Abstract
Background and Aim
Chronic hepatitis C virus (HCV) infection is relatively frequent in China. This study investigated the clinical, demographic, and viral and host genetic characteristics that may influence disease manifestations and clinical management.
Methods
In this cross-sectional observational study, treatment-naïve Han ethnic adults with recently confirmed chronic HCV infection were enrolled at 28 hospitals across China. HCV genotype and host interleukin 28B (IL28B) genotypes were determined and compared with patient demographic parameters and medical status.
Results
Among the 997 HCV-positive patients analyzed, 56.8% were infected with HCV genotype 1b, followed in prevalence by genotypes 2, 3, and 6, with substantial regional variation. Overall, 84.1% of patients were IL28B genotype CC (rs12979860), with little regional variation. Cirrhosis was reported in 10.1% of patients and was significantly associated with hepatitis B virus coinfection, low HCV viral load, low serum alanine aminotransferase, high serum aspartate aminotransferase, diabetes, and high pickled food consumption. Medical procedures were common transmission risk factors; however, lifestyle-associated risk factors, including intravenous drug abuse and tattoos or piercings, were more common in patients with HCV genotype 3 or 6.
Conclusions
Most HCV-infected Han Chinese patients were IL28B genotype CC (rs12979860). HCV genotypes varied by geographic region, and disease characteristics differed according to HCV genotype. Relatively frequent detection of advanced liver disease may reflect limitations on access to antiviral therapy, and suggests that greater awareness of factors that influence HCV-associated disease may help avoid clinical complications and improve patient outcomes.
Keywords: China, cirrhosis, epidemiology, HPV, IL28B, natural history
Introduction
China has approximately 25–50 million individuals with chronic hepatitis C virus (HCV) infection, representing 1.8–3.7% of the overall Chinese population and approximately 15–30% of the total HCV-infected population worldwide. Liver-related complications are frequent in affected individuals; thus, this large infected population comprises a significant burden on public health and patient care resources.1,2
Multiple viral and host factors contribute to HCV natural history and therapeutic response.3 HCV genotype 1 has been associated with lower rates of response to peginterferon-based regimens compared with genotypes 2 and 3;3 genotype 1a infections respond less well than genotype 1b to treatment with peginterferon/ribavirin and to triple regimens that include telaprevir or boceprevir.4–6 Host interleukin 28B (IL28B) genotype has a significant impact on the rates of spontaneous viral clearance and responses to current therapies.7–9
A few studies in China have surveyed HCV genotype frequencies in small cohorts or restricted populations, but information concerning the prevalence and distribution of viral and host genetic factors that influence HCV natural history and therapeutic outcomes is limited.2,10–12 This survey is the largest nationwide assessment of HCV genotypes and host IL28B genotypes reported in HCV treatment-naïve Han ethnic Chinese patients with chronic HCV infection. The study also included possible relationships between viral and host genotypes and HCV-associated epidemiologic and disease parameters. The purpose was to assess the burden of disease and epidemiologic parameters in relation to viral and host genotype to help guide public health policy, and to assist practitioners in evaluating patients with chronic HCV infection and in formulating cost-effective therapeutic strategies.
Methods
Patients
Han ethnic HCV treatment-naïve adults at least 18 years of age were seen at the outpatient facilities of 28 university-affiliated hospitals across China between February and June 2011. Qualified patients were enrolled into this study with stratified sampling based on the populations of five geographic regions.13 The study focused on the Han ethnic group to obtain an accurate survey of the majority (approximately 92%) Chinese population; it was not feasible to include the 55 recognized minority ethnic groups. HCV infection was confirmed or reconfirmed (anti-HCV antibody and HCV RNA positive) within 90 days prior to enrollment. Patients who had received antiviral or interferon-based therapy for hepatitis C or hepatitis B were excluded; no other exclusion criteria were applied.
Study design
In this cross-sectional observational study (ClinicalTrials.gov identifier NCT01293279), blood samples, demographic information, medical histories, and physical examinations were obtained within 9 days after enrollment. Subject interviews at this visit included collection of data pertaining to diet, lifestyle, HCV transmission risk factors, and other parameters. Routine blood biochemistry and hematology tests were completed locally. Virologic and genetic analyses, including HCV viral load (Abbott RealTime HCV; Abbott Laboratories, Des Plaines, IL, USA), HCV genotype (Versant HCV Genotype 2.0 [LiPA]; Siemens Healthcare Diagnostics, Tarrytown, NY, USA), and IL28B genotype (iPLEX Gold; Sequenom, San Diego, CA, USA), were conducted centrally (CapitalBio, Beijing, China).
