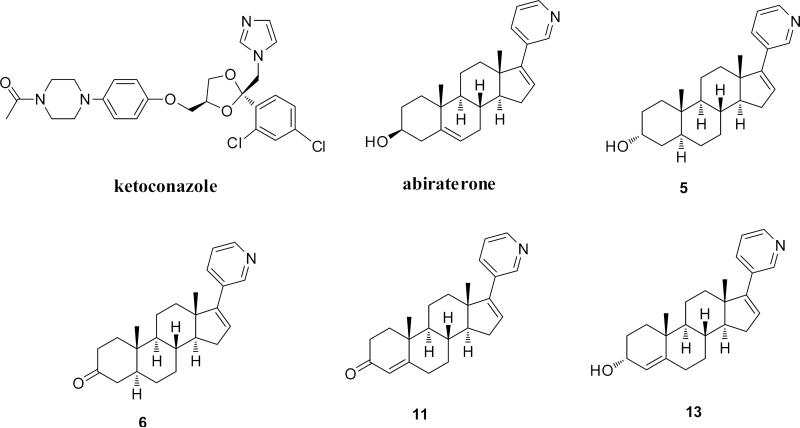
Abstract
Abiraterone acetate is a potent inhibitor of human cytochrome P450c17 (CYP17A1, 17α-hydroxylase/17,20-lyase) and is clinically used in combination with prednisone for the treatment of castration-resistant prostate cancer. Although many studies have documented the potency of abiraterone (Abi) in a variety of in vitro and in vivo systems for several species, the exact potency of Abi for human CYP17A1 enzyme has not yet been determined, and the structural requirements for high-potency steroidal azole inhibitors are not established. We synthesized 4 Abi analogs differing in the A-B ring substitution patterns: 3α-hydroxy-Δ4-Abi (13), 3-keto-Δ4-Abi (11), 3-keto-5α-Abi (6), and 3α-hydroxy-5α-Abi (5). We measured the spectral binding constants (Ks) using purified and modified human CYP17A1 along with the determination constants (Ki) applying a native human CYP17A1 enzyme in yeast microsomes for these compounds as well as for ketoconazole. For Abi, 3-keto-Δ4-Abi, 3-keto-5α-Abi, and 3α-hydroxy-5α-Abi, the type 2 spectral changes gave the best fit for a quadratic equation, since in these experiments Ks values were 0.1-2.6 nM, much lower than that for ketoconazole and 3α-hydroxy-Δ4-Abi (Ks values were 140 and 1660 nM, respectively). Inhibition experiments showed mixed inhibition patterns with Ki values of 7-80 nM. Abi dissociation from the CYP17A1-Abi complex was incomplete and slow; the t1/2 for dissociation was 1.8 hour, with 55% of complex remaining after 5 hours. We conclude that Abi and the 3 related steroidal azoles (3-keto-Δ4-Abi, 3-keto-5α-Abi, and 3α-hydroxy-5α-Abi), which also mimic natural substrates, are extraordinarily potent inhibitors of human CYP17A1, whereas the 3α-hydroxy-Δ4-Abi is moderately potent and comparable to ketoconazole.
1. Introduction
It has been 70 years since Huggins first introduced the concept of androgen-deprivation therapy (ADT) for advanced prostate cancer [1], and at the present time, ADT is the primary treatment for advanced prostate cancer. In parallel to this, there has been 30 years of off-label use of the antimycotic drug ketoconazole for the treatment of androgen resistant prostate cancer, which is also called castration-resistant prostate cancer (CRPC) [2], because of its off-target inhibition of CYP17A1. This enzyme has both 17α- hydroxylase and 17,20-lyase activities, thus mediating the biosynthesis of both cortisol and sex steroids [3]. Recently, the FDA approved abiraterone acetate (Zytiga), a prodrug for the selective CYP17A1 inhibitor abiraterone (Abi), which is about 20 times more potent than ketoconazole, for the treatment of CRPC. Abi significantly suppresses serum androgen concentrations derived from both adrenal and testicular sources within the prostate cells, thereby improving the survival in men with metastatic CRPC both before and after docetaxol failure [4]. Abi inhibition, however, also impairs cortisol synthesis, leading to a compensatory increase in ACTH and mineralocorticoid excess, a clinical syndrome of 17-hydroxylase deficiency, which includes edema, hypokalemia and hypertension[5]. As a result, Abi must be co-administered with prednisone to prevent endocrine side effects. Nevertheless, the significant reduction in androgens by Abi might also be useful in the management of hormone-dependent breast cancer and in androgen excess disorders, including polycystic ovary syndrome and congenital adrenal hyperplasia.
A general approach for the synthesis of potent steroidal CYP17A1 inhibitors is to modify the steroid scaffold by attaching a heterocycle as a functional group onto the 17-position, which forms a strong complex with the heme iron of the enzyme. Abi has a 17-(3-pyridyl) substituent together with a 16,17-double bond, and these structural features are a stringent requirement for potent inhibition. Substituents having a 2- or 4-pyridyl function instead of the currently used 3-pyridyl group showed a poor inhibitory activity; on the other hand, a reduction of the 16,17-double bond also diminished potency [6]. The crystal structure of the CYP17A1 complex with Abi at 2.6 Å resolution demonstrated that the C-17-(3-pyridyl) group of the inhibitor binds to the heme iron, and forms a 60° angle with the steroid nucleus above the heme plane and packs against the central I helix [7]. Furthermore, it was suggested that the hydrogen bonding interaction between the 3β-OH of Abi and the conserved polar asparagine 202 in the F helix is crucial for high-affinity binding. Nevertheless, the A ring stereochemistry and functionality of the substituent at C-3 of the Abi skeleton on the inhibitory activity of CYP17A1 enzyme has not yet been explored. In the present study, we report the design, the synthesis and characterization of CYP17A1 inhibitors of a series of 16-dehydro-17-(3-pyridyl) steroidal derivatives (compounds 5, 6, 11 and 13) based on known substrate tolerance (Figure 1).
Figure 1.
Compounds studied as CYP17A1 inhibitors herein.
