience also suggests that a shared neural system is involved in the processing of both physical pain and interpersonal distress. Functional Magnetic Resonance Imaging (fMRI) studies show that the neural circuits involved in the processing of physical pain (e.g., ACC, insula) also process experimentally induced feelings of social rejection (14, 78-83). (This involvement is specific to feelings of interpersonal distress vs. negative emotions in general (81)). The prefrontal cortex (PFC) appears to down-regulate the distress associated with both pain and social exclusion (80, 81). In an experimental study of this shared neural system, among participants who reported higher levels of rejection sensitivity in their daily lives, pain threshold decreased disproportionately after an experience of experimentally induced feelings of social rejection (84). Administration of acetaminophen both reduces subjective feelings of social rejection and alters activation of the ACC and insula during social exclusion, further supporting the existence of a shared neural system for social and somatic distress (85).
Patients with SP (86), chronic low back pain (87), and fibromyalgia (88) have a hypersensitivity to experimentally induced pain and augmented central processing of pain in affective-motivational nociceptive circuits (ACC, insula), and in areas known to modulate affects (mPFC) as demonstrated by fMRI studies (86-90). Single photon emission computed tomography of SP patients revealed regional cerebral blood flow alterations consistent with those findings (91). Future research should examine whether in addition to the aberrantly activated affective pain neural circuits, the extent of sensory pain neurocircuitry activation helps define clinically distinct subgroups of patients. The same affective pain-modulating regions are also linked to alexithymia in a Positron Emission Tomography (PET) study (92) and to emotional awareness in a fMRI study (93). A structural MRI study demonstrated that the size of ACC inversely correlates with alexithymia (94).
Mu-opioid receptors mediate both attachment behaviors (95) and pain perception (79), thus providing further evidence of a shared pain-interpersonal distress neural system. Variations in the μ-opioid receptor gene (OPRM1) were associated with sensitivity to social rejection and with activation of the ACC in response to experimentally induced feelings of social rejection in fMRI (96). Reduced μ-opioid receptor binding potential was demonstrated by a PET study of patients with fibromyalgia (97). Consistent with the findings of problems with emotional awareness in SP, a defensive-repressive coping style was shown to correlate with pain sensitivity, and this relationship was mediated by altered activity of the endogenous opioid system (98). Evidence that oxytocin plays a role in nociception as well as in attachment, interpersonal trust, and social cognition provides further support for a shared interpersonal affect regulation-pain system (77, 99).
Genetics and Epigenetics
Genetic predisposition
Potential mechanisms of genetic predisposition to SP include polymorphisms that influence: a) the sensitivity to pain, b) sensitivity and reactivity to interpersonal stress, predisposing to insecure attachment, and c) affect expression and regulation capacities. Genes that code for proteins in the opioid system (such as OPRM1) are implicated in both the processing of somatic pain and the formation of social attachments (95, 100). Polymorphisms in the μ-opioid receptor gene, d-opioid receptor subtype 1 gene, and catechol-O-methyltransferase gene were all associated with increased sensitivity to experimentally induced pain (101, 102). Mice lacking μ-opioid receptor genes exhibited less attachment-related behaviors, such as separation vocalizations and reactions to maternal cues (95). Consistent with these findings, the expression of OPRM1 in fibromyalgia patients positively correlates with the severity of pain symptoms (103). In addition, genetic factors may predispose a child to be sensitive to particular types of parental interaction(104). For example, dopamine DRD4 gene variations have been reported to differentially interact with maternal attachment style (105) and parenting quality (106) to predict the attachment styles and interpersonal behaviors of human infants.
Epigenetic influences
Multiple studies show that the early life environment influences gene expression and regulation. Disruptions and/or insufficiency of maternal care affect gene methylation patterns, thus altering the gene expression in brain cells, and leading ultimately to changes in attachment and altered development. These epigenetic changes can be transmitted to the next generation but are reversed if offspring are placed in an enriched environment (70). Organisms are particularly susceptible to such epigenetic changes early in life, as neurophysiologic pathways for multiple regulatory processes are established at that time.
Psychoneuroimmunology
Environmental influences on the developing immune system can have lasting consequences, affecting a person's illness vulnerability and health resiliency throughout life (107). Frequent exposure to ‘danger signals’ early in development might stimulate immune responses, lead to the overproduction of proinflammatory cytokines by the brain, and to sensitization of the immune system to danger (108). From an evolutionary perspective, feeling alone or rejected by others may be a danger signal for an infant who would not be able to survive without others (14). In fact, isolation is often used in animal models of the effects of stress on development, which show that isolation may lead to altered cytokine levels peripherally and in the brain, which in turn increases the susceptibility to sickness behavior, infections, pain sensitivity and allodynia post injury (109-112). Interestingly, administration of oxytocin – a neuropeptide associated with social bonding – to isolated rats reversed this effect, reducing the level of the inflammatory cytokine IL-1 (111). Supporting the interpersonal regulation-immunology link, increased social rejection sensitivity as measured by fMRI, was associated with an inflammatory response to social stressors (113).
Challenges to the immune system early in development may also lead to sensitization to somatic pain: for example, neonatal activation of the immune system by exposure of rat pups to lipopolysaccharide resulted in heightened nociception in adulthood to both thermal and mechanical stimulations (114). Studies of immune markers in humans with SSD are limited in scope and have produced conflicting results(115). One study however showed elevated levels of IL-6, IL-10, and Immunoglobulin E in SSD patients compared to healthy controls as well as an inverse relationship between IL-2 and alexithymia; the authors interpret these findings as supporting activated Th2 and reduced Th1 pathways among patients with SSD and high alexithymia (116). More basic and clinical research is needed to explore the effects of early relational stressors on the development of the immune system and SP.
