Abstract
Germination of Bacillus subtilis spores is normally initiated when nutrients from the environment interact with germinant receptors (GRs) in the spores' inner membrane (IM), in which most of the lipids are immobile. GRs and another germination protein, GerD, colocalize in the IM of dormant spores in a small focus termed the “germinosome,” and this colocalization or focus formation is dependent upon GerD, which is also essential for rapid GR-dependent spore germination. To determine the fate of the germinosome and germination proteins during spore germination and outgrowth, we employed differential interference microscopy and epifluorescence microscopy to track germinating spores with fluorescent fusions to germination proteins and used Western blot analyses to measure germination protein levels. We found that after initiation of spore germination, the germinosome foci ultimately changed into larger disperse patterns, with ≥75% of spore populations displaying this pattern in spores germinated for 1 h, although >80% of spores germinated for 30 min retained the germinosome foci. Western blot analysis revealed that levels of GR proteins and the SpoVA proteins essential for dipicolinic acid release changed minimally during this period, although GerD levels decreased ∼50% within 15 min in germinated spores. Since the dispersion of the germinosome during germination was slower than the decrease in GerD levels, either germinosome stability is not compromised by ∼2-fold decreases in GerD levels or other factors, such as restoration of rapid IM lipid mobility, are also significant in germinosome dispersion as spore germination proceeds.
INTRODUCTION
Spores of Bacillus subtilis are metabolically dormant and resistant to harsh environmental conditions, allowing them to survive for many years (1). However, the presence of specific nutrients triggers the process of germination, in which spores lose their dormancy and resistance and then outgrow into vegetative cells. Normally, for germination to occur, nutrients must cross the coat, outer membrane, cortex, and germ cell wall of the dormant spore to reach nutrient germinant receptors (GRs) located in the spore's inner membrane (IM) (1–3). Lipid molecules in the B. subtilis spore IM are largely (∼70%) immobile, but upon completion of spore germination, the volume encompassed by the IM expands ∼1.5-fold, and the amount of the immobile lipid fraction decreases to ∼25%, similar to the value for vegetative cells, all in the absence of detectable ATP synthesis (4). Binding of specific germinants to their cognate GRs leads to the release of the spore core's large store (∼25% of core dry weight) of dipicolinic acid (DPA) chelated to divalent cations, mostly Ca2+ (CaDPA); CaDPA release is accompanied by some water uptake into the core and some loss of spore resistance. CaDPA release also triggers the degradation of the spore's peptidoglycan (PG) cortex by cortex-lytic enzymes (CLEs), allowing for core expansion, further core water uptake, completion of germination, and, ultimately, outgrowth to generate a vegetative cell (1).
Three functional GRs have been identified in B. subtilis spores, GerA, GerB, and GerK, each encoded by a homologous tricistronic operon producing two polytopic integral transmembrane proteins (A and B subunits) and a peripheral membrane lipoprotein (C subunit). Other spore IM proteins involved in germination include GerD, a lipoprotein involved in GR-dependent germination, and the 7 SpoVA proteins, most of which are integral membrane proteins, involved in CaDPA movement into and out of the spore during sporulation and germination, respectively (5–7). It is known that some GRs cooperate during spore germination, but overall, the mechanisms for signal transduction in spore germination are unknown, and this is one of the largest gaps in our understanding of spore germination (1, 8).
A recent study showed that GerD and all GRs in B. subtilis spores colocalize in a small focus in the spores' IM in a structure termed the germinosome (9). Upon deletion of gerD, the GRs fail to colocalize and instead are diffusely distributed throughout the IM, suggesting that GerD facilitates germinosome formation, and GerD forms a small IM focus in dormant spores even in the absence of all GRs (9). The loss of GerD also causes a significant decrease in spore germination rates with nutrient germinants, furthering the significance of the germinosome and GerD in spore germination (10). Spores lacking GerD germinate normally with nonnutrient germinants that bypass the GRs, suggesting that GerD is required for efficient signal transduction from the nutrient-GR complex; however, the exact signaling mechanism remains unknown (10). The crystal structure of two-thirds of Geobacillus stearothermophilus GerD was solved recently, and the protein forms a rod-like trimer that is largely hydrophilic with a hydrophobic lipid anchor (11). Interestingly, a previous study as well as very recent work indicate that levels of GerD decrease markedly in the IM fraction from germinating and early outgrowing spores, suggesting that the protein becomes soluble or is degraded during this period (12, 13).
The mechanism by which GerD interacts with the germinosome is unknown, and our knowledge of this protein complex is limited only to its features in dormant spores (9). If GerD does act as a scaffold that GRs use to assemble into the germinosome, as GerD levels decrease in germinating/early outgrowing spores, the scaffold for the germinosome might erode away, and as a result, this complex might disassociate during germination/early outgrowth. The aim of this study was to test this hypothesis by examining the dynamics of the germinosome as well as various germination proteins during the germination and outgrowth of B. subtilis spores. Studying the fates of the germinosome, GR subunits, and GerD during spore germination and outgrowth might further the understanding of these processes and perhaps provide clues to how signal transduction works in spore germination.
MATERIALS AND METHODS
B. subtilis strains and spore preparation.
B. subtilis strains used for this work are listed in Table 1 and are isogenic with strain PS832, a prototrophic 168 laboratory strain. The fluorescent fusion strains are all isogenic derivatives of PS4150, derived from PS832 and lacking two genes essential for spore coat assembly, cotE and gerE. Loss of these two genes results in a greatly reduced autofluorescence background due to the absence of most coat proteins in the PS4150 spores, allowing visualization of fluorescence from green fluorescent protein (GFP) derivatives fused to germination proteins. The fusion strains are (i) KGB72, with an mCherry sequence fused to the C-terminal coding end of the gerKB gene at the gerK locus and with a wild-type gerKB gene that is most likely not transcribed; (ii) KGB73, with a gfp sequence fused to the C-terminal coding end of the gerD gene at the gerD locus and which retains an expressed wild-type gerD gene; (iii) KGB80, with the gerKB-mCherry fusion in strain KGB72 and the gerD-gfp fusion in strain KGB73; (iv) KGB94, which is strain KGB73 that has been transformed to Cmr by using chromosomal DNA from strain PS2307 (ΔcwlD) that carries a deletion of the cwlD gene essential for modification of spore cortex PG such that CLEs can recognize and degrade the cortex during stage II of spore germination (15, 19); and (v) KGB114, with a gerAA-mCherry sequence inserted at the amyE locus downstream of the native promoter for the gerA operon encoding the GerA GR. Spores of all fusion strains germinate relatively normally with nutrients, indicating that the fusion proteins are functional (9).
TABLE 1.