Cirrhosis was diagnosed by liver biopsy or the presence of ascites, hepatic encephalopathy, upper gastrointestinal bleeding, or Child-Turcotte-Pugh score > 7, or by any two of the following criteria: radiologic imaging showing nodular liver or splenomegaly, platelet count < 100 000 in the absence of other explanations, liver stiffness > 13 kPA, or endoscopic detection of gastroesophageal varices. Decompensated cirrhosis was defined by the presence of ascites, hepatic encephalopathy, upper gastrointestinal bleeding, or Child-Turcotte-Pugh score > 7. Hepatocellular carcinoma, fatty liver disease, and type 2 diabetes were diagnosed using established guidelines.14–16 Diagnoses of ascites, splenomegaly, and portal hypertension were based on ultrasonography.
Study oversight
Peking University People's Hospital and Bristol-Myers Squibb designed the protocol, in collaboration with academic authors, with approval by a central review board and the institutional review boards of each participating center, and by the China National Human Genetic Resource Management Office (2010)074. The study was conducted in accordance with the International Society for Pharmacoepidemiology guidelines for Good Epidemiology Practices and applicable regulatory requirements. All patients provided written informed consent before participating.
Statistical analysis
A sample size of 1000 patients was chosen to achieve adequate precision, based on assumed overall prevalence of 45% HCV genotype 1b and 81% IL28B genotype CC, and on assumed homogeneity of patient characteristics across geographic regions. Categorical variables were tabulated with counts and percents; continuous variables were summarized with univariate statistics. Descriptive analyses were conducted to describe patient demographic characteristics, frequency distributions of viral and host genotypes, and HCV viral load. Stratifications were conducted by geographic region, status of complications, and HCV viral load. Prevalence estimates for host and viral genotypes were expressed as proportions and compared by chi-squared analysis. Single nucleotide polymorphism (SNP) genotype frequencies from host genotyping tests were examined for departures from Hardy–Weinberg equilibrium.17 Cirrhosis risk factors were investigated by logistic regression; variables significant at P < 0.2 in univariate analysis were entered into a multivariate model. Backward selection was applied, removing the least significant parameters from the model until all parameters were significant at P < 0.05. HCV genotype was added to the final multivariate model.
Results
Study patients
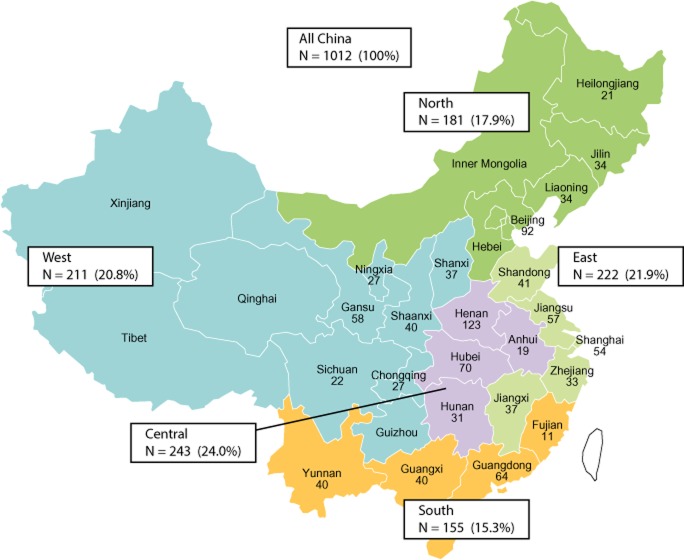
The regional distribution of the 1012 enrolled patients (Fig. 1) was consistent with that of the overall Chinese population.13 Protocol criteria for inclusion in the analysis were met by 997 patients: 15 were excluded for protocol violations (one patient had two violations) that included HCV infection not confirmed within 90 days before enrollment (10 patients); physical examinations or blood draws not completed within 9 days after enrollment (four patients); and not Han ethnic and/or < 18 years old (two patients).
Figure 1.
Distribution of enrolled patients by province and region. Chinese regions as defined in this study are color-coded; the number of patients enrolled in each province is shown with regional totals and proportions of the total enrolled population.
Distribution of viral and host genotypes
Genotype 1b was the most common HCV genotype across China, comprising 56.8% of the overall population, followed by genotypes 2, 3, and 6 (Fig. 2a). Genotypes 4 and 5 were not found. The greatest HCV genotypic diversity was observed in the southern and western regions, which had significantly lower proportions of genotype 1, and correspondingly higher proportions of genotypes 3 and 6 (south) or 2 and 3 (west) (P < 0.001). Genotype 1a was rare, comprising 1.4% overall. A small proportion (2.1%) was infected with multiple genotypes, most frequently 1 and 2.
Figure 2.