Treatment Studies
Psychopharmacology
Medication studies of SP without comorbid disorders have been rare and more studies are needed to illuminate the role of neurotransmitter systems in SP regulation. For example, a meta-analysis of 11 antidepressant treatment studies of SP (with or without depression) revealed variable improvement in pain symptoms (117, 118). Two studies have suggested that improvement in SP may be more effectively mediated by agents that affect serotonergic rather than noradrenergic function (one such study used amitriptyline with or without flupentixol (119) and the other compared citalopram vs reoboxetine (120)).
Psychotherapy
Numerous studies provide evidence that psychotherapy helps to alleviate pain and improve quality of life of SP patients (117, 121-123). While in-depth discussion of the relative efficacy of various therapeutic approaches is beyond the scope of this review, this body of research supports the developmental theory of SP proposed here by demonstrating that the experience of pain can be changed by a process of consistent work on affect, cognition and behavior conducted in a supportive interpersonal environment (124). Studies that address mechanisms of change in psychotherapy would be particularly useful and may provide further insight regarding the etiology of SP. However, these studies have been rare. Studies that explored mechanisms of change in SP suggest that a focus on affect regulation in the interpersonal context contributes to pain symptom improvement. One of the most relevant to this review, is a study of 40 SP patients randomized to an affect- and interpersonal relationships–focused psychotherapy or to treatment as usual (125). The pain was reduced significantly more in the therapy group both immediately post-treatment and at one-year follow-up. In fact, 50% of the patients in the therapy group reported complete alleviation of pain after the treatment compared to 15% of controls (125). Most importantly for this review, the study explored the mechanism of change and showed that, after treatment, the SP patients' awareness of emotions and ability to express affect in the interpersonal context differentially increased while the tendency to express interpersonal stress in somatic terms significantly decreased. This change was observed in the active treatment group but not in the control group. In another study, an increase in emotional awareness after inpatient treatment was associated with post-treatment improvement in SSD symptoms, including SP, once again highlighting the relevance of affect awareness and regulation to treatment of SP (126). More studies of mechanisms of change as well as comparative psychotherapy studies are needed. In sum, psychotherapy is helpful for SP, and the focus on affect regulation and interpersonal functioning might be particularly beneficial for the SP patients.
Discussion
This translational integration of research findings supports the developmental theory of SP (Table 1, Figure 1). Genetic predispositions that affect attachment, affect regulation and pain processing may all interact with poor mother-infant attunement to compromise the maturation of interpersonal regulation of affect and somatic distress. Failure of these regulatory capacities to develop adequately may contribute to the persistence into adulthood of the primarily somatic way of experiencing emotional distress. This is supported by multiple studies showing that SP patients tend to have less mature levels of emotional awareness and regulation. Animal models also demonstrate how compromised early interactions with caregivers lead to an increased susceptibility to disease, somatic distress and altered immune regulation in the offspring; human studies are beginning to support this finding.
Moreover, multiple human and animal neuroscience studies demonstrate that interpersonal distress and the experience of somatic pain are mediated by common neural systems (brain neurocircuitry, as well as neuroendocrine and neuroimmune systems). Therefore, compromised development of these shared systems may lead to an increase of both somatic and interpersonal distress in childhood and adulthood. This may explain the frequently reported comorbidity of SP, interpersonal difficulties and problems with interpersonal affect regulation. The symptom formation mechanisms in SP may thus include: (a) a heightened sensitivity to pain and to interpersonal interactions, (b) problems down-regulating pain and interpersonal distress, or (c) a combination of both. Additionally, because the experiences of both somatic and interpersonal distress depend on the same shared brain circuits, the predisposition to experiencing interpersonal relationships as rejecting or hurtful may activate somatic pain pathways, and somatic pain may in turn activate pathways involved in feelings of interpersonal rejection. A high frequency of distressing interpersonal interactions throughout life may also contribute to increased pain sensitivity and somatoform pain.
The symptom formation mechanisms of SP suggested here are inherently developmental. Multiple research studies point to the association of SP with a history of growing up in an environment suggestive of misattunement between an infant and its caregivers. Therefore, the ongoing vicious cycle of failures in effective distress regulation shape the developing brain, its neuroendocrine and neuroimmune systems, and their interaction with other body systems. The optimal maturation of these systems depends on learning processes spanning multiple levels, from the psychological level to neurocircuitry development to epigenetics.
This compromised developmental process predisposes an adult to increased somatic and interpersonal reactivity as well as to decreased self-regulatory abilities. Adults with SP continue to experience interpersonal emotional distress on the somatic level. Their interpersonal world is colored by a desire for closeness with others combined with a fear of rejection and abandonment. This inner representation of relationships makes it difficult for them to engage in interactions that otherwise might help an effective interpersonal regulation of affect, hence perpetuating the cycle of somatic and interpersonal distress. The apparent benefit of psychotherapies that address affect regulation in interpersonal contexts is consistent with the central pathogenic role of interpersonal affect regulation problems in the development of SP.
This developmental theory of SP is pointing to a potential specific mechanism of symptom formation. It is important, however, to acknowledge its limited current specificity. More research is needed to understand particularly what kind of early interpersonal-affective developmental processes may lead to SP or other symptoms in adulthood. The main purpose of the formulation of this theory is to suggest a hypothesis and a direction of research which may further refine or redirect the developmental ideas proposed herein. In addition, this theory is suggesting one of the symptom formation mechanisms of SP that may be applicable to a subgroup but not all patients suffering from somatoform pain. Future investigation should address the variability in the pathogenesis of SP.