B. subtilis strains useda
| Strain | Genotype and phenotype | Reference or source |
|---|---|---|
| PS533 | Wild type; pUB110 Kmr | 14 |
| PS2307 | PS832 ΔcwlD Cmr | 15 |
| PS3476 | PS832 PsspD::gerA MLSr | 16 |
| PS3665 | FB10 ΔgerA gerB* ΔgerK MLSr Spr | 17 |
| PS4150 | PS832 ΔgerE ΔcotE Spr Tcr | 18 |
| KGB09 | PS832 ΔgerD amyE::gerD-gfp Cmr Spr | Keren Griffiths |
| KGB72 | PS4150 gerKB-mCherry Cmr | 9 |
| KGB73 | PS4150 gerD-gfp Kmr | 9 |
| KGB80 | PS4150 gerKB-mCherry gerD-gfp Cmr Kmr | 9 |
| KGB94 | PS4150 gerD-gfp ΔcwlD Cmr Kmr | This study |
| KGB114 | PS4150 amyE::gerAA-mCherry Cmr | 9 |
Abbreviations: Cmr, resistance to chloramphenicol (10 μg/ml); Kmr, resistance to kanamycin (10 μg/ml); MLSr, resistance to erythromycin (1 μg/ml) and lincomycin (25 μg/ml); Spr, resistance to spectinomycin (100 μg/ml); Tcr, resistance to tetracycline (10 μg/ml).
Spores of B. subtilis strains were prepared at 37°C on 2× Schaeffer's glucose agar plates without antibiotics, and spores were incubated, harvested, and cleaned as described previously (20). All spores in this work were free (>98%) of growing or sporulating cells, germinated spores, and cell debris, and spores were stored at 4°C protected from light.
Spore germination and outgrowth.
For Western blot analysis, spores at an optical density at 600 nm (OD600) of 125 in water were heat activated at 75°C for 30 min and cooled on ice for ∼15 min. Heat-activated spores were germinated at an OD600 of ∼1.5 at 37°C in 200 ml of 10 mM l-valine plus 25 mM K-HEPES buffer (pH 7.4). Spore germination kinetics were determined by measuring the OD600 of germinating cultures, and 65-ml samples were harvested by centrifugation (10 min at 12,000 × g) after 15, 60, and 120 min of germination. The pellets from these samples were washed in phosphate-buffered saline (PBS) (50 mM NaH2PO4, 150 mM NaCl [pH 7.2]), suspended in 5 μl PBS, and washed three times with PBS by centrifugation at 12,000 × g. The final spores were suspended in 200 μl 20% Histodenz (Sigma-Aldrich Corporation, St. Louis, MO), loaded onto a Histodenz density cushion (1 ml of 50% Histodenz), and centrifuged for 15 min at 15,000 × g to separate germinated spores from nongerminated spores (21). Germinated spores were isolated by pipetting off the 20% Histodenz layer, diluted in 1 ml PBS, and cleaned by centrifugation at 12,000 × g for 10 min and washing of the pellet five times with PBS to remove Histodenz. Samples of germinated spores were suspended in 250 μl PBS and stored at −80°C.
To isolate outgrown spores, heat-activated spores were germinated at an OD600 of 1, as described above. After 2 h of germination, the spores were run on a Histodenz gradient to separate germinated and dormant spores, and germinated spores were isolated and washed as described above. These germinated spores were suspended in 15 ml of LB medium (20) at an OD600 of 0.5. Immediately upon suspension in LB medium, a 5-ml sample was harvested by centrifugation (15 min at 12,000 × g); this sample served as the 2-h germination sample in outgrowth experiments. The remaining 10 ml of germinated spores was incubated at 37°C with vigorous shaking. Samples (5 ml) of these cultures were harvested by centrifugation (15 min at 12,000 × g) at OD600s of 1 and 2, and microscopic analysis confirmed that the germinated spores had outgrown into vegetative cells. All three samples were washed with PBS three times to remove growth medium, suspended in 0.5 ml PBS, and stored at −80°C.
Preparation of spore lysates and Western blot analysis.
Samples of dormant, germinating, and outgrowing/growing spores prepared and harvested as described above were treated with lysozyme (0.5 mg/ml) in TEP buffer (50 mM Tris-HCl [pH 7.4], 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1% Triton X-100) for 5 min at 37°C and then for 20 min on ice. After incubation, the lysed samples were sonicated on ice with 100 mg of glass beads for five 15-s periods, with 30-s intervals between sonication periods. After sonication, samples were added to 1% SDS and 150 mM 2-mercaptoethanol and incubated for 2 h at 23°C (22). The samples were then bath sonicated for 2 min and centrifuged for 5 min at 16,000 × g, and the supernatant fluid collected was termed the total lysate (L) fraction. The pellet fractions were suspended in TEP buffer containing 1% SDS and 150 mM 2-mercaptoethanol, bath sonicated for 2 min, and centrifuged at 16,000 × g, and the resulting supernatant fluid was termed the washed lysate fraction. Invariably, levels of germination proteins in this washed lysate fraction were <10% of the levels found in the L fraction (data not shown).
In each experiment, equal aliquots from the same amounts of various spore fractions were run on SDS-polyacrylamide (12%) gel electrophoresis (PAGE) gels, and proteins were transferred onto polyvinylidene difluoride (PVDF) membranes and probed with antisera specific for various germination proteins, as described previously (22–24). In some experiments, after the use of one antiserum, the PVDF membranes were stripped with Restore Western blot stripping buffer (Thermo Scientific, Rockford, IL) for 15 to 20 min at 37°C and then probed again with antiserum against another germination protein. ImageJ software was used to compare the intensities of protein bands obtained from Western blots on X-ray film, which were further compared to bands from Western blot analysis of serial dilutions of samples of germinating spores.
Microscopy.
For fluorescence microscopy of germinating spores, dormant spores suspended in water at an OD600 of ∼35 were heat activated for 30 min at 75°C and then cooled on ice for 15 min. The heat-activated spores (10 μl) were spread onto 0.1% polylysine-coated glass-bottom culture dishes (MatTek, Ashland, MA), and to germinate the spores, a mixture with final concentrations of 10 mM l-valine and 25 mM K-HEPES buffer (pH 7.4) was added. Germination on the microscope platform was done at 37°C by using a heated slide chamber. Epifluorescence and differential interference microcopy (DIC) images of the dormant spores were taken at various germination times, as described previously (9), and images were processed by using ImageJ and Adobe Photoshop software. Enlarged images were scaled at 300 pixels/in. by using a bicubic function. Due to the relatively weak signal-to-noise ratio for the spores, we summed 30 to 50 consecutive fluorescent images at each time point with an acquisition time of ∼70 ms. Increasing the number of images taken or increasing the acquisition time led to significant photobleaching. Images used in subsequent analyses were generated as described previously (9). In determining the colocalization of the signal from two fluorescent proteins, if the maximum intensities of the two signals were ≤1 pixel apart (corresponding to 160 nm), they were scored as colocalized, since the positions of the mCherry and GFP foci can be partially shifted because of the signal noise and the wavefront alteration caused by the changing of dichroic mirrors (9). Fluorescence distribution curves along the long axis of spores were generated as described previously (9). Plots of σ, or the width of the Gaussian distribution at 1/e of the maximum fluorescence intensity, were generated, and P values were calculated by using the Student two-tailed t test.