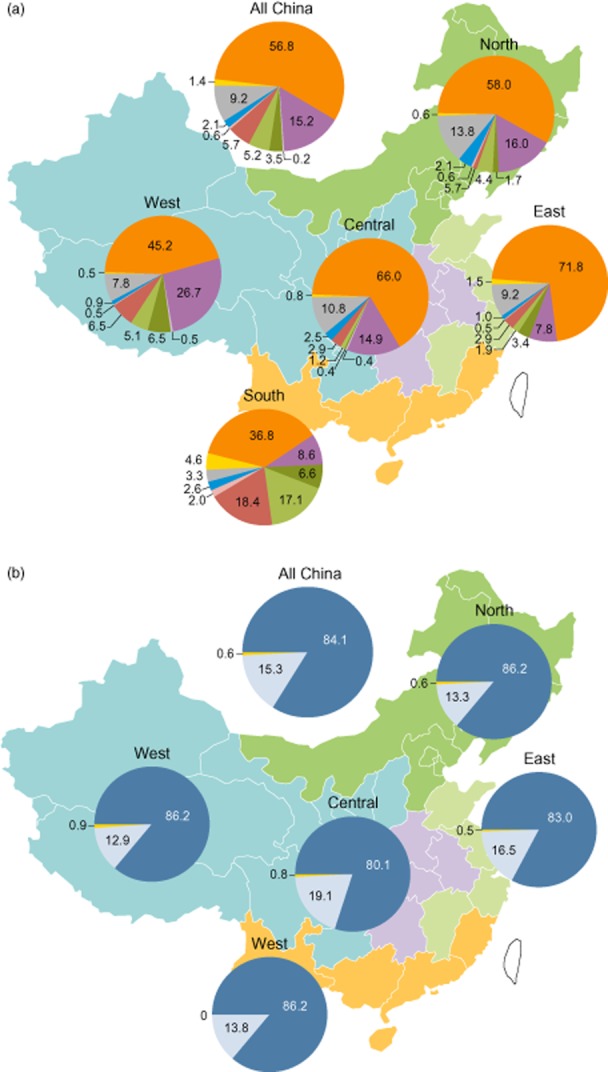
Distribution of hepatitis C virus (HCV) and IL28B genotypes by region. Data indicate percentages of patients with the indicated (a) HCV or (b) IL28B genotype in each region, and in the total study population. HCV genotype:  , 1b;
, 1b;  , 2b;
, 2b;  , 3b;
, 3b;  , 6c;
, 6c;  , 1a;
, 1a;  , 2a or 2c;
, 2a or 2c;  , 3a;
, 3a;  , 6a or 6b;
, 6a or 6b;  , multiple genotypes;
, multiple genotypes;  , subtype unidentifiable. IL28B genotype rs12979860:
, subtype unidentifiable. IL28B genotype rs12979860:  , CC;
, CC;  , CT;
, CT;  , TT.
, TT.
Across China, 84.1% of the study population was IL28B genotype CC (rs12979860), with little regional variation (P > 0.05; Fig. 2b). Similar distributions of genotypes for other IL28B SNPs associated with response to peginterferon/ribavirin therapy were observed (Tables S1 and S2).18–20 IL28B genotype CC was found in 79%, 89%, 96%, and 92% of patients with HCV genotypes 1, 2, 3, and 6 infections, respectively.
Patient demographics and clinical status
Enrolled patients had a median of 46 years of age and 54.8% male; 62% had HCV RNA levels exceeding 600 000 IU/mL. Patients with HCV genotype 3 or 6 infections had younger median ages and higher proportions were male, compared with genotype 1 or 2 (P < 0.001; Table 1). Current alcohol consumption and tobacco smoking were reported by 25.8% and 24.3% of patients, respectively; 7.6% of patients had a history of intravenous drug abuse. Evidence of liver disease was frequent. Alanine aminotransferase (ALT) levels were above the upper limit of normal in 63.4% of patients and more than twice the upper limit of normal in 28.4%. Cirrhosis was reported in 10.1% of patients, with evidence of hepatic decompensation in more than half of these. Among the 101 cirrhotic patients, 59.4%, 33.7%, and 6.9% were Child-Turcotte-Pugh categories A, B, and C, respectively. International normalized ratio and direct bilirubin level were elevated in 12.1% and 23.3% of patients, respectively.
Table 1.