Clinical Implications
The reviewed research findings and the proposed symptom formation mechanism of SP suggest that a tailored psychotherapy approach for SP would address developmental deficits that contribute to maladaptive interpersonal affect regulation in SP patients. Therapeutic techniques that help the individual learn to modulate interpersonal distress more effectively would be expected to reduce the reactivity of pain-related circuits and enhance the engagement of prefrontal regions in distress regulation. This can be achieved by psychotherapies that help a patient work through insecure attachment and learn to establish close, safe, and supportive interpersonal connections. With effective treatment that focuses on emotional regulation in the interpersonal context, SP patients will be able to express emotions to others in more mature ways, yielding more effective interactions which can in turn ameliorate interpersonal affect regulation. Pharmacologic treatments that address the dysregulation in the somatic-interpersonal neurocircuitry may potentially augment the psychotherapeutic process. Opioid and oxytocin systems may be good initial targets for this approach.
Understanding of SP as rooted in neurobiology may also help to stop the dismissive and invalidating labeling of SP as “not real” which exacerbates patients' feelings of being misunderstood. It may also help patients to see the connection between their pain and emotional distress, and to become more accepting of a referral to psychotherapy.
Another important implication of the theory proposed here is that an awareness of the role of interpersonal dysregulation among SP patients and its developmental origins may help clinicians regulate their own frustration and feelings of helplessness when working with these patients (127). Moreover, it may help physicians to be more effective in alleviating patients' distress and in facilitating referral to psychotherapists who would then use techniques that address interpersonal affect dysregulation. SP patients are very sensitive to interpersonal interactions and are more likely to perceive a clinician as bored or uninterested, which would reinforce their feelings that “no one cares about them,” diminish their trust in the clinician, and lead to more pain, perpetuating a vicious cycle. Clinicians' direct attention to such feelings and attunement to SP patients' heightened need for a safe interpersonal environment could help break this maladaptive cycle.
The pivotal importance of the patient-doctor relationship in enhancing treatment compliance and improved outcome (including alleviation of pain symptoms) has been demonstrated by prior researchers (128) and efforts to incorporate these results into medical education programs are underway (129). Our theory would suggest that educational modules focused specifically on enhancing the interpersonal encounter and interpersonal affect regulation may be particularly helpful for the clinical care of patients with somatoform pain and medically unexplained symptoms.
The research reviewed here also suggests that early-life interventions would be of great importance for preventing and alleviating SP. In particular, interventions focused on the caregiver-infant interaction and the interpersonal affect regulation could alter the developmental trajectory of SP (outlined in Figure 1), and help restore a more optimal interpersonal environment for a maturing infant. Early parent-level interventions for mothers of socioeconomic disadvantage have been shown to be effective in improving the health of their children (130, 131). A similar approach may be helpful for the prevention of SP. Due to the important contribution of interpersonal functioning to the development of SP, infants, children and adults suffering from SP may also greatly benefit from family therapy interventions (132).
Implications for Future Research
Translational research is on the verge of revealing the pathogenic mechanisms of SP. To address the developmental hypotheses proposed, longitudinal studies addressing genetic, immune, neural systems development, parenting environment, child-parent mutual affect regulation and interpersonal functioning in the same patients are needed. The identification of early markers for SP (whether biomarkers, environmental factors, or psychological markers) could contribute to early treatment and prevention of SP. It is necessary to develop effective targeted treatments for SP and to study the mechanisms of change. Future research may also identify subgroups of patients for whom targeted psychosocial and pharmacological treatments can be devised.
Acknowledgments
We thank Drs. Myron Hofer and Jenifer Nields for helpful comments on earlier drafts of this manuscript.
Disclosures: This research review was supported in part by a T32 NIMH Training Grant (Landa) and by R01 MH 071456 (Fallon).
Acronyms
- SP
Somatoform Pain
- SSD
somatization spectrum disorders
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ACC
anterior cingulate cortex
- PFC
prefrontal cortex
- fMRI
functional magnetic resonance imaging
- OPRM1
μ-opioid receptor 1 gene
- PET
Positron Emission Tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alla Landa, Developmental Neuroscience Division, Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY
Bradley S. Peterson, Director of Child and Adolescent Psychiatry, Director of MRI Research, Columbia University College of Physicians and Surgeons, New York, NY
Brian A. Fallon, Center for the Study of Neuroinflammatory Disorders and Biobehavioral Medicine, Columbia University College of Physicians and Surgeons, New York, NY.