2. Experimental procedures
2.1. Materials and methods
NMR spectra were obtained using Varian instruments at frequencies for 1H and 13C as specified for each compound in the experimental section. High-resolution mass spectra were obtained on a Micromass AutoSpec Ultima instrument. Chemical shifts were referenced to chloroform peak in the 1H NMR and are assigned at 7.26 ppm; in the 13C NMR, this signal was assigned at 77.16 ppm. Reaction progress was determined either by TLC monitoring, or an aliquot analysis by NMR. The purity of compounds 5, 6, 11, and 13 was assessed by HPLC analysis using the method in section 2.3.2. NMR spectra, mass spectra, and HPLC tracings are provided in the supplementary data file. Progesterone and all other reagents and solvents were purchased from Sigma Aldrich (St. Louis, MO), Steraloids (Newport, RI), ThermoFisher Scientific (Pittsburgh, PA), or as specified. Protein determinations used the Coomassie Plus Reagent (Pierce, Rockford, IL). Modified human CYP17A1 was expressed in E. coli JM109 cells and purified to homogeneity as described [8]. Abi was synthesized as described [9].
2.2. Chemical synthesis
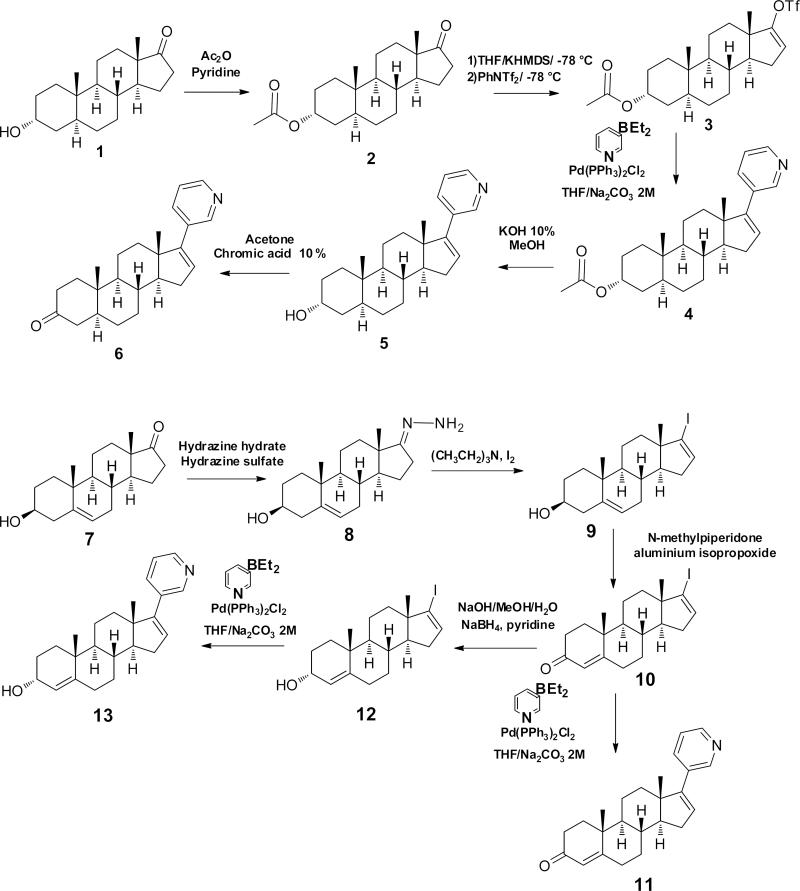
2.2.1. 3α-Acetoxy-5α-androsta-16-ene-17-yl-trifluoromethanesulfonate (3)
A solution of compound 2 (250 mg, 0.752 mmol) in tetrahydrofuran (3 mL) at -78 °C was treated with potassium bis(trimethylsilyl)amide (KHMDS, 0.5 M, 1.5 mL, 0.75 mmol) and stirred for 1 h at −78 °C. N-phenylbis-(triflouromethanesulfonamide) (323 mg, 0.905 mmol), was added, and the reaction was stirred at −78 °C for 2 h, then slowly warmed to room temperature and quenched with saturated NH4Cl. The compound was extracted with ethyl acetate. The organic phase was washed with water, dried over Na2SO4 and concentrated under reduced pressure. The compound was purified by silica gel column chromatography (hexanes to 10% ethyl acetate in hexanes). Yield: 58%. 13H NMR (400 MHz, CDCl3) δ: 5.5 (s, 1H), 4.9 (s, 1H), 1.9 (s, 3H), 0.9 (s, 3H), 0.7 (s, 3H).
2.2.2. 3α-Acetoxy-17-(3-pyridyl)-5α-androsta-16-ene (4)
A suspension of compound 3 (550 mg, 1.18 mmol), diethyl-3-pyridylborane (265 mg, 1.81 mmol), bis(triphenylphosphine)palladium (II) chloride (8.12 mg, 0.0116 mmol) in THF (8 mL) was added to an aqueous solution of sodium carbonate (2 M, 5 mL). The mixture was refluxed for 4 h under N2. The reaction was concentrated under reduced pressure, and the residue was extracted with diethylether; the organic phase was washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The compound was purified on florisil column chromatography (hexanes to 50% ethyl acetate in hexanes). Yield: 42%. 13C NMR (400 MHz, CDCl3) δ: 170.6, 151.7, 147.8, 143.7, 140.8, 133.6, 129.1, 122.9, 69.9, 21.5, 11.3, 9.1.
