Abstract
The Gram-negative commensal bacterium nontypeable Haemophilus influenzae (NTHI) can cause respiratory tract diseases that include otitis media, sinusitis, exacerbations of chronic obstructive pulmonary disease, and bronchitis. During colonization and infection, NTHI withstands oxidative stress generated by reactive oxygen species produced endogenously, by the host, and by other copathogens and flora. These reactive oxygen species include superoxide, hydrogen peroxide (H2O2), and hydroxyl radicals, whose killing is amplified by iron via the Fenton reaction. We previously identified genes that encode proteins with putative roles in protection of the NTHI isolate strain 86-028NP against oxidative stress. These include catalase (HktE), peroxiredoxin/glutaredoxin (PgdX), and a ferritin-like protein (Dps). Strains were generated with mutations in hktE, pgdX, and dps. The hktE mutant and a pgdX hktE double mutant were more sensitive than the parent to killing by H2O2. Conversely, the pgdX mutant was more resistant to H2O2 due to increased catalase activity. Supporting the role of killing via the Fenton reaction, binding of iron by Dps significantly mitigated the effect of H2O2-mediated killing. NTHI thus utilizes several effectors to resist oxidative stress, and regulation of free iron is critical to this protection. These mechanisms will be important for successful colonization and infection by this opportunistic human pathogen.
INTRODUCTION
During aerobic respiration, the suboptimal metabolic reduction of molecular oxygen to water generates reactive oxygen species that are profoundly toxic to bacterial cells. Bacteria have therefore evolved multiple mechanisms to combat such lethal insults. These include enzymatic scavenging of oxidative stress-inducing agents by superoxide dismutases (SODs) that breakdown superoxide, as well as catalases, alkylhydroperoxides, periplasmic thiol peroxidases, and bacterioferritin-comigratory proteins that decompose peroxides. In addition to a bacterium's need to protect itself from endogenously generated reactive oxygen species, bacteria that reside within a host must also contend with the release of extracellular reactive oxygen species (ROS) by phagocytes, copathogens, and the host's flora. The mechanisms that abrogate the effects of externally derived oxidative stress within a host have been well described in the model organism Escherichia coli (1). These defenses overlap the defenses used to combat endogenously generated oxidative stress.
Bacteria can also use nonenzymatic mechanisms to protect against oxidative stress. Protection against the effects of free iron is especially critical. Hydrogen peroxide (H2O2) can react with intracellular free iron to generate toxic hydroxyl radicals via the Fenton reaction. The hydroxyl radicals generated can then damage DNA. For example, when an E. coli strain unable to scavenge H2O2 was repeatedly passaged under aerobic conditions, DNA damage mediated by H2O2 was readily observed (2, 3). As iron is critical for the generation of hydroxyl radicals via the Fenton reaction, bacteria protect themselves through the sequestration of iron. The role of ferritins and ferritin-like proteins, such as Dps, in this iron sequestration process is well understood (3–5). For example, Dps protects E. coli from the effects of H2O2 in stationary phase (6). Moreover, there is strong conservation among Dps orthologs, with several core conserved amino acids forming an iron-binding core. Within this core, the bound ferrous iron is oxidized by H2O2, but critically, hydroxyl radicals are not produced (7). The expression of Dps is regulated by the anoxic redox control regulator ArcA and protects anaerobically grown Haemophilus influenzae strain Rd from H2O2 (8). Dps has also been shown to have a role in biofilm formation and in vivo survival of the nontypeable H. influenzae (NTHI) strain 86-028NP (9).
NTHI is a major cause of otitis media (OM), sinusitis, exacerbations of chronic obstructive pulmonary disease, and bronchitis, as well as other infections (10). Thus, illness due to NTHI is a major societal burden. NTHI resides as a commensal in the nasopharynx, along with host flora. In certain instances, for example following a viral infection, NTHI can then move to the middle ear, where it causes OM. Infection in the middle ear brings an increase in polymorphonuclear leukocytes (PMNs), with an increased possibility of insult by ROS. In addition, Streptococcus pneumoniae, a species that can cocolonize the nasopharynx, produces extracellular H2O2 at concentrations that are bactericidal toward H. influenzae in vitro. H. influenzae therefore has a number of proteins that protect against oxidative stress. The genes that encode catalase and PgdX were previously identified in H. influenzae strain Rd. Subsequent analyses of the genome of the NTHI strain 86-028NP identified homologues of genes encoding catalase, PgdX, Dps, and the alkylhydroperoxidase TsaA (11, 12).
We hypothesized that expression of the numerous oxidative stress protection systems is essential for an NTHI cell, both as a commensal and during the course of infection (11). In the current studies, we generated mutant strains of 86-028NP that do not express catalase, TsaA, PgdX, or Dps. We also generated double mutants that do not express catalase and PgdX, catalase and Dps, or PgdX and Dps. Finally we generated mutations in catalase, PgdX, and Dps in a strain that lacked the ferric uptake regulator, Fur. These strains were tested for their ability to withstand the effects of oxidative stress induced by in vitro treatment with H2O2. Our data indicate that strain 86-028NP possesses multiple, overlapping oxidative stress defense mechanisms with critical roles in protection against iron-exacerbated oxidative stress. We hypothesize that regulation of these mechanisms is critical for NTHI survival in vivo.
MATERIALS AND METHODS
Bacterial strains and culture media used.