References
- 1.Rief W, Hessel A, Braehler E. Somatization symptoms and hypochondriacal features in the general population. Psychosomatic Medicine. 2001;63(4):595–602. doi: 10.1097/00006842-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. International Journal of Methods in Psychiatric Research. 2003;12(1):34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirmayer LJ, Robbins JM. Three forms of somatization in primary care: Prevalence, co-occurrence, and sociodemographic characteristics. Journal of Nervous and Mental Disease. 1991;179(11):647–55. doi: 10.1097/00005053-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Toft T, Fink P, Oernboel E, Christensen K, Frostholm L, Olesen F. Mental disorders in primary care: prevalence and co-morbidity among disorders. results from the functional illness in primary care (FIP) study. Psychological Medicine. 2005;35(8):1175–84. doi: 10.1017/s0033291705004459. [DOI] [PubMed] [Google Scholar]
- 5.Grabe HJ, Meyer C, Hapke U, Rumpf H-J, Freyberger HJ, Dilling H, John U. Somatoform pain disorder in the general population. Psychotherapy & Psychosomatics. 2003;72(2):88–94. doi: 10.1159/000068681. [DOI] [PubMed] [Google Scholar]
- 6.Harris AM, Orav EJ, Bates DW, Barsky AJ. Somatization increases disability independent of comorbidity. Journal of General Internal Medicine. 2009;24(2):155–61. doi: 10.1007/s11606-008-0845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink P. The use of hospitalizations by persistent somatizing patients. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 1992;22(1):173–80. doi: 10.1017/s0033291700032827. [DOI] [PubMed] [Google Scholar]
- 8.Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Archives of General Psychiatry. 2005;62(8):903–10. doi: 10.1001/archpsyc.62.8.903. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K, Spitzer RL, deGruy FV, 3rd, Hahn SR, Linzer M, Williams JB, Brody D, Davies M. Multisomatoform disorder. An alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Archives of General Psychiatry. 1997;54(4):352–8. doi: 10.1001/archpsyc.1997.01830160080011. [DOI] [PubMed] [Google Scholar]
- 10.Escobar JI, Waitzkin H, Silver RC, Gara M, Holman A. Abridged somatization: A study in primary care. Psychosomatic Medicine. 1998;60(4):466–72. doi: 10.1097/00006842-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Noyes R, Jr, Stuart SP, Watson DB. A reconceptualization of the somatoform disorders. Psychosomatics. 2008;49(1):14–22. doi: 10.1176/appi.psy.49.1.14. [DOI] [PubMed] [Google Scholar]
- 12.Mayou R, Kirmayer LJ, Simon G, Kroenke K, Sharpe M. Somatoform disorders: time for a new approach in DSM-V. American Journal of Psychiatry. 2005;162(5):847–55. doi: 10.1176/appi.ajp.162.5.847. [DOI] [PubMed] [Google Scholar]
- 13.Engel GL. The clinical application of the biopsychosocial model. American Journal of Psychiatry. 1980;137(5):535–44. doi: 10.1176/ajp.137.5.535. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson SL, Rhodes RE. Parental Correlates of Physical Activity in Children and Early Adolescents. Sports Medicine. 2006;36(1):79–97. doi: 10.2165/00007256-200636010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Chesin MS, Jeglic EL, Stanley B. Pathways to high-lethality suicide attempts in individuals with borderline personality disorder. Archives of Suicide Research. 2010;14(4):342–62. doi: 10.1080/13811118.2010.524054. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz LM. Interpersonal Motive as an Explanatory Construct. 2004:9–24. [Google Scholar]
- 18.Craig TK, Boardman AP, Mills K, Daly-Jones O, Drake H. The South London Somatisation Study. I: Longitudinal course and the influence of early life experiences. British Journal of Psychiatry. 1993;163:579–88. doi: 10.1192/bjp.163.5.579. [DOI] [PubMed] [Google Scholar]
- 19.Bass C, Murphy M. Somatoform and personality disorders: syndromal comorbidity and overlapping developmental pathways. Journal of Psychosomatic Research. 1995;39(4):403–27. doi: 10.1016/0022-3999(94)00157-z. [DOI] [PubMed] [Google Scholar]
- 20.Violon A. Family etiology of chronic pain. International Journal of Family Therapy. 1985;7(4):235–46. [Google Scholar]
- 21.Imbierowicz K, Egle UT. Childhood adversities in patients with fibromyalgia and somatoform pain disorder. European Journal of Pain: Ejp. 2003;7(2):113–9. doi: 10.1016/S1090-3801(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 22.Mallouh SK, Abbey SE, Gillies LA. The role of loss in treatment outcomes of persistent somatization. General Hospital Psychiatry. 1995;17(3):187–91. doi: 10.1016/0163-8343(95)00026-n. [DOI] [PubMed] [Google Scholar]
- 23.de Leon J, Saiz-Ruiz J, Chinchilla A, Morales P. Why do some psychiatric patients somatize? Acta Psychiatrica Scandinavica. 1987;76(2):203–9. doi: 10.1111/j.1600-0447.1987.tb02885.x. [DOI] [PubMed] [Google Scholar]
- 24.van der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, Herman JL. Dissociation, somatization, and affect dysregulation: the complexity of adaptation of trauma. American Journal of Psychiatry. 1996;153(7 suppl):83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- 25.Lackner JM, Gudleski GD, Blanchard EB. Beyond abuse: the association among parenting style, abdominal pain, and somatization in IBS patients. Behaviour Research & Therapy. 2004;42(1):41–56. doi: 10.1016/s0005-7967(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 26.Brown RJ, Schrag A, Trimble MR. Dissociation, childhood interpersonal trauma, and family functioning in patients with somatization disorder. American Journal of Psychiatry. 2005;162(5):899–905. doi: 10.1176/appi.ajp.162.5.899. [DOI] [PubMed] [Google Scholar]