RESULTS
Levels of GR subunits in the L fraction from germinating wild-type spores.
Since the fate of all GR subunits during spore germination and outgrowth has not been thoroughly studied (13), we first examined levels of GR subunits in dormant and germinating spores to determine how long GR subunits persist following initiation of germination. GRs localize to the IM in spores (1–3, 9), but recent work found that only ∼10% of GR subunits are found in isolated IMs, since ∼90% of the subunits sediment upon low-speed centrifugation of a spore lysate made without SDS and 2-mercaptoethanol and ∼90% of the IM lipids also sediment upon the same low-speed centrifugation (22). Consequently, to ensure that we analyzed all GR subunits in spores, we added SDS plus mercaptoethanol to spore lysates and incubated them for ∼2 h at 23°C prior to centrifugation, conditions that give ≥90% of germination proteins in a soluble form (22). Western blot analysis of these samples with antisera against GR subunits (Fig. 1) showed that in wild-type spores, the levels of all GR subunit proteins were essentially the same in dormant and germinating spores, with the exception of GerBC and GerKA. GerBC band intensities decreased slightly during germination, and GerKA levels decreased more significantly (Fig. 1), although this was not seen in all experiments (data not shown; see also below). However, since significant levels of GR subunits as well as GerD (see below) persisted even after 2 h of germination, it is certainly possible that a germinosome could persist in germinating spores for extended periods.
FIG 1.

Western blot analysis of germination protein levels in the L fraction of dormant and germinating PS4150 spores. The L fraction from spores was isolated, equal amounts of protein were run on SDS-PAGE gels, and Western blots were probed with various antisera. Lanes: 1, dormant spores; 2, spores after 15 min of germination; 3, spores after 60 min of germination; 4, spores after 120 min of germination. MW, molecular weight markers (in thousands). The band running just below SpoVAD is likely a breakdown product of this protein, which was reported previously (6, 7).
Dynamics of GerD and SpoVA proteins in dormant and germinating wild-type spores.
As noted above, GerD plays an important role in spore germination, likely because this protein is essential for the formation of the germinosome in dormant spores. Previous studies suggested that levels of GerD in inner spore membrane fractions decrease during germination (12, 13), but those studies did not report what happens to GerD in a total-spore lysate. When we probed the L fraction of wild-type spores with antisera against GerD, we found that the levels of this protein decreased significantly during germination, >50% after 15 min (Fig. 1; see also below). We confirmed this finding by Western blotting using serial dilutions and by ImageJ density analysis (data not shown). This suggests that, initially, the germinosome could remain intact in germinating spores, but as GerD levels decrease, the germinosome might disperse in germinating/outgrowing spores.
Levels of some members of another important group of proteins involved in germination, the SpoVA proteins, in dormant and germinating spores were also examined. These proteins are not found in the germinosome (9) but play an important role in the overall germination process by facilitating the movement of CaDPA out of the spore core (6, 7). Like GerD and total GRs, the SpoVA proteins are present in the IM at several thousand molecules per spore (22, 23). Western blot analyses of the L fraction of wild-type spores with antisera against SpoVAD or SpoVAEa showed no significant differences in the levels of these proteins between dormant and germinating spores (Fig. 1).
Germinosome foci disperse during germination of spores with single fluorescent fusions.
To study the germinosome in B. subtilis spore germination, we first examined spores with single fluorescent fusions to germination proteins to determine if these individual germination proteins exhibited any change in their distribution or location during germination. Specifically, we examined KGB73 (PS4150 gerD-gfp) and KGB114 (PS4150 gerAA-mCherry) spores using DIC and fluorescence microscopy, analyzing both dormant and 2-h-germinated spores. Germinated spores can be distinguished from dormant spores by the large drop in their refractive index due to CaDPA release from the spore core and its replacement by water as well as core swelling and further water uptake following cortex PG hydrolysis (1, 5) (Fig. 2Ai and 3Ai). Spores of both KGB73 (Fig. 2Aii) and KGB114 (Fig. 3Aii) exhibited similar fluorescence changes after 2 h of germination; while dormant spores exhibited distinct fluorescent foci, germinated spores exhibited a more dispersed fluorescence signal and a lower peak fluorescence intensity (Fig. 2Bii and 3Bii).
FIG 2.
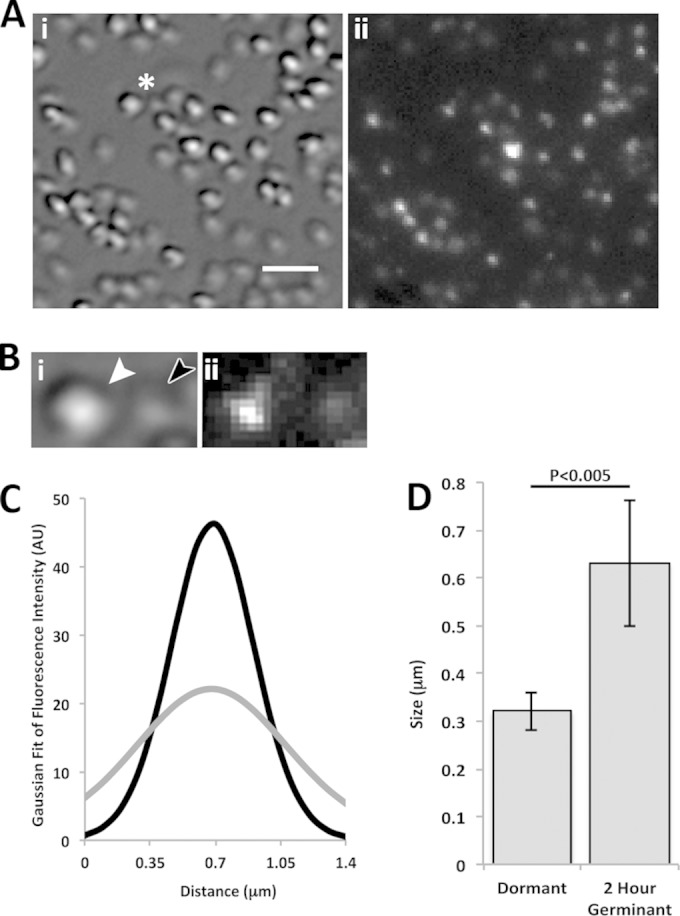
DIC and fluorescence microscopic analysis of germinating KGB73 (PS4150 gerD-gfp) spores. (A) DIC (i) and fluorescence (ii) images of spore populations 2 h after germinant addition, as described in Materials and Methods. The bar in panel i is 2 μm, and the magnifications of panels i and ii are identical. (B) Higher magnification (×2.5) of the area marked by an asterisk in panel A. Shown are a DIC image of a dormant spore (white arrowhead) and a germinated spore (dark arrowhead) (i) and a fluorescence image (ii). (C) Average Gaussian fit of the background-subtracted fluorescence intensity along the long axis of 20 dormant spores (black curve) and the same spores after 2 h of germination (gray curve). All germinated spores had Tlys values of <1 h. AU, arbitrary units. (D) Graph of the change in σ of Gaussian distributions from panel C in dormant and 2-h-germinating spores. Error bars represent one standard deviation, and the P value for the difference between dormant and germinated spores is given above the graph.