2.2.3. 17-(3-pyridyl)-5α-androsta-16-ene-3α-ol (5)
Compound 4 (470 mg, 1.2 mmol) was dissolved in methanol (5 mL) at room temperature. A solution of KOH (10%) in methanol (3.5 mL) was added, and the mixture was stirred for 1.5 h, then concentrated under reduced pressure. Dichloromethane (25 mL) and water (25 mL) were added, and the mixture was stirred for 1 h. The organic phase was dried over Na2SO4. The compound was purified on florisil column chromatography (hexanes to 50% ethyl acetate in hexanes). Yield: 68%, purity 99.3% by HPLC. 1H NMR (400 MHz, CDCl3) δ: 8.6 (s, 1H), 8.4 (d, J = 4.8, 1H), 7.6 (d, J = 7.6, 1H), 7.2 (dd, J1 = 4.8 Hz and J2 = 7.6 Hz, 1H), 5.9 (d, J = 1.6 Hz), 4.0 (m, 1H), 2.2 (d, 1H), 2.0 (t, 2H), 1.2-1.8 (m, 17H), 0.9 (s, 3H), 0.8 (s, 3H). 13C NMR (400 MHz, CDCl3) δ: 151.5, 147.4, 147.3, 133.9, 133.1, 129.3, 123.1, 66.3, 57.5, 54.5, 47.5, 39.3, 36.2, 35.3, 34.0, 31.8, 31.7, 29.0, 28.4, 20.7, 16.7, 11.2. Measured m/z for [M+H] = 352.2642 and predicted m/z = 352.2635.
2.2.4. 17-(3-pyridyl)- 5α-androsta-16-ene-3-one (6)
To a solution of compound 5 (50 mg, 0.14 mmol) in acetone (4 mL), chromic acid 10% (w/v) (4.5 mL) was drop-wise added at 0 °C. The mixture was stirred at room temperature for 3 h, and a solution of sodium bicarbonate was then added to a pH = 7. The reaction mixture was extracted with ethyl acetate, and the organic phase was washed with water and dried over Na2SO4. The compound was purified on silica gel column chromatography (hexanes to 40% ethyl acetate in hexanes). Yield: 30%, purity 99.4%. 1H NMR (400 MHz, CDCl3) δ: 8.6 (s, 1H), 8.4 (d, J = 4.8, 1H), 7.6 (d, J = 7.6, 1H), 7.2 (dd, J1 = 4.8 Hz and J2 = 7.6 Hz, 1H), 5.9 (d, J = 1.6 Hz), 2.4-1.2 (m, 20H), 1.1 (s, 3H), 1.0 (s, 3H). 13C NMR (400 MHz, CDCl3) δ: 211.8, 151.5, 147.4, 147.3, 134.2, 133.1, 129.3, 123.1, 66.3, 57.5, 54.5, 47.5, 39.3, 36.2, 35.3, 34.0, 31.8, 31.7, 29.0, 28.4, 20.7, 16.7, 11.2. Measured m/z for [M+H] = 350.2489 and predicted m/z = 350.2478.
2.2.5. 17-Iodoandrosta-5,16-dien-3β-ol (9)
Into a 100 mL round-bottomed flask, fitted with a magnetic stirring bar, was placed dehydroepiandrosterone (7) (1 g, 3.47 mmol) and ethanol (18 mL). Hydrazine hydrate solution (4.5 mL, 67.3 mmol) was added followed by a solution of hydrazine sulfate (0.05 g, 0.384 mmol) in water (0.5 mL). After stirring at room temperature during 18 h, the mixture was poured into cold water. The precipitate was collected by filtration and dried at room temperature to yield the hydrazone 8 (96% yield). To a solution of compound 8 (2.3 g, 7.61 mmol) and triethylamine (9 mL, 64.82 mmol) in dioxane (42 mL) was added iodine (4.117 g, 16.22 mmol) in portions over 30 min. The mixture was stirred at room temperature for an additional 1 h, it was poured into 10% Na2SO3 (50 mL), and the precipitate was collected by filtration, washed with cold water, and recrystallized from ethanol to yield the iodo derivative 9. Yield: 50%. 1H NMR (400 MHz, CDCl3) δ: 6.1 (d, 1H), 5.3 (d, 1H), 3.5 (m, 1H), 1.(s, 3H), 0.7 (s, 3H).
2.2.6. 17-Iodoandrosta-4,16-dien-3-one (10)
A solution of 9 (300 mg, 0.7531 mmol) in toluene (60 mL) and N-methylpiperidone (12 mL) was refluxed; 35 mL of toluene was distilled off using a Dean-Stark apparatus. Aluminium isopropoxide (800 mg, 3.9 mmol) was added, and the reaction was refluxed for 6 h. The reaction mixture was extracted with ethyl acetate, it was washed with brine, dried over Na2SO4, and concentrated under reduced pressure. The crude compound was purified by silica gel column chromatography (hexanes to 15% ethyl acetate in hexanes). Yield: 75%. 1H NMR (400 MHz, CDCl3) δ: 6.1 (d, 1H), 5.7 (s, 1H), 1.1 (s, 3H), 0.7 (s, 3H).
2.2.7. 17-(3-pyridyl)androsta-4,16-dien-3-one (11)
A stirred solution of 10 (0.1 g, 0.2729 mmol) in THF (1.2 mL) was purged with nitrogen, and bis(triphenylphosphine)palladium (II) chloride catalyst (0.0017 g, 0.0024 mmol) was added, followed by diethyl(3-pyridyl)borane (0.048 g, 0.3264 mmol). To the resultant orange solution was added an aqueous solution of sodium carbonate (2M, 0.8 mL). After refluxing for 7 h, diethyl ether was added, and the organic phase was washed with water and dried over sodium sulfate. The compound was purified on florisil column chromatography (hexanes to 40% ethyl acetate in hexanes). Yield: 39%, purity 99.2%. 1H NMR (400 MHz, CDCl3) δ: 8.6 (s, 1H), 8.4 (d, J = 4.8, 1H), 7.6 (d, J = 7.6, 1H), 7.2 (dd, J1 = 4.8 Hz and J2 = 7.6 Hz, 1H), 5.9 (d, J = 1.6 Hz), 5.7 (s, 1H), 2.5-1.2 (complex, 16H), 1.2 (s, 3H), 1.0 (s, 3H). 13C NMR (400 MHz, CDCl3) δ: 199.3, 170.8, 151.3, 147.5, 147.4, 133.9, 132.8, 129.2, 123.9, 123.1, 56.7, 53.9, 38.6, 35.5, 35.0, 34.1, 33.9, 32.7, 31.7, 31.6, 20.8, 17.2, 16.5. Measured m/z for [M+H] = 348.2328 and predicted m/z = 348.2322.