NTHI strain 86-028NP, recovered from the nasopharynx of a child with chronic OM, has been well characterized in vitro (13, 14) and in a chinchilla model of OM (15–17). The genome sequence has been published (12). A streptomycin-resistant derivative of strain 86-028NP with a mutation in rpsL,86-028NPrpsL, was used as the parent strain in mutant construction, as described in Carruthers et al. (18).
For routine growth, NTHI strains were cultured on chocolate II agar plates (Fisher Scientific, Pittsburgh, PA). For counterselection, strain 86-028NPrpsL was cultured on chocolate agar plates containing 1,000 μg streptomycin/ml. The complemented NTHI strains were cultured on chocolate agar plates containing 200 μg spectinomycin/ml. For routine liquid culture, NTHI cells were grown in brain heart infusion broth supplemented with 2 μg NAD/ml and 2 μg heme/ml (sBHI). Unless stated otherwise, all growth was with shaking at 180 rpm at 37°C. Cell growth was tracked by measuring optical density at 600 nm.
Construction of mutant strains.
All mutant strains were generated using a recombineering method developed for strain 86-028NP (18). Mutants were selected on medium containing 200 μg spectinomycin/ml. The spectinomycin resistance antibiotic cassette was then removed by site-specific recombination. After the removal of the spectinomycin resistance antibiotic cassette, additional mutations could be introduced using the same method. The identity of each mutant was confirmed by PCR and sequencing.
Construction of complemented mutant strains.
Each complemented strain was generated as follows. The coding sequence, as well as the upstream sequence that contained a predicted OxyR-binding site, was amplified by PCR. The primer upstream from the gene contained a BamHI restriction site. The primer downstream from the gene contained an EcoRI restriction site. The PCR amplicon was cloned between the BamHI and EcoRI sites in pSPEC1, a derivative of the Haemophilus-Actinobacillus pleuropneumoniae shuttle vector pGZRS-39A, in which the kanamycin resistance gene was replaced by a spectinomycin resistance gene (16, 19). Each construct was transformed into its respective mutant by electroporation, and transformants selected on chocolate agar containing 200 μg spectinomycin/ml. Strains are listed on Table 1.
TABLE 1.
Bacterial strains
| Strain | Description | Reference or source |
|---|---|---|
| 86-028NP | Nontypeable H. influenzae strain from a child with chronic otitis media | 12 |
| 86-028NPrpsL | Streptomycin-resistant derivative of 86-028NP | 18 |
| 86-028NPrpsL ΔtsaA | Derivative of 86-028NPrpsL with a deletion mutation of tsaA | This study |
| 86-028NPrpsL ΔhktE | Derivative of 86-028NPrpsL with a deletion mutation of hktE | This study |
| 86-028NPrpsL ΔhktE(phktE) | 86-028NPrpsL ΔhktE transformed with pSPEC1-hktE | This study |
| 86-028NPrpsL ΔpgdX | Derivative of 86-028NPrpsL with a deletion mutation of pgdX | This study |
| 86-028NPrpsL ΔpgdX(ppgdX) | 86-028NPrpsL ΔpgdX transformed with pSPEC1-pgdX | This study |
| 86-028NPrpsL Δdps | Derivative of 86-028NPrpsL with a deletion mutation of dps | This study |
| 86-028NPrpsL Δdps(pdps) | 86-028NPrpsL Δdps transformed with pSPEC1-dps | This study |
| 86-028NPrpsL Δdps(phktE) | 86-028NPrpsL Δdps transformed with pSPEC1-hktE | This study |
| 86-028NPrpsL Δfur | Derivative of 86-028NPrpsL with a deletion mutation of fur | This study |
| 86-028NPrpsL ΔhktEΔpgdX | Derivative of 86-028NPrpsL with deletion mutations of hktE and pgdX | This study |
| 86-028NPrpsL ΔhktEΔpgdX(phktE) | 86-028NPrpsL ΔhktEΔpgdX transformed with pSPEC1-hktE | This study |
| 86-028NPrpsL ΔhktEΔpgdX(ppgdX) | 86-028NPrpsL ΔhktEΔpgdX transformed with pSPEC1-pgdX | This study |
| 86-028NPrpsL ΔdpsΔhktE | Derivative of 86-028NPrpsL with deletion mutations of dps and hktE | This study |
| 86-028NPrpsL ΔdpsΔhktE(pdps) | 86-028NPrpsL ΔdpsΔhktE transformed with pSPEC1-dps | This study |
| 86-028NPrpsL ΔdpsΔhktE(phktE) | 86-028NPrpsL ΔdpsΔhktE transformed with pSPEC1-hktE | This study |
| 86-028NPrpsL ΔhktEΔfur | Derivative of 86-028NPrpsL with deletion mutations of hktE and fur | This study |
| 86-028NPrpsL ΔhktEΔfur(phktE) | 86-028NPrpsL ΔhktEΔfur transformed with pSPEC1-hktE | This study |
| 86-028NPrpsL ΔpgdXΔfur | Derivative of 86-028NPrpsL with deletion mutations of pgdX and fur | This study |
| 86-028NPrpsL ΔpgdXΔfur(ppgdX) | 86-028NPrpsL ΔpgdXΔfur transformed with pSPEC1-pgdX | This study |
| 86-028NPrpsL ΔdpsΔfur | Derivative of 86-028NPrpsL with deletion mutations of dps and fur | This study |
| 86-028NPrpsL ΔdpsΔfur(pdps) | 86-028NPrpsL ΔdpsΔfur transformed with pSPEC1-dps | This study |
Quantitative RT-PCR.