FIG 3.
DIC and fluorescence microscopic analysis of germinating KGB114 (PS4150 gerAA-mCherry) spores. (A) DIC (i) and fluorescence (ii) images of spore populations 2 h after germinant addition. The bar in panel i is 2 μm, and the magnifications of panels i and ii are identical. (B) Higher magnification (×2.5) of the area marked by an asterisk in panel A. Shown are a DIC image of a dormant spore (white arrowhead) and a germinated spore (dark arrowhead) (i) and a fluorescence image (ii). (C) Average Gaussian fit of the fluorescence intensity along the long axis of 20 dormant spores (black curve) and the same 20 spores after 2 h of germination (gray curve). All spores had Tlys values of <1 h. (D) Graph of the change in σ of Gaussian distributions from panel C in dormant and 2-h-germinating spores. Error bars represent one standard deviation, and the P value is given above the graph.
In order to compare the spores' fluorescence quantitatively, the fluorescence intensities of 20 individual spores, when dormant and then after 2 h of germination, were measured along their long axis. The full width at half-maximal intensity for dormant KGB114 spores was ∼300 nm (data not shown), as seen previously (9), indicative of the maximum germinosome size and the resolution limit of the microscope. When these fluorescence intensities were fit to a Gaussian distribution and averaged (Fig. 2C and 3C, black curves), the resulting curves were similar to those seen previously for dormant spores with comparable fluorescent fusions (9). However, there was a significant change in the signal distribution after 2 h of germination (Fig. 2C and 3C, gray curves). To compare the change in Gaussian distributions between dormant and 2-h-germinated spores, we determined σ, or the width of the curve at 1/e of the maximum fluorescence intensity, at both time points (Fig. 2D and 3D). For each fluorescent fusion, σ increased notably in 2-h-germinated spores, and this change was statistically significant (Fig. 2D and 3D). This change is in agreement with our fluorescence imaging that indicated that the germinosome becomes disperse and the fluorescence distribution broadens as the spore germinates. Thus, even though levels of GerD in wild-type spores decreased by as much as 50% at 15 min after germinant addition, GerD-GFP and GerAA-mCherry fluorescence was still detectable in spores 2 h after germinant addition, not in discrete foci but rather in a more disperse distribution in which the average maximum fluorescence intensity decreased 2- to 3-fold (Fig. 2C and 3C; see also below).
Germinosome foci disperse during germination of spores with two fluorescent fusions.
To further understand the fate of the germinosome in B. subtilis spore germination, dormant and germinating KGB80 (PS4150 gerKB-mCherry gerD-gfp) spores were also examined by using DIC and fluorescence microscopy. Images were taken before a nutrient germinant was added (dormant) and 1 and 2 h after germinant addition (Fig. 4A). Time-lapse microscopy of these spores showed that upon germination, the germinosome again displayed two different appearances: discrete foci (Fig. 4Ci) in which GRs and GerD are colocalized, as seen previously in dormant spores (9), and a more diffuse signal in which GRs and GerD are dispersed (Fig. 4Cii). However, in some germinating spores, ∼20% after 2 h of germination, no fluorescence signal was seen (see below). Loss of the fluorescence signal due to photobleaching was not apparent (Fig. 4Axii, asterisk), and this was confirmed with control photobleaching experiments (data not shown). KGB80 spores germinated effectively in glass-bottom dishes, with ∼75% of spores being germinated after 2 h (see below), although this germination was slower than in solution (data not shown). In this work, we define the time of completion of the fall in the DIC intensity of germinating spores due to complete CaDPA release as well as completion of cortex hydrolysis and core swelling as Tlys (25). Spores with a Tlys of <1 h (Fig. 4A, black arrowheads) as well as those with a Tlys of ≥1 h (Fig. 4A, white arrowheads) displayed a similar dispersed germinosome appearance, suggesting that germinosome foci remain intact for less than an hour following initiation of spore germination and then disperse.
FIG 4.
Time-lapse microscopy of germinating double-fluorescent-fusion (PS4150 gerKB-mCherry gerD-gfp) spores. (A) DIC images (i, v, and ix) and fluorescence images of GFP (ii, xi, and x), mCherry (iii, vii, and xi), and GFP/mCherry overlays (iv, viii, and xii). The field of view was kept stationary to track the same spores throughout the experiment. The bar in panel i is 2 μm, and the magnifications of panels i to xii are identical. White arrowheads indicate spores with Tlys values of <1 h, and black arrowheads indicate spores with Tlys values of >1 h. The asterisk in panel xii indicates a dormant spore 2 h after germinant addition, with no photobleaching being apparent during the 2-h experiment. (B) Gaussian fit of overlay fluorescence intensity along the long axis of 30 dormant spores (black curve) and the same 30 spores 1 or 2 h after germinant addition that had germinated before 1 h (dark gray and light gray curves, respectively). (C) Higher magnification (×3.5) of spores from the 2-h-germination fluorescence overlay in panel Axii. We define the germinosome phenotype as a discrete focus (i) or a dispersed signal (ii). Shown are a dormant spore (Axii, asterisk) (i) and a germinated spore (ii) with a Tlys of <1 h (Axii, black arrowhead). (D) Graph of the change in σ of Gaussian distributions from panel B. Error bars represent one standard deviation, and P values are shown above the graph.
To further quantitate germinosome dispersion in germinating spores, the total fluorescence intensity along multiple individual dormant spores' long axis and in these same spores after 1 and 2 h of germination was measured, and the data were fit to a Gaussian distribution and averaged (Fig. 4B). The latter analysis allowed determination of values for σ for these three data sets (Fig. 4D). As was the case with spores with single fluorescent fusions, there were marked decreases in peak fluorescence intensity (Fig. 4B) and significant increases in the value for σ (Fig. 4D) as germination of spores with the two fluorescent fusions proceeded. However, the maximum σ value of 600 nm for the 2-h-germinated spores was still lower than that for all spores, consistent with the fluorescent proteins remaining in the IM but being dispersed.
Levels of GR subunits and GerD in L fractions of dormant and germinating spores with single fluorescent fusions.