- 27.Waller E, Scheidt CE. Somatoform disorders as disorders of affect regulation: a development perspective. International Review of Psychiatry. 2006;18(1):13–24. doi: 10.1080/09540260500466774. [DOI] [PubMed] [Google Scholar]
- 28.Mattila AK, Kronholm E, Jula A, Salminen JK, Koivisto AM, Mielonen RL, Joukamaa M. Alexithymia and somatization in general population. Psychosomatic Medicine. 2008;70(6):716–22. doi: 10.1097/PSY.0b013e31816ffc39. [DOI] [PubMed] [Google Scholar]
- 29.Grabe HJ, Spitzer C, Freyberger HJ. Alexithymia and personality in relation to dimensions of psychopathology. The American Journal of Psychiatry. 2004:1299–301. doi: 10.1176/appi.ajp.161.7.1299. [DOI] [PubMed] [Google Scholar]
- 30.Millard RW, Kinsler BL. Evaluation of constricted affect in chronic pain: an attempt using the Toronto Alexythymia Scale. Pain. 1992;50(3):287–92. doi: 10.1016/0304-3959(92)90033-8. [DOI] [PubMed] [Google Scholar]
- 31.Cox BJ, Kuch K, Parker JD, Shulman ID, Evans RJ. Alexithymia in somatoform disorder patients with chronic pain. Journal of Psychosomatic Research. 1994;38(6):523–7. doi: 10.1016/0022-3999(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 32.Landa A. Beyond the unexplainable pain: Relational dynamics and alexithymia in somatization. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2009;70(6-B):2009. [Google Scholar]
- 33.Besharat MA. Attachment styles and alexithymia. Psychological Research. 2010;12(3-4):63–80. [Google Scholar]
- 34.Pedrosa Gil F, Scheidt CE, Hoeger D, Nickel M. Relationship between attachment style, parental bonding and alexithymia in adults with somatoform disorders. International Journal of Psychiatry in Medicine. 2008;38(4):437–51. doi: 10.2190/PM.38.4.d. [DOI] [PubMed] [Google Scholar]
- 35.Vanheule S, Desmet M, Meganck R, Bogaerts S. Alexithymia and interpersonal problems. Journal of Clinical Psychology. 2007;63(1):109–17. doi: 10.1002/jclp.20324. [DOI] [PubMed] [Google Scholar]
- 36.Montebarocci O, Codispoti M, Baldaro B, Rossi N. Adult attachment style and alexithymia. Personality and Individual Differences. 2004;36(3):499–507. [Google Scholar]
- 37.Lemche E, Klann-Delius G, Koch R, Joraschky P. Mentalizing language development in a longitudinal attachment sample: implications for alexithymia. Psychotherapy & Psychosomatics. 2004;73(6):366–74. doi: 10.1159/000080390. [DOI] [PubMed] [Google Scholar]
- 38.Spitzer C, Siebel-Jurges U, Barnow S, Grabe HJ, Freyberger HJ. Alexithymia and interpersonal problems. Psychotherapy & Psychosomatics. 2005;74(4):240–6. doi: 10.1159/000085148. [DOI] [PubMed] [Google Scholar]
- 39.Wearden AJ, Lamberton N, Crook N, Walsh V. Adult attachment, alexithymia, and symptom reporting: an extension to the four category model of attachment. Journal of Psychosomatic Research. 2005;58(3):279–88. doi: 10.1016/j.jpsychores.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Joukamaa M, Luutonen S, von Reventlow H, Patterson P, Karlsson H, Salokangas RK. Alexithymia and childhood abuse among patients attending primary and psychiatric care: results of the RADEP Study. Psychosomatics. 2008;49(4):317–25. doi: 10.1176/appi.psy.49.4.317. [DOI] [PubMed] [Google Scholar]
- 41.Egger HL, Costello EJ, Erkanli A, Angold A. Somatic complaints and psychopathology in children and adolescents: stomach aches, musculoskeletal pains, and headaches. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(7):852–60. doi: 10.1097/00004583-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Subic-Wrana C, Beutel ME, Knebel A, Lane RD. Theory of mind and emotional awareness deficits in patients with somatoform disorders. Psychosomatic Medicine. 2010;72(4):404–11. doi: 10.1097/PSY.0b013e3181d35e83. [DOI] [PubMed] [Google Scholar]
- 43.Maunder RG, Hunter JJ. Attachment and psychosomatic medicine: developmental contributions to stress and disease. Psychosomatic Medicine. 2001;63(4):556–67. doi: 10.1097/00006842-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Ciechanowski PS, Walker EA, Katon WJ, Russo JE. Attachment theory: a model for health care utilization and somatization. Psychosomatic Medicine. 2002;64(4):660–7. doi: 10.1097/01.psy.0000021948.90613.76. [DOI] [PubMed] [Google Scholar]
- 45.Taylor R, Mann A, White N, Goldberg D. Attachment style in patients with unexplained physical complaints. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2000;30(4):931–41. doi: 10.1017/s0033291799002317. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt S, Strauss B, Braehler E. Subjective physical complaints and hypochondriacal features from an attachment theoretical perspective. Psychology & Psychotherapy: Theory, Research & Practice. 2002;75(Pt 3):313–32. doi: 10.1348/147608302320365217. [DOI] [PubMed] [Google Scholar]
- 47.Waldinger RJ, Schulz MS, Barsky AJ, Ahern DK. Mapping the road from childhood trauma to adult somatization: the role of attachment. Psychosomatic Medicine. 2006;68(1):129–35. doi: 10.1097/01.psy.0000195834.37094.a4. [DOI] [PubMed] [Google Scholar]
- 48.Stuart S, Noyes R., Jr Attachment and interpersonal communication in somatization. Psychosomatics. 1999;40(1):34–43. doi: 10.1016/S0033-3182(99)71269-7. [DOI] [PubMed] [Google Scholar]
- 49.Waller E, Scheidt CE, Hartmann A. Attachment representation and illness behavior in somatoform disorders. Journal of Nervous & Mental Disease. 2004;192(3):200–9. doi: 10.1097/01.nmd.0000116463.17588.07. [DOI] [PubMed] [Google Scholar]
- 50.Hagekull B, Bohlin G. Predictors of Middle Childhood Psychosomatic Problems: An Emotion Regulation Approach. Infant and Child Development. 2004;13(5):389–405. [Google Scholar]