Demographic and disease parameters by HCV genotype†
| Parameter | Genotype 1 (N = 582) | Genotype 2 (N = 240) | Genotype 3 (N = 91) | Genotype 6 (N = 63) | All genotypes (N = 997) |
|---|---|---|---|---|---|
| Age, median years (Q1, Q3) | 47.0 (39.0, 57.0) | 48.0 (39.0, 57.5) | 38.0 (32.0, 42.0) | 35.0 (31.0, 41.0) | 46.0 (37.0, 56.0) |
| Age at HCV diagnosis, mean years (SD) | 44.5 (12.9) | 44.6 (13.2) | 37.1 (8.6) | 38.5 (11.5) | 43.5 (12.8) |
| Male sex, n (%) | 303 (52.1) | 122 (50.8) | 69 (75.8) | 42 (66.7) | 546 (54.8) |
| Body mass index, mean kg/(m2) (SD) | 23.4 (3.3) | 23.6 (3.3) | 22.3 (3.1) | 23.1 (3.5) | 23.3 (3.3) |
| HCV RNA, mean log10 IU/mL (SD) | 5.9 (0.9) | 5.8 (1.0) | 5.9 (1.0) | 6.2 (0.9) | 5.8 (1.0) |
| ALT, median U/L (Q1, Q3) | 51.0 (32.0, 84.4) | 59.5 (34.0, 109) | 81.5 (52.0, 140) | 55.0 (31.0, 102) | 55.0 (33.0, 95.0) |
| Compensated cirrhosis, n (%) | 33 (5.7) | 12 (5.0) | 1 (1.1) | 1 (1.6) | 47 (4.7) |
| Decompensated cirrhosis, n (%) | 38 (6.5) | 9 (3.8) | 6 (6.6) | 1 (1.6) | 54 (5.4) |
| CTP score, mean (SD) | 5.3 (0.8) | 5.2 (0.7) | 5.3 (0.9) | 5.1 (0.4) | 5.2 (0.7) |
| CTP score in cirrhotics, mean (SD) | 6.3 (1.4) | ||||
| CTP score ≥ 7, n (%) | 42 (7.3) | 11 (4.6) | 6 (6.6) | 2 (3.2) | 62 (6.2) |
| Direct bilirubin, mean ìmol/L (SD) | 6.2 (13.1) | 5.4 (5.2) | 6.3 (4.8) | 5.0 (4.2) | 6.1 (12.3) |
| Portal hypertension present, n (%) | 16 (2.7) | 4 (1.7) | 1 (1.1) | 0 | 21 (2.1) |
| Ascites present, n (%) | 18 (3.1) | 7 (2.9) | 1 (1.1) | 1 (1.6) | 27 (2.7) |
| Splenomegaly present, n (%) | 62 (10.7) | 15 (6.3) | 3 (3.3) | 2 (3.2) | 83 (8.3) |
| Hepatocellular carcinoma, n (%) | 5 (0.9) | 0 | 0 | 0 | 5 (0.5) |
| Fatty liver history, n (%) | 48 (8.2) | 29 (12.1) | 14 (15.4) | 2 (3.2) | 95 (9.5) |
| Type 1 diabetes present, n (%) | 3 (0.5) | 4 (1.7) | 0 | 0 | 7 (0.7) |
| Type 2 diabetes present, n (%) | 44 (7.6) | 20 (8.3) | 7 (7.7) | 2 (3.2) | 74 (7.4) |
| Insulin resistance | 14 (2.4%) | 2 (0.8) | 4 (4.4) | 0 | 20 (2.0) |
| Hyperlipidemia, n (%) | 37 (6.4) | 7 (3.0) | 3 (3.3) | 4 (6.3) | 53 (5.3) |
Eighteen patients were infected with genotypes 1 + 2, and one patient each with genotypes 1 + 3, 1 + 6, or 1 + 2 + 6.
ALT, alanine aminotransferase; CTP, Child–Turcotte–Pugh; HCV, hepatitis C virus.
Indicators of advanced liver disease, such as cirrhosis, elevated bilirubin, portal hypertension, ascites, and splenomegaly, were generally more frequent in patients with HCV genotype 1 and 2 infections, compared with genotypes 3 or 6 (Table 1). The frequencies of some HCV-related and extra-hepatic disease parameters varied by host genotype; however, differences were inconsistent. Among evaluable patients (n = 997), five patients (0.5%) had hepatocellular carcinoma, and all of these five patients were genotype 1 and with decompensated cirrhosis. Overall, 10.3% of patients had abnormalities of glucose homeostasis (diabetes, insulin resistance); 5.3% had hyperlipidemia.
HCV transmission risk factors
HCV transmission risk factors varied by HCV genotype, with a general distinction between patients infected with genotype 1 or 2 versus genotype 3 or 6 (Fig. 3). Overall, 27% of patients reported exposure to more than one risk factor. Among patients with genotype 1 or 2, the most common reported risk factors were medical procedures such as blood transfusion (67% and 62% of patients, respectively), surgery (19.9% and 20.4%), and dental treatment (14.6% and 17.5%). Blood transfusion was a much less common risk factor for patients with HCV genotype 3 or 6 infection, reported by 15.4% and 17.5% of patients, respectively. In contrast, for patients with genotype 3 or 6, lifestyle-associated risk factors were more frequent, including IV drug abuse (40.7% and 34.9% of patients, respectively), and tattoos and piercings (19.8% and 11.1%). These lifestyle-associated risk factors were less common in patients with genotype 1 or 2; together, they were reported by 7.0% and 7.9% of patients, respectively.
Figure 3.