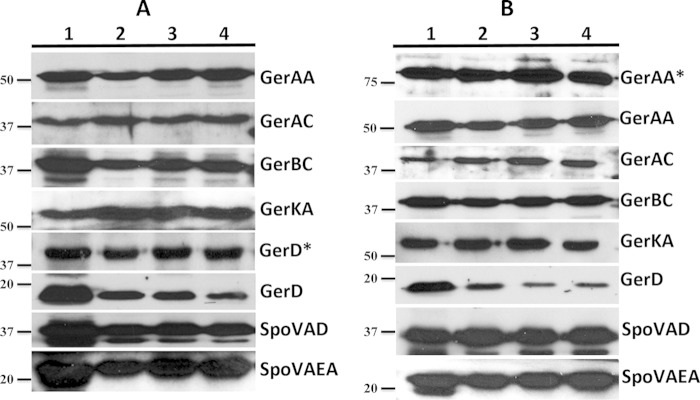
In order to prove that the changes in germinosome appearance described above are not a result of changes in levels of GR proteins, we also examined levels of GR subunits in dormant and germinating KGB73 (PS4150 gerD-gfp) and KGB114 (PS4150 gerAA-mCherry) spores (Fig. 5). As seen with wild-type spores, Western blots of L fractions from dormant and germinating KGB73 and KGB114 spores showed that except for GerBC, GR subunit levels changed minimally during germination. It was also notable that the GerD antigen was detected at two molecular masses in Western blots of L fractions of KGB73 spores, the normal protein at ∼18 kDa and GerD-GFP at ∼47 kDa; surprisingly, while levels of the normal protein decreased during germination, GerD-GFP levels remained unchanged. We confirmed the decrease in endogenous GerD levels by Western blotting of serial dilutions and ImageJ density analysis. L fractions of KGB114 spores also contained two forms of the GerAA antigen, that of the normal protein of ∼60 kDa and the ∼82-kDa GerAA-mCherry form, both of which were stable for ∼2 h of germination. Since the GR subunits were present throughout germination in these fusion protein strains, we believe that changes in their levels do not influence the dispersion of the germinosome seen as germination proceeds.
FIG 5.
Western blot analysis of germination protein levels in spores of strains KGB73 (PS4150 gerD-gfp) (A) and KGB114 (PS4150 gerAA-mCherry) (B). The L fraction of dormant and germinating spores was isolated, and equal amounts of protein were run on SDS-PAGE gels and subjected to Western blot analysis with various antisera. Lanes: 1, dormant-spore L fraction; 2, L fraction at 15 min of germination; 3, L fraction at 60 min of germination; 4, L fraction at 120 min of germination. The migration positions of molecular mass markers (in kDa) are shown to the left of the panels. Proteins labeled with asterisks indicate a fluorescent fusion protein that ran at a higher molecular mass than the native protein.
These same Western blots showed that levels of SpoVAD and SpoVAEa were also relatively unchanged during germination of KGB73 and KGB114 spores (Fig. 5). In contrast, as seen with wild-type spores, levels of normal GerD decreased >50% by 15 min after germinant addition and generally even more, and this was confirmed by Western blotting of serial dilutions of L fractions from spores of both strains (data not shown). However, as noted above, GerD-GFP was detected at ∼47 kDa and remained at dormant spore levels throughout germination, indicating that the fusion protein is not degraded or processed during germination. This explains why a GerD-GFP signal could still be seen by fluorescence microscopy 2 h after germinant addition to KGB73 spores although not in foci but as a more disperse signal (Fig. 2).
Timing of germinosome dispersion during spore germination.
While it was clear from analyses of spores germinating for 2 h that the germinosome ultimately disperses during germination, it was important to determine when this change took place, as this might identify germination events correlated with germinosome dispersion. There are two stages in spore germination (1): stage I, characterized by CaDPA release, followed by stage II, in which cortex PG is degraded by CLEs and the spore core takes up water and expands. Events in these two stages can be examined separately by using spores of strains that cannot degrade their cortex PG. One such strain lacks the CwlD protein, which is essential for a specific cortex PG modification that allows CLEs to specifically recognize and degrade this modified PG (1, 15, 19). Spores of a cwlD strain go through stage I of germination relatively normally, but further germination events do not take place, including the large decrease in the immobile fraction of IM lipid (4, 14). DIC and fluorescence microscopy of KGB94 (PS4150 gerD-gfp ΔcwlD) spores indicated that >95% of dormant spores contained fluorescent GFP foci (Table 2). Germinated spores of this cwlD strain could be identified by their decreased DIC image contrast, although this decrease was not quite as great as that in spores that could go through stage II of germination (Fig. 6A). Strikingly, almost all the germinated KGB94 spores retained their fluorescent foci even after 2 h of germination, with no increase in the number of spores with a dispersed germinosome (Fig. 6B and Table 2). Thus, events in stage I of germination are not sufficient to trigger germinosome dispersion.
TABLE 2.
Germinosome appearance in dormant and germinating cwlD sporesa
| Germinosome appearance | No. (%) of spores |
||
|---|---|---|---|
| Dormant | At germination time of: |
||
| 1 h | 2 h | ||
| Foci | 157 (95) | 120 (90) | 135 (89) |
| Disperse | 2 (1) | 3 (2) | 3 (2) |
| No signal | 6 (4) | 11 (8) | 14 (9) |
Fluorescence and DIC images of KGB94 (PS4150 gerD-gfp ΔcwlD) in dormant-spore populations and in spore populations after 1 and 2 h of germination were taken as described in Materials and Methods, and the germinosome appearance in individual spores was scored as foci or disperse, as shown in Fig. 2 to 4.
FIG 6.
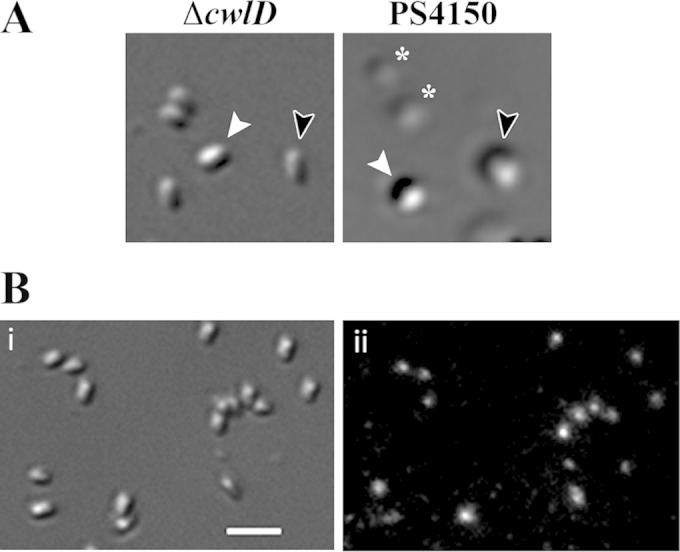
Differential interference contrast microscopy of spores after 2 h of germination. (A) KGB94 (PS4150 gerD-gfp ΔcwlD) (ΔcwlD) and PS4150 spores were germinated for 2 h at 37°C in glass-bottom dishes, as described in Materials and Methods, and imaged by DIC microscopy. White arrowheads indicate a dormant spore (high refractive index), black arrowheads indicate a germinating spore after completion of CaDPA release (low refractive index), and asterisks indicate germinating spores after the completion of cortex hydrolysis (lowest refractive index). (B) KGB94 spores were germinated for 2 h at 37°C in glass-bottom dishes as described above for panel A and imaged by DIC (i) and epifluorescence (ii) microscopy. The bar in panel i is 2 μm.