- 51.Solano L, Toriello A, Barnaba L, Ara R, Taylor GJ. Rorschach interaction patterns, alexithymia, and closeness to parents in psychotic and psychosomatic patients. Journal of the American Academy of Psychoanalysis. 2000;28(1):101–16. doi: 10.1521/jaap.1.2000.28.1.101. [DOI] [PubMed] [Google Scholar]
- 52.Blaustein JP, Tuber SB. Knowing the unspeakable: somatization as an expression of disruptions in affective-relational functioning. Bulletin of the Menninger Clinic. 1998;62(3):351–65. [PubMed] [Google Scholar]
- 53.Hilbert A, Martin A, Zech T, Rauh E, Rief W. Patients with medically unexplained symptoms and their significant others: Illness attributions and behaviors as predictors of patient functioning over time. Journal of Psychosomatic Research. 2010;68(3):253–62. doi: 10.1016/j.jpsychores.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Hofer MA. In: Developmental Psychobiology of Early Attachment. Casey BJ, editor. 2004. pp. 1–28. [Google Scholar]
- 55.Harlow HF. The nature of love. American Psychologist. 1958;13(12):673–85. doi: 10.1037/h0029383. [DOI] [PubMed] [Google Scholar]
- 56.Eisenberger NI. Identifying the Neural Correlates Underlying Social Pain: Implications for Developmental Processes. Human Development. 2006;49(5):273–93. [Google Scholar]
- 57.Kuhn CM, Schanberg SM. Responses to maternal separation: mechanisms and mediators. International Journal of Developmental Neuroscience. 1998;16(3-4):261–70. doi: 10.1016/s0736-5748(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 58.Hofer MA. In: Developmental neuroscience. Berntson Gary G., editor. Vol. 1. 2009. pp. 12–31. [Google Scholar]
- 59.Dickinson AL, Leach MC, Flecknell PA. Influence of early neonatal experience on nociceptive responses and analgesic effects in rats. Laboratory Animals. 2009;43(1):11–6. doi: 10.1258/la.2007.007078. [DOI] [PubMed] [Google Scholar]
- 60.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2002;282(2):G307–16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 61.Bernardi M, Genedani S, Tagliavini S, Bertolini A. Effects on long-term sensitivity to pain and morphine of stress induced in the newborn rat by pain or manipulation. Physiology & Behavior. 1986;37(5):827–31. doi: 10.1016/0031-9384(86)90191-5. [DOI] [PubMed] [Google Scholar]
- 62.Kalinichev M, Easterling KW, Holtzman SG. Repeated neonatal maternal separation alters morphine-induced antinociception in male rats. Brain Research Bulletin. 2001;54(6):649–54. doi: 10.1016/s0361-9230(01)00485-3. [DOI] [PubMed] [Google Scholar]
- 63.Barreau F, de Lahitte JD, Ferrier L, Frexinos J, Bueno L, Fioramonti J. Neonatal maternal deprivation promotes Nippostrongylus brasiliensis infection in adult rats. Brain, Behavior, & Immunity. 2006;20(3):254–60. doi: 10.1016/j.bbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Skolnick NJ, Ackerman SH, Hofer MA, Weiner H. Vertical transmission of acquired ulcer susceptibility in the rat. Science. 1980;208(4448):1161–3. doi: 10.1126/science.7189606. [DOI] [PubMed] [Google Scholar]
- 65.Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Developmental Psychobiology. 1989;22(1):29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- 66.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Research Molecular Brain Research. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 67.Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology & Behavior. 1999;66(2):293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- 68.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22(3):219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 69.Kuhn CM, Pauk J, Schanberg SM. Endocrine responses to mother-infant separation in developing rats. Developmental Psychobiology. 1990;23(5):395–410. doi: 10.1002/dev.420230503. [DOI] [PubMed] [Google Scholar]
- 70.Champagne FA. Epigenetic influence of social experiences across the lifespan. Developmental Psychobiology. 2010;52(4):299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- 71.Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67(12):1137–45. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biological Psychiatry. 1978;13(5):607–18. [PubMed] [Google Scholar]
- 73.Kehoe P, Blass EM. Opioid-mediation of separation distress in 10-day-old rats: reversal of stress with maternal stimuli. Developmental Psychobiology. 1986;19(4):385–98. doi: 10.1002/dev.420190410. [DOI] [PubMed] [Google Scholar]
- 74.Kalin NH, Shelton SE, Lynn DE. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20(7):735–42. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 75.MacDonald G, Leary MR. Why Does Social Exclusion Hurt? The Relationship Between Social and Physical Pain. Psychological Bulletin. 2005;131(2):202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- 76.Gu XL, Yu LC. Involvement of opioid receptors in oxytocin-induced antinociception in the nucleus accumbens of rats. Journal of Pain. 2007;8(1):85–90. doi: 10.1016/j.jpain.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Progress in Brain Research. 2008;170:337–50. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 78.Apkarian A, Bushnell M, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9(4):463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Rainville P. Brain mechanisms of pain affect and pain modulation. Current Opinion in Neurobiology. 2002;12(2):195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 80.Eisenberger NI, Lieberman MD, Williams KD. Does Rejection Hurt? An fMRI Study of Social Exclusion. Science. 2003;302(5643):290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 81.Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19(6):945–56. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- 82.Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- 83.Sher L, Stanley BH, Cooper TB, Malone KM, Mann J, Oquendo MA. Serotonergic responses in depressed patients with or without a history of alcohol use disorders and healthy controls. European Neuropsychopharmacology. 2008;18(9):692–9. doi: 10.1016/j.euroneuro.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126(1-3):132–8. doi: 10.1016/j.pain.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 85.DeWall C, MacDonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychological Science. 2010;21(7):931–7. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- 86.Gundel H, Valet M, Sorg C, Huber D, Zimmer C, Sprenger T, Tolle TR. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137(2):413–21. doi: 10.1016/j.pain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Giesecke T, Gracely RH, Grant MAB, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis & Rheumatism. 2004;50(2):613–23. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 88.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis & Rheumatism. 2002;46(5):1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 89.Stoeter P, Bauermann T, Nickel R, Corluka L, Gawehn J, Vucurevic G, Vossel G, Egle UT. Cerebral activation in patients with somatoform pain disorder exposed to pain and stress: an fMRI study. Neuroimage. 2007;36(2):418–30. doi: 10.1016/j.neuroimage.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 90.Horowitz LM, Rosenberg SE. In: Stanford University collaborative: Assessing interpersonal problems in psychodynamic treatment. Beutler Larry E., editor. 1991. pp. 299–304. [Google Scholar]
- 91.Karibe H, Arakawa R, Tateno A, Mizumura S, Okada T, Ishii T, Oshima K, Ohtsu M, Hasegawa I, Okubo Y. Regional cerebral blood flow in patients with orally localized somatoform pain disorder: a single photon emission computed tomography study. Psychiatry & Clinical Neurosciences. 2010;64(5):476–82. doi: 10.1111/j.1440-1819.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- 92.Kano M, Fukudo S, Gyoba J, Kamachi M, Tagawa M, Mochizuki H, Itoh M, Hongo M, Yanai K. Specific brain processing of facial expressions in people with alexithymia: an H2 15O-PET study. Brain. 2003;126(Pt 6):1474–84. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- 93.McRae K, Reiman EM, Fort CL, Chen K, Lane RD. Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. Neuroimage. 2008;41(2):648–55. doi: 10.1016/j.neuroimage.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gundel H, Lopez-Sala A, Ceballos-Baumann AO, Deus J, Cardoner N, Marten-Mittag B, Soriano-Mas C, Pujol J. Alexithymia correlates with the size of the right anterior cingulate. Psychosomatic Medicine. 2004;66(1):132–40. doi: 10.1097/01.psy.0000097348.45087.96. [DOI] [PubMed] [Google Scholar]
- 95.Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304(5679):1983–6. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 96.Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences of the United States of America; 2009; pp. 15079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta J-K. Decreased central mu-opioid receptor availability in fibromyalgia. Journal of Neuroscience. 2007;27(37):10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jamner LD, Leigh H. Repressive/defensive coping, endogenous opioids and health: how a life so perfect can make you sick. Psychiatry Research. 1999;85(1):17–31. doi: 10.1016/s0165-1781(98)00134-6. [DOI] [PubMed] [Google Scholar]
- 99.Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, Dayanithi G. Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neuroscience & Therapeutics. 2010;16(5):e138–e56. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proceedings of the National Academy of Sciences of the United States of America; 2008; pp. 5277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Limer KL, Nicholl BI, Thomson W, McBeth J. Exploring the genetic susceptibility of chronic widespread pain: the tender points in genetic association studies. Rheumatology. 2008;47(5):572–7. doi: 10.1093/rheumatology/ken027. [DOI] [PubMed] [Google Scholar]
- 102.Buskila D. Genetics of chronic pain states. Best Practice & Research in Clinical Rheumatology. 2007;21(3):535–47. doi: 10.1016/j.berh.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 103.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. Genetic influences on the dynamics of pain and affect in fibromyalgia. Health Psychology. 2010;29(2):134–42. doi: 10.1037/a0018647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ayduk O, Downey G, Testa A, Yen Y, Shoda Y. Does rejection elicit hostility in rejection sensitive women? Social Cognition. 1999;17(2):245–71. [Google Scholar]
- 105.Van Ijzendoorn MH, Bakermans-Kranenburg MJ. DRD4 7-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attachment & Human Development. 2006;8(4):291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]
- 106.Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development & Psychopathology. 2007;19(4):1039–46. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- 107.Lane RD, Waldstein SR, Chesney MA, Jennings J, Lovallo WR, Kozel PJ, Rose RM, Drossman DA, Schneiderman N, Thayer JF, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, Part I: Historical context, methods, and relevant basic science. Psychosomatic Medicine. 2009;71(2):117–34. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- 108.Dantzer R. Somatization: a psychoneuroimmune perspective. Psychoneuroendocrinology. 2005;30(10):947–52. doi: 10.1016/j.psyneuen.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 109.Hermes GL, Rosenthal L, Montag A, McClintock MK. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. American Journal of Physiology Regulatory Integrative & Comparative Physiology. 2006;290(2):R273–82. doi: 10.1152/ajpregu.00368.2005. [DOI] [PubMed] [Google Scholar]