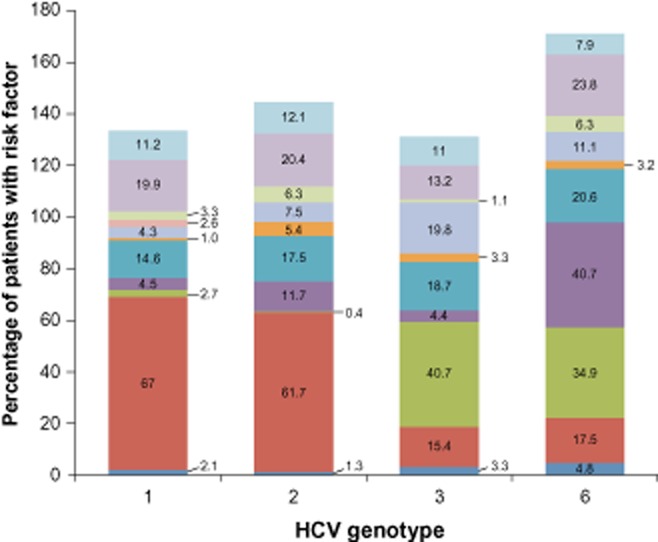
hepatitis C virus (HCV) transmission risk factors by HCV genotype. The proportions of patients with self-reported transmission risk factors are shown by HCV genotype. Some patients reported multiple factors.  , other;
, other;  , surgery, organ transplant;
, surgery, organ transplant;  , interventional exams and treatments;
, interventional exams and treatments;  , dialysis;
, dialysis;  , tattoos, piercings;
, tattoos, piercings;  , long-term HCV exposure;
, long-term HCV exposure;  , dental treatment;
, dental treatment;  , IV infusion;
, IV infusion;  , IV drug abuse;
, IV drug abuse;  , blood transfusion;
, blood transfusion;  , sex.
, sex.
Among patient's self reported possible infection routes (or exposures), the most common reported risk factors were medical procedures, but with substantial geographic regional variation: for instance, blood transfusion as a possible infection transmission route was found 69.3% in central region, 60.4% in West, 59.7% in East, 57.5% in North, and 40.1% in South; surgery as a possible route was reported 15.4%, 15.7%, 12.1%, 34.8%, and 27.6%, in the central region, West, East, North, and South, respectively; and dental treatment was reported 11.6%, 6.5%, 12.6%, 33.7%, and 23.0%, among the five geographic regions, respectively. IV drug abuse was a much more common risk factor for patients in western and southern regions, reported by 12.9% and 19.7% of patients, respectively. Interventional exams (endoscopy exam for example) and treatments and long-term HCV exposure (living together with patients with hepatitis C over a year) were more common in patients in southern than other regions, reported by 11.2% and 9.2% of patients. From this patient's self reported information, it is also noticed that blood transfusion as a possible route of HCV infection changed dramatically before and after 1993, 72% versus 47%, respectively; however, other possible routes were increased after 1993—for example, dental service as a possible infection route was reported 9% and 21%, before and after 1993, respectively.
The time of first potential exposure to HCV varied according to risk factor. First potential exposure tended to occur earlier for blood transfusion (mean 18.4 years before study enrollment) and surgeries (15.5 years before enrollment) than first exposure to dental treatment (11.8 years) or IV drug abuse (11.3 years).
Association of cirrhosis with potential risk factors
Among the 101 cirrhotic patients, HCV genotype was not significantly associated with cirrhosis in age-adjusted multivariate analysis (Fig. 4). Significant disease-related factors included low HCV viral load, hepatitis B virus coinfection, history of diabetes, low serum ALT, and high serum aspartate aminotransferase (AST). Among demographic and lifestyle parameters, high consumption of alcohol and pickled food was associated with cirrhosis. High alcohol consumption was defined as ≥ 40 g/day (men) or ≥ 20 g/day (women). Pickled food (salted/smoked/preserved food) consumption was defined as high (at least twice daily), average (twice weekly to once daily), or low (at most once weekly). There was a trend toward a negative association between high tea consumption and cirrhosis, but this did not reach statistical significance. IL28B genotype was not significantly associated with cirrhosis when added to the multivariate analysis. Factors not significant at P < 0.2 in the univariate analysis or were excluded before the final multivariate analysis included years since first exposure to HCV, age at first exposure, body mass index, hyperlipidemia, active smoking status, coffee consumption, protein consumption, weight loss, and appetite loss.
Figure 4.
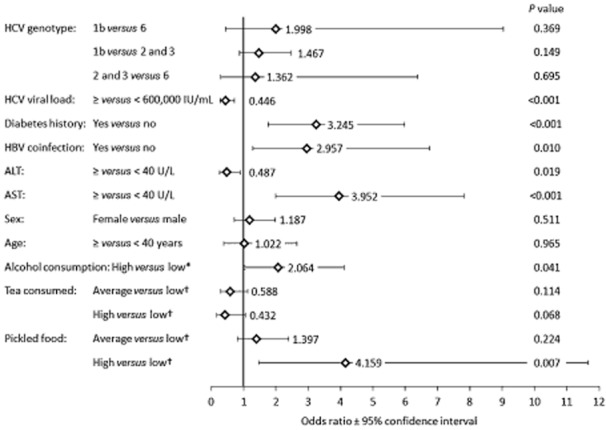
Correlates of cirrhosis. Odds ratios and 95% confidence intervals are shown for parameters entered into the multivariate model. *High alcohol consumption was defined as ≥ 40 g/day (men) or ≥ 20 g/day (women). †Tea and pickled food consumption defined as high (at least twice daily), average (twice weekly to once daily), or low (at most once weekly).