2.2.8. 17-Iodoandrosta-4,16-dien-3α-ol (12)
An ice-cold solution of sodium hydroxide (10 mg, 0.250 mmol) in water (0.1 mL) and methanol (2.5 mL) was added to a vigorously stirred ice-cold solution of compound 10 (50 mg, 0.126 mmol) in methanol (3 mL). An ice-cold solution of sodium borohydride (20 mg, 0.538 mmol) in pyridine (1.1 mL) was added, and the mixture was stirred at room temperature for 1 h. The reaction was quenched with 5% HCl and extracted with diethyl ether. The organic phase was washed with a saturated solution of sodium bicarbonate and dried over anhydrous sodium sulfate. The compound was purified on silica gel column chromatography (hexanes to 30% ethyl acetate in hexanes). Yield: 46%. 1H NMR (400 MHz, CDCl3) δ: 6.1 (s, 1H), 5.3 (s, 1H), 4.1 (bs, 1H), 1.0 (s, 3H), 0.7 (s, 3H). 13C NMR (400 MHz, CDCl3) δ: 146.6, 137.4, 123.8, 112.9, 67.5, 18.8, 15.2.
2.2.9. 17-(3-pyridyl)androsta-4,16-dien-3α-ol (13)
To a stirred solution of 12 (500 mg), bis(triphenylphosphine)palladium(II) chloride catalyst (10 mg), and diethyl-(3-pyridyl)borane (250 mg) in 6 mL of THF was added 5 mL of 2 M aqueous sodium carbonate. The mixture was refluxed for 4 h, diethyl ether was added, and the organic phase was washed with water and dried over sodium sulfate. The crude compound was purified on florisil column chromatography (hexanes to 40% ethyl acetate in hexanes). Yield: 66%. 1H NMR (400 MHz, CDCl3) δ: 8.6 (s, 1H), 8.5 (d, J = 4.8, 1H), 8.1 (d, J = 7.6, 1H), 7.6 (dd, J1 = 4.8 Hz and J2 = 7.6 Hz, 1H), 6.2 (d, J = 1.6 Hz), 5.3 (s, 1H), 4.1 (bs, 1H), 2.4-0.9 (m, 16H), 1.1 (s, 3H), 1.0 (s, 3H). 13C NMR (400 MHz, CDCl3) δ: 13C NMR (400 MHz, CDCl3) δ: 151.5, 147.4, 147.3, 134.2, 133.1, 129.3, 123.5, 123.1, 66.3, 57.5, 54.5, 47.5, 39.3, 36.2, 35.3, 34.0, 31.8, 31.7, 29.0, 28.4, 20.8, 18.3, 16.5. Measured m/z for [M+H]= 350.2481 and predicted m/z = 350.2478.
2.3. Enzymology studies
2.3.1. Spectral assay for ligand binding
Spectral binding titrations with Abi and related compounds were carried out with purified CYP17A1 from E coli at room temperature using a UV-visible scanning spectrophotometer (UV-2600; Shimadzu Scientific Instruments, Columbia, MD). The CYP17A1 protein was diluted to 200 nM in 100 mM potassium phosphate buffer, pH 7.4, containing 20% glycerol. The diluted protein was equally divided between two 1.0 mL quartz cuvettes (1-cm path length), then aliquots of the steroidal ligand dissolved in 100% methanol were added to the sample cuvette, and an equal volume of methanol was added to the reference cuvette. After the solution was mixed, spectra were recorded at 350-490 nm with a 1 min interval until no further increase in the Δ(A426 - A392) value was observed (typically 3–5 min). During the spectral titrations, the total amount of methanol added did not exceed 1.5% by volume. GraphPad Prism 6.02 software (GraphPad Software Inc., San Diego, CA) was used to calculate spectral binding constants (Ks) by fitting spectral data into one of the following equations:
| (1) |
or
| (2) |
Where ΔA is the spectral response at different ligand concentrations [L], ΔAmax is the maximal amplitude of the spectral response, and [E] is the total enzyme concentration. Equation 1 was applied when the value of Ks was lower than the enzyme concentration, and equation 2 was used when Ks was similar or higher than the enzyme concentration, assuming 1:1 binding stoichiometry.
2.3.2. CYP17A1 activity assays
The hydroxylase activity of CYP17A1 was determined by measuring the conversion of progesterone to 17-hydroxyprogesterone and 16α-hydroxyprogesterone. Microsomes were prepared from an yeast strain that expresses human CYP17A1 and human P450-oxidoreductase (POR), according to the methods described [10]. The test inhibitor (Scheme 1, 5–80 nM, delivered in <5 μL methanol) and microsomes (0.5–1.5 μL, 5-15 pmol P450) were pre-incubated in 0.25 mL 50 mM potassium phosphate (pH 7.4) at 37 °C for 3 min. Progesterone (0.4-40 μM) and [3H]-progesterone (0.1 μCi/μL) were added to the mixture, which was incubated at 37 °C for 3 min. NADPH (1 mM) was added, and an additional incubation was carried out at 37 °C for 20 min. The reaction was stopped by the addition of 1 mL of dichloromethane, and the organic phase was collected and dried under a nitrogen flow. The steroids were analyzed using an Agilent 1260 Infinity HPLC system with UV detector and a β-RAM4 in-line scintillation counter (LabLogic, Brandon, FL). Steroids were dissolved in 20 μL of methanol, and 5 μL injections were resolved with a 50 mm x 2.1 mm, 26 μm, C8 Kinetex column (Phenomenex, Torrence, CA) equipped with a guard column at a flow of 0.4 mL/min. Aqueous methanol linear gradients were employed (27% methanol from 0 to 0.5 min, jump to 39% methanol, and gradient from 39 to 75% methanol over 30 min). Products were identified by retention times of external standards chromatographed at the beginning of the experiment using radiochemical detection of [3H]-labeled steroids with Bio-safe II scintillation cocktail (Research Products International, Mount Prospect, IL) at a flow rate of 1.2 mL/min. The data were processed with Laura 4 (LabLogic) program. Inhibitory constants Ki were determined by fitting v vs [S] using Origin 7.0 (OriginLab, Northampton, MA) to determine Km and Vm max constants and with Lineweaver-Burk (v− vs [S]−1) plots to determine the mode of inhibition. Ki values were calculated for competitive and noncompetitive inhibition using the following equations:
| (3) |
| (4) |
Where K′m is the apparent Km in the presence of inhibitor at [I], and V′max is the apparent Vmax in the presence of inhibitor at [I].