Quantitative reverse transcription (RT)-PCR (qRT-PCR) was used to determine the expression of genes with predicted roles in combating oxidative stress. qRT-PCR was performed with a one-step QuantiTect SYBR green RT-PCR kit (Qiagen, Valencia, CA) as outlined by Harrison et al. (20). Three biological replicates and three technical replicates were performed for each gene analyzed. Fold changes were calculated. All threshold cycle (CT) values were normalized to the value for the endogenous control gyrA. Relative quantitation was calculated from the median CT value using ΔΔCT, and statistical significance was determined using Student's two-tailed t test. A fold change in gene expression greater than 2-fold and with a P value of <0.05 was assessed as being significant.
In vitro sensitivity tests with hydrogen peroxide.
Unless stated otherwise, strain 86-026NPrpsL and each mutant to be tested were grown in sBHI with shaking at 180 rpm to mid-exponential phase. H2O2 was then added to give a final concentration of 500 μM. Growth was continued for 10 min with shaking at 180 rpm. Cells were then removed, serially diluted, and plated on chocolate agar to assess viability.
Iron chelation tests with 2,2′-bipyridine.
To chelate iron from cultures, exponentially growing cells were treated with 500 μM 2,2′-bipyridine, a treatment that we previously showed affected iron levels in sBHI (20). Cells which did not receive 500 μM 2,2′-bipyridine had ethanol added to a final concentration of 0.2% to replicate the addition of ethanol used as the vehicle for 2,2′-bipyridine. Chelation was carried out concurrently with H2O2 production, and cell viability was assessed on chocolate agar as described above.
Quantitation of peroxidase activity.
Strain 86-026NPrpsL and each mutant to be tested were grown in sBHI with shaking at 180 rpm to mid-exponential phase. Cultures were then split into two 20-ml aliquots, and H2O2 added to one aliquot of each strain to a final concentration of 500 μM. After a further 30 min of growth, 5-ml amounts of cells, with or without H2O2 treatment, were chilled on ice and then harvested by centrifugation at 3,220 × g, washed once in 5 ml cold 0.1 M potassium phosphate buffer, pH 6.8, resuspended in 1.7 ml cold 0.1 M potassium phosphate buffer, pH 6.8, and mechanically lysed using lysing matrix B (MP Biomedicals, Solon, OH). Lysates were cleared of lysing matrix by centrifugation at 27 × g for 5 min, and protein concentrations calculated using a bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL). Twenty-five-microliter amounts of the resulting lysates were then used in a catalase activity assay using an Amplex red catalase assay kit (Life Technologies, Grand Island, NY).
Statistical analyses of H2O2 sensitivity and peroxidase activity assays.
Data produced by the H2O2 sensitivity and peroxidase activity assays were tested for significance using a multiple t test with Bonferroni correction. All analyses were carried out with Prism 6 (GraphPad Software, La Jolla, CA).
RESULTS
Roles of catalase, peroxiredoxin/glutaredoxin, and alkylhydroperoxidase in protection of NTHI against hydrogen peroxide-induced oxidative stress.
We had previously determined that the loss of OxyR, the master transcriptional regulator of genes with roles in protection against oxidative stress, had an effect on the viability of cells treated with both 250 μM and 500 μM H2O2. We thus hypothesized that one or more of these OxyR-regulated genes would be critical to the survival of strain 86-028NP when subjected to H2O2-induced oxidative stress. We have previously shown that the expression of tsaA was unresponsive to treatment with H2O2, and tsaA is not a member of the OxyR regulon in strain 86-028NP (21). In concert with these data, when the tsaA mutant and its parental strain were grown to late exponential phase and treated with 500 μM H2O2, there was no significant difference in the rates of viability of the tsaA mutant and the parent (data not shown). Conversely, treatment with 500 μM H2O2 produced a slight but significant decrease in the viability of the hktE mutant compared to that of the parent strain (Fig. 1A). The catalase mutation was complemented with a plasmid-encoded parental copy of hktE downstream from its native 5′ untranslated region (UTR), which contained a predicted OxyR-binding site. Restoration of OxyR-regulated hktE in the complemented hktE mutant abrogated the susceptibility of the hktE mutant to H2O2 (Fig. 1A). This experiment was repeated with the pgdX mutant. The loss of PgdX resulted in increased viability compared to that of the parent strain after treatment with 500 μM H2O2. This increased survival was reduced by complementation of the pgdX mutation (Fig. 1B). These data suggested the compensatory effect of an additional oxidative stress protection mechanism in the absence of PgdX. Possibly an increase in catalase was compensating for the loss of PgdX, as previously demonstrated in H. influenzae strain Rd (22). This hypothesis was supported by the observation that a strain that lacked both hktE and pgdX demonstrated reduced viability, albeit nonsignificant, compared to that of the parent after treatment with 500 μM H2O2 but that the loss of viability was abrogated when the hktE pgdX double mutant strain was complemented with either hktE or pgdX (Fig. 2).
FIG 1.