Discussion
This cross-sectional epidemiologic study, with stratified sampling based on regional populations, is the largest combined assessment of HCV and host genotypes that has been completed in China. We investigated potential links between HCV and IL28B genotypes, transmission risk factors, and clinical status. Our results indicate that HCV genotype 1b is the most prevalent genotype across China, consistent with previous studies, with genotype 1a found infrequently.1,2,10,12 However, we observed marked differences between geographic regions. The higher genotypic diversity and prevalence of genotypes 3 and 6 in the south and west may, in part, reflect the changing epidemiology of HCV in China.10,21,22 Previous reports indicate that in southwest China, the relative prevalence of genotypes 3 and 6 has increased significantly, with a corresponding decline in the prevalence of genotypes 1b and 2a, particularly among young patients.23
Transmission by intravenous drug abuse is most common in southern and western China, and has become more frequent and associated with genotype 3 or 6 infection, consistent with other data from China on transmission risk factors.22,24,25 In this regard, the younger median age of patients with genotype 3 or 6 in our study, compared with genotypes 1 and 2, is consistent with data indicating that younger, more recent heroin users are more likely to become heroin injectors, with the associated risk of HCV (and HIV) infection from contaminated needles.26 Correspondingly, since the early 1990s, when measures to improve medical safety achieved widespread implementation in China, HCV transmission through unsafe medical practices has dropped substantially.27 The older median age of patients with genotype 1 or 2 infections and the predominance of medical procedure-associated infection risk factors suggest that many genotype 1 or 2 infections were acquired before the early 1990s. Thus, regional differences in HCV genotype distribution may reflect differences in the timing of acquisition of HCV infection that may in turn influence the timing of the peak burden of cirrhosis.
As a consequence of these trends, regional differences in HCV genotype prevalence and epidemiology may warrant consideration of prevention and treatment strategies that are tailored to local needs. With the increased safety of blood transfusions and recent cultural changes, surgical procedures, dental treatment, and intravenous drug use have become more common transmission risk factors. Therefore, reducing the reuse of syringes has become a critical issue for preventing HCV infections. Public health initiatives to reduce injection drug abuse and/or the use of contaminated syringes, targeted primarily toward areas in southern and western China where drug abuse is most prevalent, may help reduce transmission of both HCV and HIV.
The high prevalence of IL28B genotype CC (rs12979860) in China is consistent with previous reports.8,28,29 In Asian populations, the high frequency of the IL28B C allele, and the predominance of HCV genotypes other than 1a, may contribute to the high reported rates (61–79%) of sustained virologic response (SVR) with peginterferon/ribavirin regimens.30,31 The IL28B T allele and HCV genotype 1a are both more common in Caucasian than in Asian patients, and are likely contributors to the lower (38–41%) reported SVR rates in Caucasians receiving peginterferon/ribavirin or triple regimens containing HCV protease inhibitors.4,5,32 The predominance in China of genotype 1b and IL28B genotype CC suggests that current triple regimens are likely to be highly effective in the Chinese patient population, potentially with reduced treatment durations and relatively low rates of drug resistance and treatment failure. Recent studies of a peginterferon-free regimen containing two direct-acting antivirals indicate that the risk of resistance is less in patients infected with HCV genotype 1b versus 1a; thus, regions with a high proportion of genotype 1b may gain more benefit from such regimens.33 Treatment of HCV genotype 2 or 3 infections with peginterferon/ribavirin provides high SVR rates in both Asian and Caucasian patients, and more potent triple regimens may not be necessary for these patients. Overall, the high prevalence of favorable host and viral genotypes in Asian patients may allow treatment strategies that differ from those most commonly applied in Western countries, both with current peginterferon/ribavirin-based regimens and possibly with future interferon-free therapies.
The slightly lower frequency of IL28B genotype CC in patients with HCV genotype 1 is interesting and may relate to epidemiologic differences. Infection risk factor data suggest a high proportion of long-duration infections in patients with genotype 1; thus, disproportionate loss of patients with IL28B genotype CC may have occurred because of greater spontaneous viral clearance.8,9 Further prospective study is needed to investigate this hypothesis, and to explore the relationships between viral and IL28B genotypes and the possible clinical consequences. It was also observed that cirrhosis was present in a somewhat higher proportion of patients with IL28B genotype CT or TT (13.8%) compared with CC (9.4%), although IL28B genotype was not significantly associated with cirrhosis in multivariate analysis. However, there were only 101 cirrhotic patients in our study, most of whom were IL28B genotype CC; extended follow-up of a larger cohort may clarify whether IL28B genotype influences fibrosis progression.
Multiple factors related to disease status, HCV infection, and lifestyle were significantly associated with the presence of cirrhosis. It is important to note that this was not a longitudinal study, and that the potential causality with respect to cirrhosis development cannot be assessed, and while the methodology used to diagnose cirrhosis is consistent with Chinese clinical practice and guidelines, the lack of biopsy confirmation of liver disease stage is a limitation of this analysis. Information on previous assessments of medical complications in the 101 cirrhotic patients is not available; however, it was confirmed that all patients were HCV treatment-naïve.