To more precisely determine when the germinosome disperses during spore germination, the germinosome appearance in multiple individual germinating spores carrying fluorescent fusions at various times during germination as well as in dormant spores was determined (Table 3). This analysis showed that germinosome dispersion did not become significant until between 15 and 30 min after germinant addition, with most dispersion taking place between 30 and 60 min after germinant addition. There was also an increasing percentage of spores that had minimal if any fluorescence signal remaining as germination progressed, although this loss of fluorescence signal was not due to photobleaching, as noted above.
TABLE 3.
Germinosome appearance in dormant spores and during spore germinationa
| Germinosome appearance | No. (%) of spores |
||||
|---|---|---|---|---|---|
| Dormant | Spores at germination time (min) (% germination) ofb: |
||||
| 15 (10) | 30 (50) | 60 (70) | 120 (80) | ||
| Foci | 203 (91) | 178 (89) | 180 (81) | 25 (12) | 28 (12) |
| Disperse | 17 (8) | 19 (10) | 37 (17) | 157 (76) | 161 (67) |
| No signal | 4 (2) | 4 (2) | 5 (2) | 24 (12) | 51 (21) |
Quantitation of spore germination and germinosome appearance in dormant KGB72 (PS4150 gerKB-mCherry) spores and germinated (low refractive index) KGB72 (30-, 60-, and 120-min samples) spores or KGB72 and KGB80 (PS4150 gerKB-mCherry gerD-gfp) (15-min sample) spores germinated in glass-bottom dishes was performed as described in Materials and Methods. Numbers for 15-min samples are from both KGB72 and KGB80 spores to ensure that sufficient numbers of spores that germinated at this time point were obtained; germinated spores of both strains gave essentially identical results. Fluorescence images of dormant spores and spores after various germination times were analyzed, and germinosome appearance in individual spores was scored as described in Table 2.
The percentage of spore germination was determined by examining the DIC image intensities of >500 individual spores at each time point. Values shown are for KGB72 spores, and values for KGB80 spores were essentially identical.
Levels of germination proteins in outgrowing spores.
While levels of GR and SpoVA proteins were relatively constant throughout spore germination, it seemed unlikely that these proteins would be retained indefinitely once spores had outgrown and resumed vegetative growth. To determine when these proteins disappeared following spore germination, wild-type, gerD-gfp, and gerAA-mCherry spores were germinated, outgrown, and grown vegetatively, and samples were analyzed by Western blotting to determine the levels of various germination proteins. With germinated spores of these three strains grown to an OD600 of 2, the levels of GR subunits, SpoVAEa, and SpoVAD decreased 30 to 85%, 50 to 60%, and 50 to 90%, respectively (Fig. 7). Most notably, GerD was found at minimal levels, if at all, at an OD600 of 1 (Fig. 7). With the GerD-GFP fusion strain, we were unable to come to a definitive conclusion about changes in GerD-GFP levels due to high levels of nonspecific binding in the GerD-GFP region of Western blots with samples from spores giving vegetative cells at an OD600 of 1 to 2 (data not shown). However, GerAA-mCherry levels decreased ∼75% at an OD600 of 2 (Fig. 7C). The universal decrease in levels of native germination proteins seen during outgrowth and subsequent growth as well as the complete loss of GerD indicate that as spores complete germination and outgrowth and begin to grow vegetatively, levels of all germination proteins decrease, most notably GerD. Consequently, the germinosome cannot persist in wild-type spores once outgrowing spores become growing cells.
FIG 7.
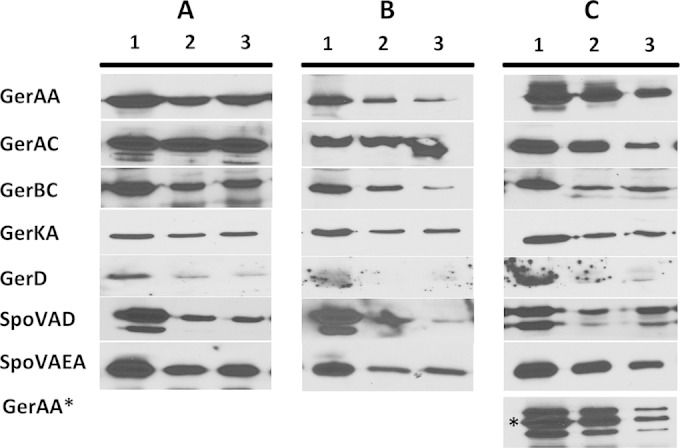
Western blot analysis of germination protein levels in germinated and outgrowing spores of strains PS4150 (wild type) (A), KGB73 (PS4150 gerD-gfp) (B), and KGB114 (PS4150 gerAA-mCherry) (C). The L fractions of equal volumes of 2-h-germinated as well as outgrowing spores were isolated, and equal volumes of sample were run on SDS-PAGE gels and subjected to Western blot analysis with various antisera. Lanes: 1, spore lysates at 2 h of germination; 2, spore lysates outgrown to an OD600 of 1; 3, spore lysates outgrown to an OD600 of 2. All proteins ran at the expected molecular mass. Proteins labeled with asterisks indicate a fusion protein that ran at a higher molecular mass than the normal protein.
DISCUSSION
How signals are transferred from the GRs to downstream signaling molecules during B. subtilis spore germination remains unknown. In dormant spores, germination proteins coalesce into the germinosome, and the formation of this multiprotein complex is dependent on the accessory germination protein GerD (9). In this work, the use of fluorescently labeled germination proteins revealed that the germinosome has a different appearance in dormant and germinated spores, with discrete foci in dormant spores that become dispersed as spore germination proceeds. Levels of GR subunits and SpoVA proteins remained relatively unchanged after 2 h of germination, although upon germinated-spore outgrowth and conversion into vegetative cells at an OD600 of 2, the levels of the majority of these proteins had decreased >50%.