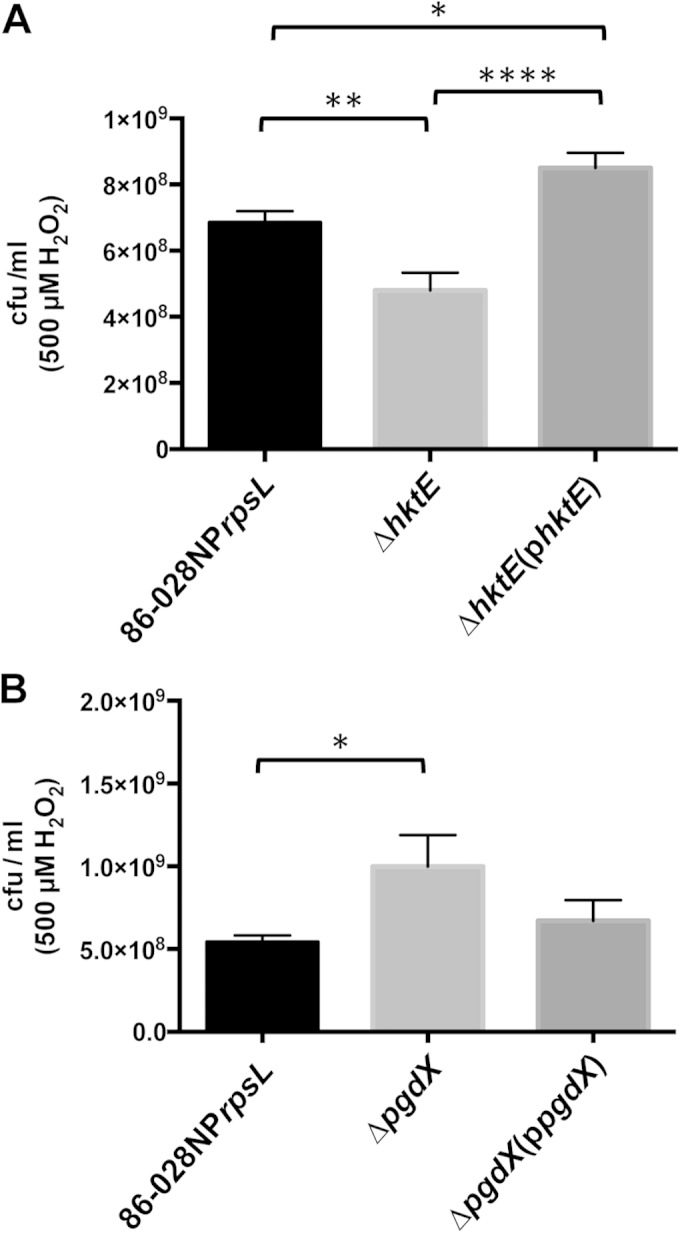
Both the catalase HktE and the peroxidase PgdX protect NTHI strain 86-028NP from H2O2-induced oxidative stress. Strains of 86-028NP that lacked either hktE (A) or pgdX (B) were grown exponentially and then treated with 500 μM H2O2 for 10 min. The cells were harvested and plated to assess viability relative to that of the parent strain. Loss of hktE produced a slight but significant loss in cell viability, while the strain that lacked pgdX exhibited increased viability. Complementation of the mutated gene restored the parental phenotype in both cases. Error bars show standard errors of the means. *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001 (n = 9 [A]; n = 6 [B]).
FIG 2.
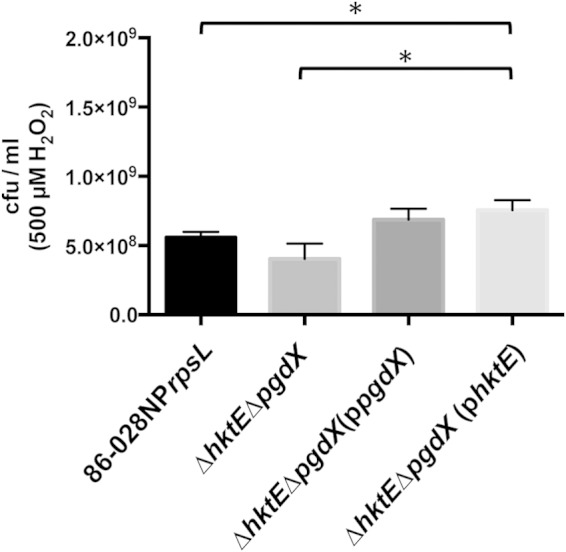
Catalase and the peroxidase PgdX together protect NTHI strain 86-028NP from H2O2-induced oxidative stress. A strain of 86-028NP that lacked both hktE and pgdX was grown exponentially and then treated with 500 μM H2O2 for 10 min. The cells were harvested and plated to assess viability. Loss of both hktE and pgdX produced a slight loss in cell viability. Complementation of the double mutant with either hktE or pgdX restored the parental phenotype. Error bars show standard errors of the means. *, P ≤ 0.05 (n = 7).