Scheme 1.
Synthetic pathway for abiraterone derivatives
2.3.3. Dissociation kinetics
Purified CYP17A1 from E coli (800 nM) was incubated with increasing amount of Abi (20-900 nM) to achieve complete binding of the inhibitor to the enzyme. The enzyme-Abi complex was then rapidly diluted 5-fold into 100 mM potassium phosphate buffer (pH 7.4) containing 20 μM of allopregnanolone. The loss of Abi binding was followed by a recording spectra as a function of time over 300 min after dilution to monitor conversion from type II to type I binding and to estimate the rate constant (koff) for Abi-CYP17A1 dissociation.
3. Results
3.1. Synthesis of compounds
The synthetic approach to novel 16-dehydro-17-(3-pyridyl) steroidal derivatives are outlined in Scheme 1. Our strategy employed Suzuki coupling of diethyl-(3-pyridyl)borane with a steroidal vinyl triflate or iodide, similar to the synthesis of Abi. The commercially available steroidal starting materials, androsterone and dehydroepiandrosterone facilitated the synthesis of the final products—compounds 5, 6, 11 and 13—using standard reactions such as: oxidation, reduction and protecting group removal.
3.2. Inhibitor binding spectra and Ks determinations
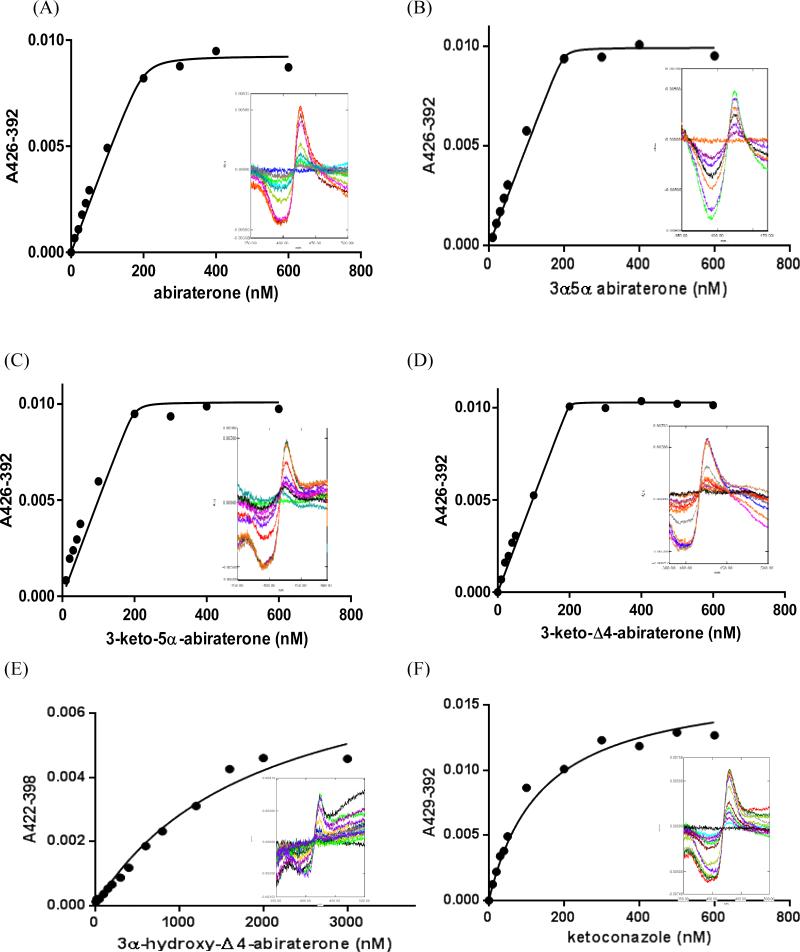
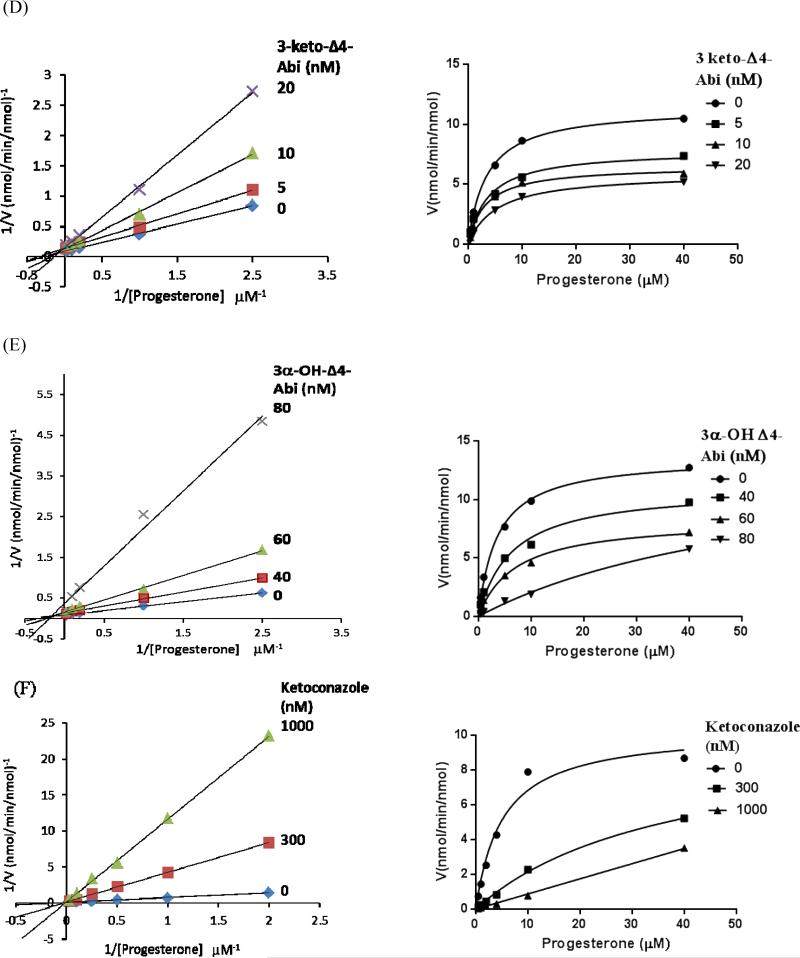
The binding of the steroidal inhibitors to the heme iron of CYP17A1 was investigated using UV-visible difference spectroscopy. Compounds 5, 6, 11, and 13, as well as Abi and ketoconazole, each induced a type II difference spectrum as shown in Figure 2, indicating coordination of pyridine-ring nitrogen to the heme iron of CYP17A1, with formation of low-spin iron. The Soret peak maximum was centered at 426 nm for the enzyme complex with Abi and for compounds 5, 6, and 11, while for compound 13 and ketoconazole, it was shifted to 422 and 429 nm, respectively. These spectral binding constants (Ks) for compounds 5, 6, and 11 were in the subnanomolar-to-low nanomolar range and similar to that of Abi (0.1-2.6 nM, Table 1). In fact, these inhibitors bind to CYP17A1 with such high affinity that the population of free inhibitor molecules is significantly depleted by the formation of the enzyme-inhibitor complex. For these tight binding inhibitors, the steady state approximations are no longer valid, and an alternative method as described in the experimental procedures was used for data analysis to correctly calculate