In contrast to GR subunits and SpoVA proteins, GerD levels decreased ≥50% as early as 15 min after germinant addition. This decrease in the GerD level was observed in not only germinating wild-type spores but also germinating gerD-gfp and gerAA-mCherry spores, indicating that the fusion proteins did not alter the behavior of endogenous GerD. We also tested the stability of GerD-GFP by Western blot analysis of extracts of dormant and germinating KGB09 (ΔgerD amyE::gerD-gfp) spores that lack endogenous GerD and found that <5% of the fusion protein appeared at the position of native GerD (data not shown). The decrease in total spore GerD levels early in spore germination is consistent with previous reports of decreases in GerD levels in isolated IMs from germinated spores (12, 13). In addition, levels of native GerD fell essentially to zero as germinated spores outgrew and became vegetative cells. Since germinosome formation in dormant spores requires GerD (9), it is not unreasonable that germinosome dispersion in germination might be due at least in part to decreased levels of GerD. This loss of GerD soon after the initiation of germination is presumably due to proteolysis, and GerD becomes sensitive to both an externally added protease and a biotinylation agent in germinated spores, while it is resistant to this protease and biotinylation agent in dormant spores, even decoated dormant spores (26). Perhaps, in dormant spores, GerD is shielded from protease digestion and biotinylation in the germinosome foci and becomes accessible to these treatments when the germinosome disperses. The sensitivity of GerD to a protease and an external biotinylation agent in germinated spores suggests that GerD is on the outer surface of the germinated spores' IM and presumably in dormant spores as well. This location of GerD-GFP is, however, somewhat at odds with the fact that GFP generally does not fold properly after crossing a membrane, especially the plasma membrane of Gram-negative bacteria, as most proteins fused to GFP that are secreted into the periplasm do not exhibit significant fluorescence (27). However, unlike the periplasm of Gram-negative bacteria, which is an oxidizing environment (27), the space between the developing spore's IM and outer membrane is not in direct contact with the sporulation medium and could well be a reducing environment, just as in the forespore cytoplasm. Unfortunately, there are no data on the precise conditions in this compartment of sporulating cells.
GerD is a helical trimer in vitro and may be capable of forming even higher-order oligomeric structures (11). In addition, GerD-GFP can form germinosome-like foci in dormant spores even in the absence of GRs (9). These results suggest that GerD may self-associate to form a scaffold on which the GRs bind to form the germinosome, and perhaps, this scaffold can facilitate signal transmission events during germination. However, when this GerD scaffold protein is lost, the germinosome can no longer maintain its structural integrity and disperses. Unfortunately, in vitro affinity pulldown experiments with purified GerD indicate that GerD does not associate with at least GerBC (6, 28). Possible explanations for this negative result could be that GerD and GR proteins interact exclusively within the novel environment of the IM of dormant spores or that the GerD interaction with GRs in the germinosome is not through the C subunits of the GRs.
The demonstration that a cwlD mutation prevents germinosome dispersion during spore germination indicates that germinosome dispersion requires progression through at least stage II of germination, and this is consistent with the kinetics of germinosome dispersion during spore germination. The rapid >50% decrease in the GerD level ∼15 min into spore germination suggests that the germinosome might disperse early in germination, even though levels of most other known germinosome constituents remained relatively normal for up to 2 h. However, germinosome dispersion during germination was clearly significantly slower than the loss of >50% of GerD in the first 15 min of germination, taking place 15 to 30 min after the completion of spore germination. Why might germinosome dispersion be so much later than the significant drop in the GerD level? An obvious answer to this question is that the >2-fold decrease in the GerD level early in germination alone is insufficient to cause immediate germinosome dispersion. In addition, perhaps the GerD that is rapidly degraded early in germination is not interacting with GRs. If the latter is the case, GerD might be more stable in spores with higher GR levels and might be more rapidly and completely degraded early in the germination of spores with low GR levels. However, this was not the case, as spores of strain PS3476 overexpressing the GerA GR by ∼8-fold (16); strain PS3665 containing only the GerB* GR, which has a single amino acid change allowing spore germination with l-asparagine alone (17); and strain PS533 (wild type) (14) lost GerD at essentially identical rates during spore germination (data not shown). Since the average numbers of GR molecules in spores of the above-described 3 strains are ∼10,000 (PS3476), 2,500 (PS533), and 700 (PS3665) (22, 23), it appears to be clear that the numbers of GR subunits available for interaction do not influence GerD stability during spore germination.
Another piece of evidence suggesting that the loss of GerD is not the crucial event leading to germinosome dispersion in spore germination is that germinosome dispersion took place in not only spores with an mCherry fusion to GerAA or GerKB but also spores with GFP fused to GerD, yet we found that GFP-GerD is stable throughout spore germination. Since GFP-GerD is functional in spore germination (9), either a germinosome with GFP-GerD is inherently much less stable than a germinosome with wild-type GerD or some factor or factors other than GerD levels are crucial for germinosome stability. What might this other factor or factors be? While we cannot yet answer this question definitively, we know that the environment of the IM of dormant B. subtilis spores is very different from that in a growing cell or a germinated spore. As noted in the introduction, the mobile fraction of IM lipids rapidly increases ∼2.5-fold early after the completion of stage II of B. subtilis spore germination and to a value essentially identical to that of the mobile fraction in the plasma membrane of vegetative cells (4). However, even with this change, the diffusion coefficient of mobile IM lipids in germinated spores increases only ∼2-fold over that in the IM of dormant spores and is still ∼9-fold lower than that in the plasma membrane of vegetative cells (4). Perhaps it is a change in the germinated spore's IM resulting in a further increase in the lipid diffusion coefficient in the IM to a value closer to that in the plasma membrane of vegetative cells that results in germinosome dispersion. However, when the latter change of the lipid diffusion coefficient takes place during spore germination is not known.
The kinetics of the germinosome dispersion process might also be affected by the strength of the interactions that hold proteins together in the germinosome, a structure that may exist in a transient interacting state, since the signal facilitated by the germinosome that triggers CaDPA release from the spore core occurs in a very short time period and well before the germinosome disperses after the completion of spore cortex hydrolysis. Transient protein interactions, or the “soft-wired-signaling concept,” suggest that signaling proteins translocate and have reversible binding interactions as part of their overall signaling pathway (29, 30). In B. subtilis spores, the protein-protein interactions in the germinosome, in particular between the GRs and GerD and between GRs and GRs, could be promoted by changes in the IM during sporulation. As the IM is compressed when dormant spores are formed, the amount of germination proteins present brings GerD trimers into close proximity with both other GerD trimers and GRs, promoting germinosome formation through multiple weak protein-protein interactions, and these interactions become fixed because of the minimal mobility of lipids in the IM of dormant spores. As the IM expands after Tlys and lipid mobility increases, GerD becomes accessible to proteases and is degraded, while the GRs slowly diffuse away through the milieu of the now largely mobile IM. It is also possible that GRs exist in two different conformations, one receptive to interacting with other GRs and GerD in dormant spores and one in germinated spores when the GRs change conformation and are no longer receptive to interactions with GerD. There is no direct evidence for this possibility; however, there is evidence for membrane proteins existing in two conformations (31, 32). It is clear that further experimentation is required to understand germinosome dynamics, and analysis of the kinetics of and factors required for germinosome formation during sporulation may give particularly valuable information; this work is currently in progress.