To determine whether there was increased hktE expression in the absence of PgdX, qRT-PCR analysis was used to quantify hktE expression in the pgdX mutant. Unexpectedly, when the mutant was treated with H2O2, the loss of PgdX led to a small, nonsignificant decrease in hktE expression that was reversed when the mutation was complemented. Little effect on hktE expression in the ΔpgdX background was observed in the absence of peroxide-induced stress (data not shown). We further quantified the ability of the parent strain to decompose H2O2. The hktE mutant, the pgdX mutant, and the hktE pgdX double mutant strain were tested similarly. Each strain was grown to late exponential phase and then split into two aliquots. One aliquot was treated with 500 μM H2O2, as in the sensitivity tests. The second aliquot was left untreated. Cell extracts were prepared and assayed using an Amplex red catalase activity kit from Life Technologies, which measures decomposition of H2O2 as a surrogate for catalase activity. When extracts from cells untreated with H2O2 were compared, the hktE mutant demonstrated an approximately 3-fold reduction in activity compared to the results for the parent strain. Strikingly, without H2O2 pretreatment, the pgdX mutant decomposed H2O2 with approximately 4-fold greater activity than the parent. However, this increase in enzymatic activity was lost in the strain that lacked both catalase and PgdX, suggesting that the increase in the ability to decompose H2O2 in the pgdX mutant was due to increased catalase enzymatic activity (Fig. 3). This thesis was supported by data that compared the levels of H2O2 decomposition activity in strains pretreated with H2O2 with the levels in the strains that were left untreated. The parent exhibited an approximately 2-fold increase in activity when the cells were pretreated with H2O2 compared to the activity in the cells that were untreated. When the hktE mutant was tested similarly, pretreatment with H2O2 did not produce an increase in decomposition activity. Actually, pretreatment of the hktE mutant produced a slight but nonsignificant decrease in activity compared to that in the untreated cells (Fig. 3). However, when either the pgdX mutant or the ΔhktE ΔpgdX mutant was tested, pretreatment with H2O2 had no significant effect on decomposition activity compared to the results for the untreated strains. These data are in agreement with data derived from H. influenzae strain Rd (22) and further suggest the presence of a third functional peroxidase in strain 86-028NP.
FIG 3.
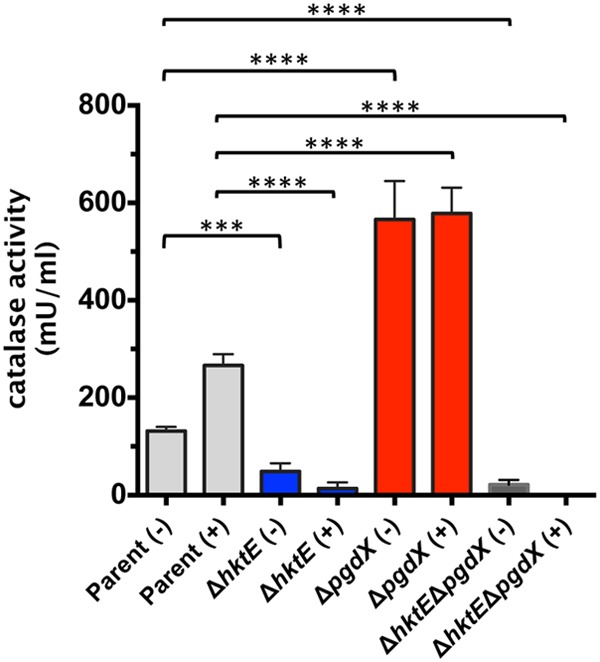
A strain of 86-028NP that lacks the peroxidase PgdX has an increased ability to decompose H2O2. Strains of 86-028NP that lacked the genes encoding the catalase HktE, the peroxidase PgdX, or both HktE and PgdX were grown exponentially and then treated with (+) 500 μM H2O2 for 30 min. A control sample was left untreated (−). The cells were harvested and lysed, and their ability to decompose H2O2 assessed using an Amplex red catalase assay kit. The addition of H2O2 to the cells produced increased H2O2 decomposition activity in the parent strain relative to the activity in the untreated cells. The hktE mutant showed a large reduction in H2O2 decomposition activity. In contrast, the pgdX mutant demonstrated H2O2 decomposition activity that was significantly greater than that of the parent. Slight activity remained in the strain that lacked both hktE and pgdX. Error bars show standard errors of the means. ***, P ≤ 0.001; ****, P ≤ 0.0001 (n = 8).
The loss of Dps increases NTHI's sensitivity to hydrogen peroxide-induced oxidative stress.
The toxic effects of H2O2 are exacerbated by the presence of iron, due to the Fenton reaction. Ferric iron participates in the reductive decomposition of H2O2. This reaction leads to the production of hydroxyl radicals, which can profoundly damage DNA (23). Bacteria have therefore evolved mechanisms to abrogate the effects of the Fenton reaction. Aside from decomposition of H2O2, bacteria can sequester free iron. Notable among the proteins involved in iron sequestration is Dps, a ferritin-like protein whose function was first elucidated in E. coli (5). A dps mutant was generated in strain 86-028NPrpsL, and the importance of Dps in resistance to H2O2-induced oxidative stress was tested. Treatment of exponentially growing cells with 500 μM H2O2 produced an approximately 22-fold decrease in the viability of the dps mutant compared to that of the parent strain (Fig. 4). In contrast, a similarly treated catalase mutant exhibited a small decrease in viability compared to the viability of the parent strain (Fig. 1A). The dps mutation was complemented with a plasmid-encoded parental copy of dps downstream from its native 5′ UTR, which contained a predicted OxyR-binding site. Restoration of OxyR-regulated dps in the complemented dps mutant abrogated the susceptibility of the dps mutant to H2O2 (Fig. 4). We reasoned that the loss of Dps led to an increase in intracellular iron and, thus, in the presence of H2O2, to a decrease in cell viability due to the Fenton reaction. To determine the importance of H2O2 in this process, the plasmid construct previously used to complement the catalase mutant (Fig. 1A) was introduced into the dps mutant. The sensitivity of this strain, 86-028NPrpsLΔdps(phktE), to 500 μM H2O2 was then assessed and found to be similar to that of the parental strain (Fig. 4). These data support the hypothesis that H2O2-mediated toxicity is due to the interplay between iron and H2O2, with the ability to chelate iron being critical to the survival of the cell. We therefore generated an additional 86-028NPrpsL strain in which both hktE and dps were mutated. After treatment of exponentially growing cells with 500 μM H2O2, there was approximately a 110-fold decrease in the viability of cells due to the loss of both Dps and catalase (Fig. 5). When either Dps or catalase was introduced into the hktE dps double mutant strain via pdps or phktE, the viability of the double mutant after H2O2 treatment returned to parental levels (Fig. 5).