the binding constants Ks for the enzyme-inhibitor complexes. Surprisingly, the Ks for compound 13 (1660 nM) is over 3 orders of magnitude higher than that for the related compounds 5 and 11, which differ only by the oxidation state and stereochemistry at carbon 3 or the saturation at the A-B ring junction of carbons 4 and 5. By comparison, the reference compound ketoconazole gave an intermediate Ks value of 140 nM. These data demonstrated that the 3β-hydroxyl group of Abi is not necessary for a tight binding to CYP17A1 and that other substitution patterns might yield better inhibitors, with the exception of the combination of a Δ4- double bond with a 3α-hydroxyl group at carbon-3.
Figure 2.
Spectrophotometric equilibrium binding titrations of CYP17A1 with abiraterone and related inhibitors. Difference spectra were obtained upon titration of 200 nM CYP17A1 in 0.1 M potassium phosphate buffer, pH 7.4, containing 20% glycerol (v/v) with inhibitors at 20-600 nM. CYP17A1 titration with (A) abiraterone, (B) compound 5, (C) compound 6, (D) compound 11, (E) compound 13, and (F) ketoconazole. Progressive shifts in the UV-visible difference spectra indicate nitrogen binding to heme iron. Plots of Δ(A426 - A392) versus concentration of inhibitor werefit to a equations (1) or (2) using GraphPad Prism (see “Experimental Procedures”). The resulting Ks values were 2.6 nM for abiraterone, less than 1 nM for compounds 5, 6, and 11, 1660 nM for compound 13, and 140 nM for ketoconazole.
Table 1.
Inhibition of CYP17A1 Hydroxylase Activity by Abiraterone and Related Compounds
| Inhibitor | K s(nM) | Ki(nM) | Typea |
|---|---|---|---|
| Abiraterone | 2.6±3.2 | 27 | M |
| 3α-hydroxy-5α-Abi (cpd 5) | 0.6±1.3 | 14 | M |
| 3-keto-5α-Abi (cpd 6) | 0.7±2.8 | 7 | M |
| 3-keto-△4-Abi (cpd 11) | 0.1±0.4 | 22 | M |
| 3α-hydroxy-△4-Abi (cpd 13) | 1660±286 | 80 | N |
| Ketoconazole | 140 ± 3 | 98 | M |
CYP17A1 activities were determined by using progesterone as a substrate
Type of inhibition: M, mixed competitive-noncompetitive inhibition; N, primarily noncompetitive inhibition. Data are the means (± SE) of 2-4 experiments.
3.3. CYP17A1 inhibition
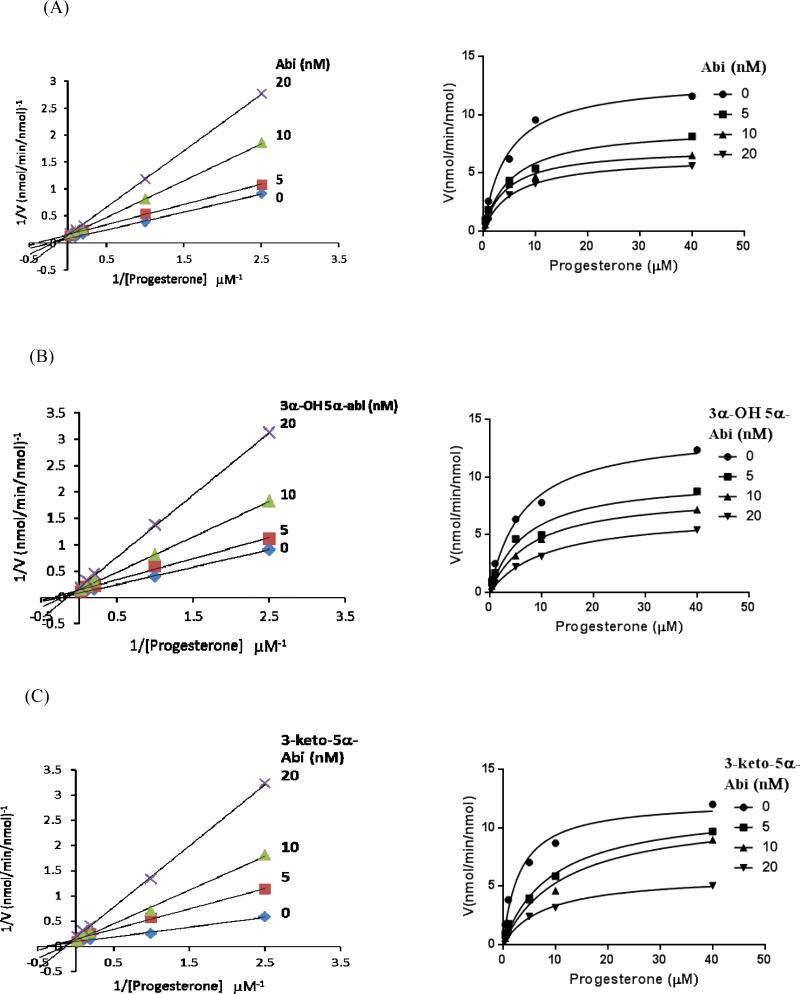
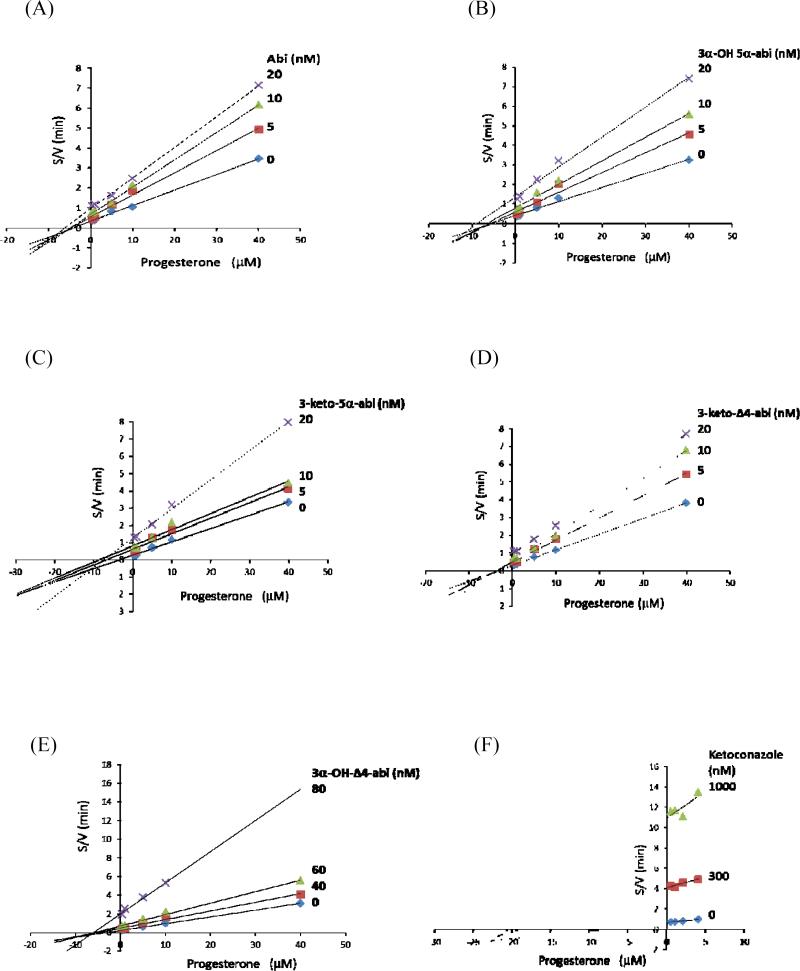