ACKNOWLEDGMENTS
We thank Keren Griffiths for creating strains KGB09, KGB72, and KGB94.
This work was supported by a Department of Defense Multi-Disciplinary University Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911F-09-1-0286 (P.S., J.Y., and A.E.C.) and by a grant from the Army Research Office under contract number W911NF-12-1-0325.
REFERENCES
- 1.Setlow P. 2003. Spore germination. Curr Opin Microbiol 6:550–556. doi: 10.1016/j.mib.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Paidhungat M, Setlow P. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J Bacteriol 183:3982–3990. doi: 10.1128/JB.183.13.3982-3990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudson KD, Corfe BM, Kemp EH, Feavers IM, Coote PJ, Moir A. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J Bacteriol 183:4317–4322. doi: 10.1128/JB.183.14.4317-4322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan AE, Olivastro EM, Koppel DE, Loshon CA, Setlow B, Setlow P. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are largely immobile. Proc Natl Acad Sci U S A 101:7733–7738. doi: 10.1073/pnas.0306859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Yi X, Li YQ, Setlow P. 2011. Germination of individual Bacillus subtilis spores with alterations in the GerD and SpoVA proteins, which are important in spore germination. J Bacteriol 193:2301–2311. doi: 10.1128/JB.00122-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Davis A, Korza G, Zhang P, Li YQ, Setlow B, Setlow P. 2012. Role of a SpoVA protein in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 194:1875–1884. doi: 10.1128/JB.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Valdespino A, Li Y, Setlow B, Ghosh S, Pan D, Korza G, Feeherry FE, Doona CJ, Li Y-Q, Hao B, Setlow P. 2014. Properties and function of the SpoVAEa and SpoVAF proteins in Bacillus subtilis spores. J Bacteriol 196:2077–2088. doi: 10.1128/JB.01546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi X, Liu J, Faeder JR, Setlow P. 2011. Synergism between different germinant receptors in the germination of Bacillus subtilis spores. J Bacteriol 193:4664–4671. doi: 10.1128/JB.05343-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths KK, Zhang J, Cowan AE, Yu J, Setlow P. 2011. Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol Microbiol 81:1061–1077. doi: 10.1111/j.1365-2958.2011.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelczar PL, Igarashi T, Setlow B, Setlow P. 2007. Role of GerD in germination of Bacillus subtilis spores. J Bacteriol 189:1090–1098. doi: 10.1128/JB.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Jin K, Gosh S, Carlson K, Devarakonda P, Davis A, Stewart KV, Cammett E, Pelczar-Rossi P, Setlow B, Lu M, Setlow P, Hao B. 2014. Structural and functional analysis of the GerD spore germination protein of Bacillus species. J Mol Biol 426:1995–2008. doi: 10.1016/j.jmb.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelczar PL, Setlow P. 2008. Localization of the germination protein GerD to the inner membrane in Bacillus subtilis spores. J Bacteriol 190:5635–5641. doi: 10.1128/JB.00670-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Ray WK, Helm RF, Melville SB, Popham DL. 2014. Levels of germination proteins in Bacillus subtilis dormant, superdormant and germinating spores. PLoS One 9:e95781. doi: 10.1371/journal.pone.0095781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popham DL, Helin J, Costello CE, Setlow P. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci U S A 93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S, Setlow B, Wahome PG, Cowan AE, Plomp M, Malkin AJ, Setlow P. 2008. Characterization of spores of Bacillus subtilis that lack most coat layers. J Bacteriol 190:6741–6748. doi: 10.1128/JB.00896-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrera-Martinez RM, Tovar-Rojo F, Vepachedu VR, Setlow P. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J Bacteriol 185:2457–2464. doi: 10.1128/JB.185.8.2457-2464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atluri S, Ragkousi K, Cortezzo DE, Setlow P. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J Bacteriol 188:28–36. doi: 10.1128/JB.188.1.28-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore ME, Bandyopadhyay D, Dean AM, Linnstaedt SD, Popham DL. 2004. Production of muramic δ-lactam in Bacillus subtilis spore peptidoglycan. J Bacteriol 186:80–89. doi: 10.1128/JB.186.1.80-89.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England. [Google Scholar]
- 21.Ghosh S, Scotland M, Setlow P. 2012. Levels of germination proteins in dormant and superdormant spores of Bacillus subtilis. J Bacteriol 194:2221–2227. doi: 10.1128/JB.00151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart K-AV, Setlow P. 2013. Numbers of individual nutrient germinant receptors and other germination proteins in spores of Bacillus subtilis. J Bacteriol 195:3575–3582. doi: 10.1128/JB.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart K-AV, Yi X, Ghosh S, Setlow P. 2012. Germination protein levels and rates of germination of spores of Bacillus subtilis with overexpressed or deleted genes encoding germination proteins. J Bacteriol 194:3156–3164. doi: 10.1128/JB.00405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez-Peralta A, Stewart K-AV, Thomas SK, Setlow B, Chen Z, Li Y-Q, Setlow P. 2012. Effects of the SpoVT regulatory protein on the germination and germination protein levels of spores of Bacillus subtilis. J Bacteriol 194:3417–3425. doi: 10.1128/JB.00504-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang J, Zhang P, Setlow P, Li YQ. 2014. High precision fitting measurements of the kinetics of size changes during germination of individual Bacillus spores. Appl Environ Microbiol 80:4606–4615. doi: 10.1128/AEM.01204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korza G, Setlow P. 2013. Topology and accessibility of germination proteins in the Bacillus subtilis spore inner membrane. J Bacteriol 195:1484–1491. doi: 10.1128/JB.02262-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dammeyer T, Tinnefeld P. 2012. Engineered fluorescent proteins illuminate the bacterial periplasm. Comp Struct Biotechnol J 3:e201210013. doi: 10.5936/csbj.201210013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Setlow B, Setlow P, Hao B. 2010. Crystal structure of the GerBC component of a Bacillus subtilis spore germinant receptor. J Mol Biol 402:8–16. doi: 10.1016/j.jmb.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teruel M, Meyer T. 2000. Translocation and reversible localization of signaling proteins: a dynamic future for signal transduction. Cell 103:181–184. doi: 10.1016/S0092-8674(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 30.Pawson T, Scott JD. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara E, Nagano K, Nikaido H. 2012. Alternative folding pathways of the major porin OprF of Pseudomonas aeruginosa. FEBS J 279:910–918. doi: 10.1111/j.1742-4658.2012.08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugawara E, Nestorovich EM, Bezrnkov SM, Nakaido H. 2006. Pseudomonas aeruginosa protein OprF exists in two different conformations. J Biol Chem 281:16220–16229. doi: 10.1074/jbc.M600680200. [DOI] [PMC free article] [PubMed] [Google Scholar]