FIG 4.
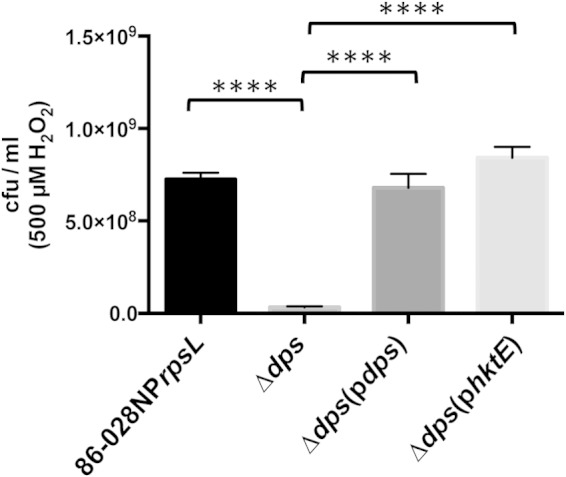
The ferritin-like protein Dps has a major role in the protection of NTHI strain 86-028NP from H2O2-induced oxidative stress. A strain of 86-028NP that lacked dps was grown exponentially and then treated with 500 μM H2O2 for 10 min. The cells were harvested and plated to assess viability. Loss of dps produced a large and significant loss in cell viability relative to that of the parent strain. Complementation of the mutated gene with either dps or hktE restored the parental phenotype in both cases. Error bars show standard errors of the means. ****, P ≤ 0.0001 (n = 6).
FIG 5.
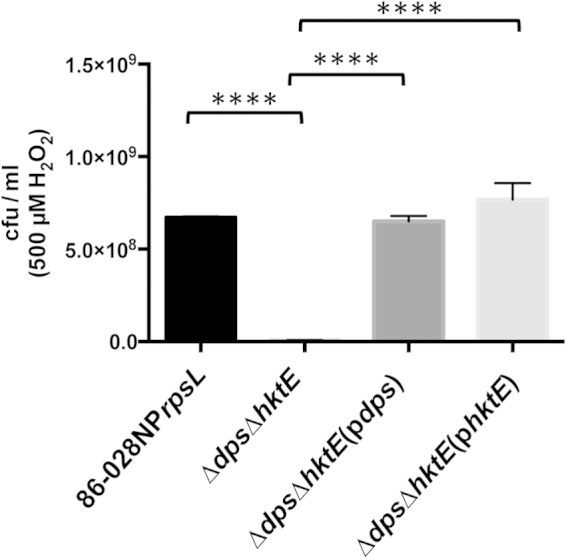
The activities of both the ferritin-like protein Dps and the catalase HktE are critical in the protection of NTHI strain 86-028NP from H2O2-induced oxidative stress. A strain of 86-028NP that lacked both hktE and dps was grown exponentially and then treated with 500 μM H2O2 for 10 min. The cells were harvested and plated to assess viability. Loss of both hktE and dps produced a large and significant loss in cell viability relative to that of the parent strain. Complementation of the double mutant with either hktE or dps restored the parental phenotype. Error bars show standard errors of the means. ****, P ≤ 0.0001 (n = 5).
We further confirmed the importance of iron in H2O2-mediated killing of strain 86-028NPrpsL by comparing the toxic effects of H2O2 on strain 86-028NPrpsL in iron-replete medium and iron-depleted medium. Briefly, exponentially grown cells were split into two aliquots, and one aliquot was treated with 500 μM 2,2′-bipyridine. Treatment with H2O2 or with 2,2′-bipyridine had no significant effect on the viability of the parental strain (data not shown). Conversely, H2O2 treatment significantly decreased the viability of the dps mutant compared to that of the dps mutant that was untreated (Fig. 6). The reduction in viability of the dps mutant due to H2O2 treatment was abrogated when the iron in the medium was depleted with 2,2′-bipyridine (Fig. 6). Finally, when the experiment was repeated with the strain in which the dps mutation was complemented, there were no significant differences in the rates of viability of the strain under all treatments tested (data not shown).
FIG 6.
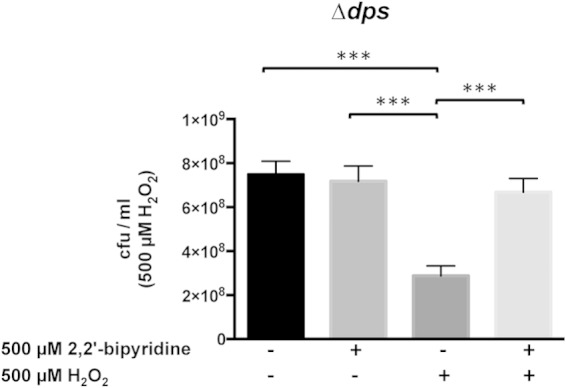
Minimizing the interplay between iron and H2O2 is critical in the protection of NTHI strain 86-028NP from H2O2-induced oxidative stress. A strain of 86-028NP that lacked dps was grown exponentially and then treated with (+) or without (−) 500 μM H2O2 for 10 min. The treatment with H2O2 was carried out in the presence (+) or absence (−) of the iron chelator 2,2′-bipyridine. The cells were harvested and plated to assess viability. The dps mutant exhibited a loss of viability in the absence of iron chelation. In contrast, iron chelation protected the dps mutant from the effects of H2O2. Complementation of the dps mutation restored the parental phenotype under all conditions tested (data not shown). Error bars show standard errors of the means. ***, P ≤ 0.001 (n = 6).