All synthesized compounds were found to inhibit CYP17A1 17- and 16α-hydroxylase activities (Figure 3). The Ki values are presented with comparison to ketoconazole and Abi in Table 1. All 3-keto compounds (detailed structures in Schemes 1) were strong inhibitors (Ki< 22 nM) with 3-keto-5α-Abi (compound 6, Ki=7 nM) being the most potent. Compound 5, 3α-hydroxy-5α-Abi remained highly potent (Ki =14 nM) in inhibiting CYP17A1 hydroxylation as compared to Abi (Ki = 27 nM), despite the opposite stereochemistry of the 3-hydroxyl group. In contrast, compound 13, 3α-hydroxy-Δ4-Abi, was a less potent inhibitor (Ki=80 nM) than the other steroidal azoles but similar to ketoconazole (Ki=98 nM). For compound 13, the discrepancy between its Ki and Ks values suggests that its mode of inhibition is largely not active-site directed. Consistent with this model, compound 13 showed primarily non-competitive inhibition at higher inhibitor concentrations, whereas all other compounds showed mixed but primarily competitive inhibition (Figure 3). Inhibition of 17,20-lyase activity was similar to that of hydroxylase activities (not shown). The mode of inhibition was confirmed after analyzing the data using Cornish-Bowden plots. This analysis showed that Abi, ketoconazole, and compounds 5, 6, and 11 all act as mixed-type inhibitors of CYP17A1, whereas 3α-hydroxy-Δ4-abi (compound 13) is a non-competitive inhibitor (Figure 4).
Figure 3.
Lineweaver-Burke plots and v versus [S] plots of human CYP17A1 17-hydroxylase activity in the presence of inhibitors (A) abiraterone, (B) compound 5, (C) compound 6, (D) compound 11, (E) compound 13, and (F) ketoconazole. Yeast microsomes containing human CYP17A1 and POR were incubated with 0.4–40 μM progesterone in the absence or presence of 5-80 nM inhibitors. The resulting Ki values are 27 nM for abiraterone, 14 nM for compound 5, 7 nM for compound 6, and 22 nM for compound 11, using inhibitor concentrations of 10-20 nM. The Ki value is 80 nM for compound 13 using inhibitor concentrations of 40-60 nM. The Ki value for ketoconazole is 98 nM using inhibitor concentration of 1000 nM. These plots show mixed competitive-noncompetitive inhibition for abiraterone, compounds 5, 6, and 11 (A-D), and ketoconazole (F). In contrast, compound 13 inhibition of CYP17A1 is primarily noncompetitive (E). Data points are the means of 2-4 experiments.
Figure 4.
Cornish-Bowden plots illustrating the mode of inhibition of CYP17A1-mediated progesterone hydroxylation by abiraterone and related compounds. V, initial rate (velocity) of the reaction; S, substrate concentration.