Several of the factors significantly associated with cirrhosis in this study have previously been shown to increase the risk of developing cirrhosis, including hepatitis B virus coinfection and high alcohol consumption.3,34 In contrast, low viral load has been associated with cirrhosis previously, most likely a consequence of reduced functional liver tissue, but is not considered a causal factor.35 A similar relationship may hold in this study. Similarly, the association of cirrhosis with high AST and low ALT levels may reflect the high proportion of decompensated liver disease in the cirrhotic population; advanced cirrhosis has been associated with progressive increases in the AST/ALT ratio.36 Type 2 diabetes is disproportionately prevalent in cirrhotics and may contribute to further disease progression, but the role of diabetes in the development of cirrhosis is uncertain.37
In the overall population, evidence of advanced liver disease was more common in patients with HCV genotype 1 than other genotypes. A relationship between HCV genotype and cirrhosis remains to be established; previous reports are inconsistent. HCV genotype 1b has been associated with increased risk of hepatocellular carcinoma in both European and Asian studies,38,39 but it remains uncertain whether this is due to accelerated fibrosis, increased oncogenic potential of this viral subtype, or to some confounding factor. In our multivariate analysis, odds ratios for genotype 1b versus other genotypes were high but not statistically significant, recommending further prospective study in a larger cohort that includes more non-genotype 1-infected patients.
The influence of high alcohol consumption on fibrosis in hepatitis C is well known; however, we also found links between cirrhosis and pickled food consumption, and a trend toward a negative relationship between cirrhosis and high consumption of tea. A beneficial effect of tea consumption has been reported previously,40 but the observed relationship with pickled food is novel. Further study of these environmental or lifestyle factors may be warranted because patients can often modify their exposure if causal relationships to fibrosis are established.
A limitation of this study's cross-sectional design is that it cannot establish cause–effect relationships between the findings and possible causes; the intention of this study is mainly to describe the demographic and clinical status of HCV patients who were naïve to HCV treatments. Nevertheless, this study has shown associations between key clinical observations and possible contributing factors that may be related to cirrhosis. These results have established a good foundation for generating hypotheses for future studies. Additionally, this study did not identify patients with possible HCV/HIV coinfection; it was due to a concern of potential impact on patient enrollment. Last, this study used a convenience sample for patient enrollment. We took this approach because HCV is generally a silent disease, and many patients would not be identified until they were seen and diagnosed; therefore, it would not be possible to assess actual patient numbers prior to enrollment. Given this situation, a randomization design was not possible. However, the study design considered populations from all geographic regions in China, and the numbers to enroll took the proportion of regional populations to the entire country to ensure that study enrollment would provide a representative sample of the regional distribution of the Han ethnic population.
Overall, these results present a clinical picture of chronic hepatitis C in treatment-naïve Han Chinese patients that varied according to Chinese region and HCV genotype, with lesser effects of host genotype. Some demographic and disease parameters appeared to differ according to HCV genotype, potentially reflecting both epidemiologic factors and HCV genotype-associated differences in natural history. The relatively frequent detection of advanced liver disease may reflect limitations on access to antiviral therapy, and suggests that earlier diagnosis and treatment, and greater awareness of factors that influence HCV-associated disease, may help avoid clinical complications and improve patient outcomes.
Acknowledgments
This work was supported by grants from the China National Science and Technology Major Project for Infectious Diseases Control during the 11th Five-Year Plan Period [grant numbers 2008ZX10002-013, 2008ZX10002-012] and 12th Five-Year Plan Period [grant number 2012ZX10002003], and from Bristol-Myers Squibb. Editorial assistance was provided by Dr Richard Boehme of Articulate Science, with funding from Bristol-Myers Squibb. Operational support and statistical analyses were provided by Research Pharmaceutical Services, Beijing, China.
Supporting Information
Table S1 Regional distribution of IL28B Genotypes.
Table S2 IL28B SNPs departures from Hardy-Weinberg Equilibrium.