The critical importance of the interplay between iron and H2O2 in the induction of oxidative stress was further demonstrated using strains in which the mutations in catalase, pgdX, and dps were introduced into a strain with a deletion of the gene that encodes the ferric uptake regulator, Fur. We had previously generated a strain of 86-028NP carrying a marked deletion of fur (20). For this study, we generated a strain of 86-028NPrpsL carrying an unmarked deletion of fur. We could then easily introduce additional mutations into this strain. We previously showed that strain 86-028NP, which lacked Fur, had upregulated expression of many genes involved in iron import (20). Microarray analysis carried out as detailed in our previous studies (20) showed concordance between the Fur-regulated genes identified using the marked fur mutant and the unmarked fur mutant (data not shown). We therefore hypothesized that a fur mutant of strain 86-028NPrpsL would have elevated amounts of intracellular iron and so be more susceptible to the effects of H2O2. Previous killing assays were carried out with exponentially growing cells that had been grown in sBHI with shaking at 180 rpm. However, the pgdX fur double mutant would not grow under these conditions (data not shown). We hypothesized that shaking at 180 rpm introduced oxygen into the medium at a level the mutants could not tolerate. This growth phenotype was partially overcome when shaking was reduced to 50 rpm. When shaken at 50 rpm, all strains except the pgdX fur double mutant had similar growth kinetics. The pgdX fur double mutant demonstrated a delayed entrance into exponential phase and had a lower rate of exponential growth than the other strains (data not shown). Despite the differences in growth kinetics, it was possible to test the viability of all strains after exposure to H2O2. The killing assays with 500 μM H2O2 were thus repeated as detailed above, with the caveat that cells were grown to exponential phase while shaking at 50 rpm. Treatment with 500 μM H2O2 produced a slight but nonsignificant increase in the survival of the hktE mutant (Fig. 7A). This stands in contrast with the results of the same experiment carried out with cells grown at 180 rpm, where there was a slight but significant decrease in the viability of the hktE mutant compared to that of the parent strain (Fig. 1A). When the fur mutant was treated similarly, there was a slight but nonsignificant reduction in the viability of the fur mutant compared to that of the parent (Fig. 7A). Conversely, when the hktE fur double mutant was treated with 500 μM H2O2, the viability of the mutant was reduced by approximately 26-fold compared to that of the parent (Fig. 7A). The sensitivity to H2O2 of the hktE fur mutant was abrogated when hktE was introduced into the mutant via the complementing plasmid. This experiment was repeated with the pgdX fur double mutant. As shown by the results described above, the loss of PgdX resulted in increased viability after treatment with 500 μM H2O2 compared to the viability of the parent strain (Fig. 7B). However, in contrast to the results for the hktE fur double mutant, which demonstrated a significant reduction in viability compared to that of the fur mutant, treatment with 500 μM H2O2 produced a slight increase in the survival of the pgdX fur double mutant compared to the survival of the fur mutant (Fig. 7B). Loss of fur in the pgdX fur double mutant led to an approximately 5-fold decrease in survival compared to that of the pgdX mutant (Fig. 7B). Finally, we determined how the loss of both fur and dps affected cell viability. When the culture was growing exponentially, treatment of the dps mutant with 500 μM H2O2 produced approximately a 5-log decrease in viability compared to that of the parent strain (Fig. 7C). This stands in contrast to the data derived from cells grown at 180 rpm, where loss of dps led to a decrease in viability of only 22-fold compared to that of the parent (Fig. 4). When the experiment was repeated with the dps fur double mutant, the number of viable cells recovered after treatment with 500 μM H2O2 was below the limit of detection (Fig. 7C).
FIG 7.