- 110.Chida Y, Sudo N, Kubo C. Social isolation stress exacerbates autoimmune disease in MRL/lpr mice. Journal of Neuroimmunology. 2005;158(1-2):138–44. doi: 10.1016/j.jneuroim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, Courtney DeVries A. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosomatic Medicine. 2010;72(6):519–26. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- 112.Horowitz LM, Rosenberg SE, Bartholomew K. Interpersonal problems, attachment styles, and outcome in brief dynamic psychotherapy. Journal of Consulting and Clinical Psychology. 1993;61(4):549–60. doi: 10.1037//0022-006x.61.4.549. [DOI] [PubMed] [Google Scholar]
- 113.Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. PNAS Proceedings of the National Academy of Sciences of the United States of America; 2010; pp. 14817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boissé Lysa, Spencer SJ, Mouihate A, Vergnolle N, Pittman QJ. Neonatal immune challenge alters nociception in the adult rat. Pain. 2005;119(1-3):133–41. doi: 10.1016/j.pain.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 115.Houtveen JH, Kavelaars A, Heijnen CJ, van Doornen LJP. Heterogeneous medically unexplained symptoms and immune function. Brain, Behavior, & Immunity. 2007;21(8):1075–82. doi: 10.1016/j.bbi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 116.Pedrosa Gil F, Nickel M, Ridout N, Schwarz MJ, Schoechlin C, Schmidmaier R. Alexithymia and interleukin variations in somatoform disorder. Neuroimmunomodulation. 2007;14(5):235–42. doi: 10.1159/000112048. [DOI] [PubMed] [Google Scholar]
- 117.Sumathipala A. What is the evidence for the efficacy of treatments for somatoform disorders? A critical review of previous intervention studies. Psychosomatic Medicine. 2007;69(9):889–900. doi: 10.1097/PSY.0b013e31815b5cf6. [DOI] [PubMed] [Google Scholar]
- 118.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Do antidepressants have an analgesic effect in psychogenic pain and somatoform pain disorder? A meta-analysis. Psychosomatic Medicine. 1998;60(4):503–9. doi: 10.1097/00006842-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 119.Van Kempen GM, Zitman FG, Linssen AC, Edelbroek PM. Biochemical measures in patients with a somatoform pain disorder, before, during, and after treatment with amitriptyline with or without flupentixol. Biological Psychiatry. 1992;31(7):670–80. doi: 10.1016/0006-3223(92)90276-6. [DOI] [PubMed] [Google Scholar]
- 120.Aragona M, Bancheri L, Perinelli D, Tarsitani L, Pizzimenti A, Conte A, Inghilleri M. Randomized double-blind comparison of serotonergic (Citalopram) versus noradrenergic (Reboxetine) reuptake inhibitors in outpatients with somatoform, DSM-IV-TR pain disorder. European Journal of Pain: Ejp. 2005;9(1):33–8. doi: 10.1016/j.ejpain.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 121.Kroenke K, Swindle R. Cognitive-behavioral therapy for somatization and symptom syndromes: A critical review of controlled clinical trials. Psychotherapy and Psychosomatics 2000. 2000 Jul-Aug;69(4) doi: 10.1159/000012395. [DOI] [PubMed] [Google Scholar]
- 122.Allen LA, Escobar JI, Lehrer PM, Gara MA, Woolfolk RL. Psychosocial treatments for multiple unexplained physical symptoms: A review of the literature. Psychosomatic Medicine. 2002;64(6):939–50. doi: 10.1097/01.psy.0000024231.11538.8f. [DOI] [PubMed] [Google Scholar]
- 123.Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychotherapy & Psychosomatics. 2009;78(5):265–74. doi: 10.1159/000228247. [DOI] [PubMed] [Google Scholar]
- 124.Lipsitt DR, Escobar J. In: Psychotherapy of somatoform disorders. Gabbard Glen O., editor. 2005. pp. 247–58. [Google Scholar]
- 125.Monsen K, Monsen JT. Chronic pain and psychodynamic body therapy: A controlled outcome study. Psychotherapy: Theory, Research, Practice, Training. 2000:257–69. [Google Scholar]
- 126.Subic-Wrana C, Bruder S, Thomas W, Lane RD, Kohle K. Emotional awareness deficits in inpatients of a psychosomatic ward: a comparison of two different measures of alexithymia. Psychosomatic Medicine. 2005;67(3):483–9. doi: 10.1097/01.psy.0000160461.19239.13. [DOI] [PubMed] [Google Scholar]
- 127.Lipsitt DR. Characteristics of patient-doctor relationships with somatizing patients. In: Kubo Chiharu, Kuboki Tomifusa., editors. (2006) Psychosomatic medicine(374-377)):Proceedings of the 18th World Congress on Psychosomatic Medicine, held in Kobe Japan, between 21 and 6 August 2005. 2006. [Google Scholar]
- 128.Horowitz LM. Introduction to the Interpersonal Approach. 2004. pp. 1–6. [Google Scholar]
- 129.Rief W, Martin A, Rauh E, Zech T, Bender A. Evaluation of general practitioners' training: how to manage patients with unexplained physical symptoms. Psychosomatics. 2006;47(4):304–11. doi: 10.1176/appi.psy.47.4.304. [DOI] [PubMed] [Google Scholar]
- 130.Koniak-Griffin D, Verzemnieks IL, Anderson NL, Brecht ML, Lesser J, Kim S, Turner-Pluta C. Nurse Visitation for Adolescent Mothers: Two-Year Infant Health and Maternal Outcomes. Nursing Research. 2003;52(2):127–36. doi: 10.1097/00006199-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 131.Kurzweil S. PLAYSPACE: A preventive intervention for infants and young children at risk from postnatal depression. The International Journal of Mental Health Promotion. 2008;10(1):5–15. [Google Scholar]
- 132.Marvin RS. Attachment- and family systems-based intervention in developmental psychopathology. Development and Psychopathology 1992. 1992;4 [Google Scholar]