References
- Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl. 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- Chen YD, Liu MY, Yu WL, et al. Hepatitis C virus infections and genotypes in China. Hepatobiliary Pancreat. Dis. Int. 2002;1:194–201. [PubMed] [Google Scholar]
- Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand-Abravanel F, Colson P, Leguillou-Guillemette H, et al. Influence of the HCV subtype on the virological response to pegylated interferon and ribavirin therapy. J. Med. Virol. 2009;81:2029–2035. doi: 10.1002/jmv.21583. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–129. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao HY, Sun DG, Jiang D, et al. IL28B genetic variants and gender are associated with spontaneous clearance of hepatitis C virus infection. J. Viral Hepat. 2012;19:173–181. doi: 10.1111/j.1365-2893.2011.01497.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Nakano T, He Y, Fu Y, Hagedorn CH, Robertson BH. Hepatitis C virus genotype distribution in China: predominance of closely related subtype 1b isolates and existence of new genotype 6 variants. J. Med. Virol. 2005;75:538–549. doi: 10.1002/jmv.20307. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Lok AS, Chan DT, Widell A. Greater diversity of hepatitis C virus genotypes found in Hong Kong than in mainland China. J. Clin. Microbiol. 1995;33:2931–2934. doi: 10.1128/jcm.33.11.2931-2934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wang Y, Xia W, et al. New trends of HCV infection in China revealed by genetic analysis of viral sequences determined from first-time volunteer blood donors. J. Viral Hepat. 2011;18:42–52. doi: 10.1111/j.1365-2893.2010.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bureau of Statistics of China. 2010. China Statistical Yearbook 2010: Total population by urban and rural residence and birth rate, death rate, natural growth rate by region (2009) . Cited 15 Oct 2010. Available from URL: http://www.stats.gov.cn/tjsj/ndsj/2009/indexch.htm.
- Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Chitturi S, Lau GK, Sollano JD Asia-Pacific Working Party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J. Gastroenterol. Hepatol. 2007;22:775–777. doi: 10.1111/j.1440-1746.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl. 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am. J. Hum. Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- Xia X, Lu L, Tee KK, et al. The unique HCV genotype distribution and the discovery of a novel subtype 6u among IDUs co-infected with HIV-1 in Yunnan, China. J. Med. Virol. 2008;80:1142–1152. doi: 10.1002/jmv.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu N, Liu J, et al. HCV subtype characterization among injection drug users: implication for a crucial role of Zhenjiang in HCV transmission in China. PLoS ONE. 2011;6:e16817. doi: 10.1371/journal.pone.0016817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang X, Mao Q, et al. Changes in modes of hepatitis C infection acquisition and genotypes in southwest China. J. Clin. Virol. 2009;46:230–233. doi: 10.1016/j.jcv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao YP, Liu ZM, Lu L. Review of HIV and HCV infection among drug users in China. Curr. Opin. Psychiatry. 2010;23:187–194. doi: 10.1097/YCO.0b013e328338658b. [DOI] [PubMed] [Google Scholar]
- Lai S, Chen J, Celentano D, et al. Adoption of injection practices in heroin users in Guangxi Province, China. J. Psychoactive Drugs. 2000;32:285–292. doi: 10.1080/02791072.2000.10400451. [DOI] [PubMed] [Google Scholar]
- Shan H, Wang JX, Ren FR, et al. Blood banking in China. Lancet. 2002;360:1770–1775. doi: 10.1016/S0140-6736(02)11669-2. [DOI] [PubMed] [Google Scholar]
- Chen JY, Lin CY, Wang CM, et al. IL28B genetic variations are associated with high sustained virological response (SVR) of interferon-alpha plus ribavirin therapy in Taiwanese chronic HCV infection. Genes Immun. 2011;12:300–309. doi: 10.1038/gene.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Chen JY, Lin TN, et al. IL28B SNP rs12979860 is a critical predictor for on-treatment and sustained virologic response in patients with hepatitis C virus genotype-1 infection. PLoS ONE. 2011;6:e18322. doi: 10.1371/journal.pone.0018322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ML, Dai CY, Huang JF, et al. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47:1884–1893. doi: 10.1002/hep.22319. [DOI] [PubMed] [Google Scholar]
- Kuboki M, Iino S, Okuno T, et al. Peginterferon alpha-2a (40 KD) plus ribavirin for the treatment of chronic hepatitis C in Japanese patients. J. Gastroenterol. Hepatol. 2007;22:645–652. doi: 10.1111/j.1440-1746.2007.04834.x. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N. Engl. J. Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- Chayama K, Takahashi S, Toyota J, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 55:742–748. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- Coffin CS, Terrault NA. Management of patients co-infected with HBV and HCV. Expert Rev. Anti Infect. Ther 2012. 2009;7:549–558. doi: 10.1586/eri.09.38. [DOI] [PubMed] [Google Scholar]
- Duvoux C, Pawlotsky JM, Bastie A, Cherqui D, Soussy CJ, Dhumeaux D. Low HCV replication levels in end-stage hepatitis C virus-related liver disease. J. Hepatol. 1999;31:593–597. doi: 10.1016/s0168-8278(99)80336-5. [DOI] [PubMed] [Google Scholar]
- Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch. Intern. Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856–1862. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- Bruno S, Crosignani A, Maisonneuve P, Rossi S, Silini E, Mondelli MU. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology. 2007;46:1350–1356. doi: 10.1002/hep.21826. [DOI] [PubMed] [Google Scholar]
- Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J. Clin. Oncol. 2010;28:4587–4593. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- Jin X, Zheng RH, Li YM. Green tea consumption and liver disease: a systematic review. Liver Int. 2008;28:990–996. doi: 10.1111/j.1478-3231.2008.01776.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Regional distribution of IL28B Genotypes.
Table S2 IL28B SNPs departures from Hardy-Weinberg Equilibrium.