3.4. Abi-CYP17A1 dissociation rate
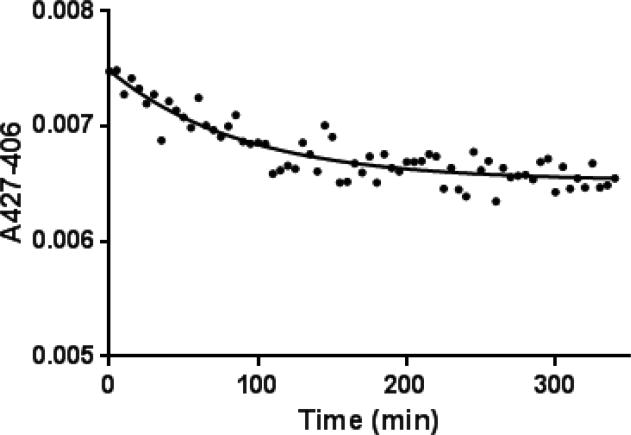
To determine the rate of ligand dissociation, the equilibrated CYP17A1-Abi complex was rapidly diluted with a competing substrate (allopregnanolone), and the amount of residual bound Abi was then spectroscopically measured as a function of time. The combination of the larger reduction in Abi concentration and the high concentration of the competing substrate caused by the jump-dilution results in conditions, which strongly disfavor Abi rebinding after dissociation. As shown in Figure 5, upon addition of allopregnanolone, 55% of the type II complex remained intact after 5 h. A plot of residual bound Abi as a function of time was then fitted to one phase decay equation and the koff was estimated to be 0.0093 min-1 or t1/2 of 1.8 h.
Figure 5.
A jump-dilution experiment to measure the dissociation rate (koff) of the CYP17A1•abiraterone complex. Initially, abiraterone and the enzyme were allowed to bind until equilibrium was reached. At that point, dissociation was initiated by introduction of excess allopregnanolone, a CYP17A1substrate with high affinity (Ks = 1.2 nM, not shown). Binding decreased over the first 3 h incubation. A koff value of 0.0093 min-1 was obtained from fitting the data to a single-phase exponential decay equation (Prism 6.02; GraphPad Software).
4. Discussion
In the present study, we synthesized four Abi analogs with various A-B ring functional groups and found that all except the 3α-hydroxy-Δ4 Abi derivative retained very high potency, based on both Ki values for native CYP17A1 in yeast microsomes and Ks values for purified, modified CYP17A1 from E coli. These compounds were designed to mimic the naturally occurring pregnane substrates pregnenolone (3β-hydroxy-Δ5-Abi), progesterone (3-keto-Δ4-Abi), 5α-dihydroprogesterone (3-keto-5α-Abi), and allopregnanolone (3α-hydroxy-5α-Abi). The very high potency of compound 5 (3α-hydroxy-5α-Abi) reflects the high affinity of CYP17A1 for steroids with this A-ring stereochemistry [11]. In fact, 5α-pregnan-3α,17α-diol-20-one (17-hydroxyallopregnanolone), which has the same A/B-ring structure as 3α-hydroxy-5α-Abi, is the best known substrate for the 17,20-lyase activity of human CYP17A1 [11]. Our results demonstrated also that the stereochemistry at C-3 of Abi (3β-hydroxy) is not a major contributor to its very high affinity for CYP17A1. The binding affinities of CYP17A1 for Abi and compounds 5, 6, and 11 were so enhanced that their spectral binding constants Ks could only be calculated using equation 1, since the Ks values were much lower than the enzyme concentration. The koff for Abi was estimated to be 0.0093 min-1, which is consistent with extremely tight binding, and more than half of the CYP17A1-Abi complex remained intact after 5 hours. In essence, every molecule of Abi or one of our potent analogs binds to and inhibits a molecule of CYP17A1 enzyme over a wide range of concentrations.
Surprisingly, compound 13 had much poorer affinity than the other steroidal azoles and a largely noncompetitive mode of inhibition. To our knowledge, steroids with similar A-B ring modifications have not been tested as substrates, but the substrate-binding pocket of CYP17A1 appears not to favor this geometry and functionality. A comprehensive understanding of which inhibitors will form a tight complex with the active site of CYP17A1 enzyme will require a more detailed structure-activity study. This work might not only provide insight to human biochemistry but could also lead to more potent CYP17A1 inhibitors, which might be useful in the treatment of androgen-dependent disorders such as prostate cancer and androgen excess states.
Supplementary Material
Highlights.
Abiraterone analogs with modified A/B-rings retain nanomolar affinity for CYP17A1
CYP17A1 inhibition by abiraterone and analogs is mixed competitive/noncompetitive
Dissociation of abiraterone from CYP17A1 is slow (koff < 0.01 min−1) and incomplete
The 3β-hydroxyl Δ5-system of abiraterone is not required for high CYP17A1 affinity
Acknowledgments
This work was supported by grant R01-GM086596 from the National Institutes of Health. Mariana Garrido thanks CONACYT (a funding agency in Mexico) and DGAPA project IN211312 for the scholarship, which permitted the realization of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann Surg. 1942;115:1192–1200. doi: 10.1097/00000658-194206000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trump DL, Havlin KH, Messing EM, Cummings KB, Lange PH, Jordan VC. High-dose ketoconazole in advanced hormone-refractory prostate cancer: endocrinologic and clinical effects. J Clin Oncol. 1989;7:1093–1098. doi: 10.1200/JCO.1989.7.8.1093. [DOI] [PubMed] [Google Scholar]
- 3.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 5.Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22:243–258. doi: 10.1016/j.beem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017α (17α-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 7.DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–119. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng HM, Auchus RJ. The action of cytochrome b5 on CYP2E1 and CYP2C19 activities requires anionic residues D58 and D65. Biochemistry. 2013;52:210–220. doi: 10.1021/bi301384n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Evaul K, Sharma KK, Chang KH, Yoshimoto J, Liu J, Auchus RJ, Sharifi N. Abiraterone inhibits 3β-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin Cancer Res. 2012;18:3571–3579. doi: 10.1158/1078-0432.CCR-12-0908. [DOI] [PubMed] [Google Scholar]
- 10.Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem. 2003;278:48563–48569. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 11.Gupta MK, Guryev OL, Auchus RJ. 5α-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003;418:151–160. doi: 10.1016/j.abb.2003.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.