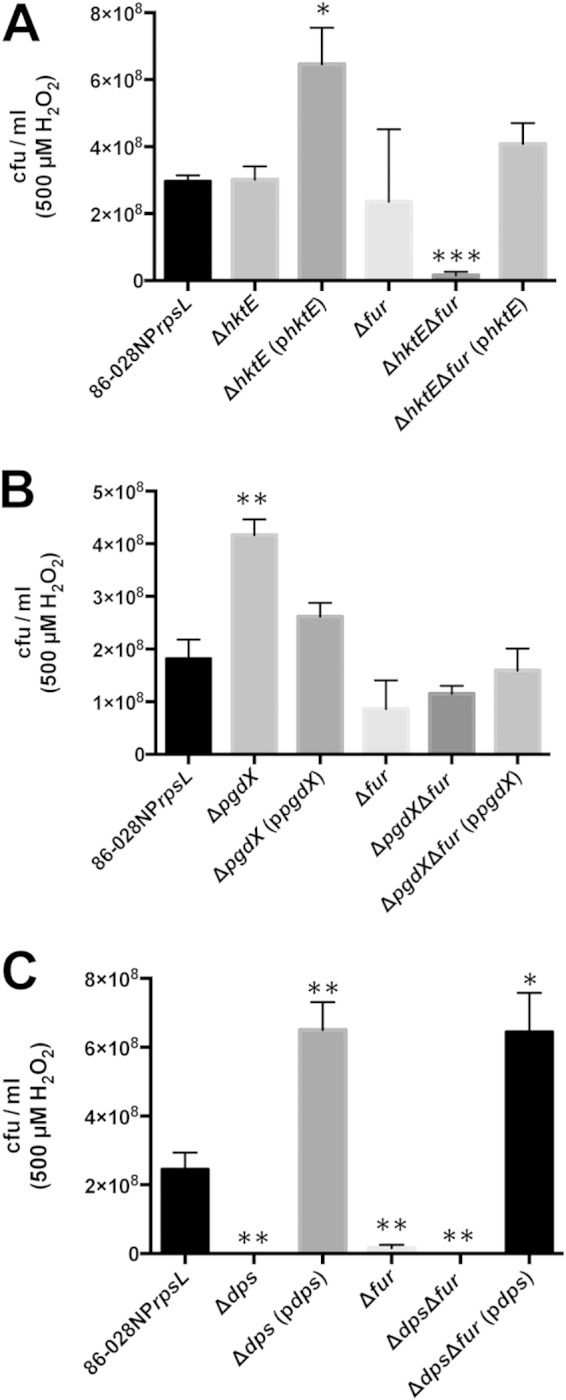
Unregulated import of iron into NTHI strain 86-028NP exacerbates the effects of H2O2-induced killing. The genes encoding catalase (hktE) (A), peroxiredoxin glutaredoxin (pgdX) (B), or the ferritin-like protein Dps (dps) (C) were deleted in a strain of 86-028NP that lacked the ferric uptake regulator, Fur. All strains were grown exponentially and then treated with 500 μM H2O2 for 10 min. The cells were harvested and plated to assess viability. (A, B) Loss of the ability to decompose H2O2 in the face of unrestricted iron import produced significant losses in cell viability relative to that of the parent strain or the respective single mutant strain. (C) Loss of the ability to bind iron in the dps mutant produced a nearly total loss of cell viability when iron import was unregulated and the cells were treated with H2O2. Complementation of the double mutants with hktE, pgdX, or dps restored cell viability. Significance was calculated relative to the results for the parent strain. Error bars show standard errors of the means. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001 (n = 3 [A]; n = 4 [B, C]).
DISCUSSION
H. influenzae can grow both aerobically and as a facultative anaerobe. During aerobic growth, the metabolism of H. influenzae will generate oxidative stress. H. influenzae will also endure oxidative stress derived from host defense cells (24, 25) and from copathogens (primarily Streptococcus pneumoniae [26]). In all niches, NTHI is also in an iron-restricted environment and so must have avid and well-regulated mechanisms to import iron. NTHI must therefore carefully balance the need to acquire scarce sources of iron while minimizing the generation of hydroxyl radicals via the iron-mediated Fenton reaction. To mediate this balance, H. influenzae has evolved multiple overlapping defenses that allow the organism to survive in such a hostile environment.
Enzymatic decomposition of H2O2 is protective in NTHI.
To determine the respective roles of the products of genes presumed to protect against oxidative stress in NTHI strain 86-028NP, we generated strains that lacked the genes that encode catalase, PgdX, TsaA, and Dps. Using these strains, we demonstrated that TsaA had no role in protection against H2O2-induced oxidative stress. This is not unexpected, as we previously showed that the peroxide stress regulator OxyR does not regulate the expression of TsaA (21). The E. coli ortholog of TsaA, AhpC, has a primary role in the protection against endogenously generated oxidative stress. Concentrations of exogenously applied H2O2 in excess of 20 μM overwhelm AhpC's ability to decompose the H2O2, so E. coli must rely on additional mechanisms of protection (27). NTHI strain 86-028NP lacks an ortholog of AhpC's cognate reductase AhpR. TsaA may well be reduced promiscuously by another reductase, but the lack of a cognate reductase and the insensitivity of tsaA expression to OxyR regulation suggest that TsaA's function in strain 86-028NP is different from that of AhpC in E. coli.
The results of hydrogen peroxide killing assays did, however, show a requirement for catalase, PgdX, and Dps. As expected, catalase protected against endogenously applied oxidative stress. However, the protection was not absolute. The catalase mutant only showed a slight reduction in viability compared to the viability of the parent. Other mechanisms generated an additional level of protection. This idea of an overlap in protective mechanisms against peroxide-induced stress was supported by data generated using the strain of 86-028NP that lacked pgdX. The pgdX mutant exhibited an increase in strain viability relative to that of the parent after H2O2 treatment. Increased resistance to H2O2 by a pgdX mutant was also observed by Pauwels et al. (22). In H. influenzae strain Rd, the absence of PgdX leads to suboptimal decomposition of H2O2, leading to an increased intracellular concentration of H2O2. This increase in H2O2 produces a greater response in OxyR activity, with a concomitant upregulation of genes in the OxyR regulon (22). However, when the expression of hktE was assessed in the strain 86-028NP pgdX mutant, we observed no significant changes in hktE expression when oxidative stress was induced by H2O2. Similarly, there was no significant change in the expression of dps in the pgdX mutant (data not shown). hktE and dps are members of the OxyR regulon in strain 86-028NP (21). The lack of PgdX does not appear to lead to a change in gene expression of the members of the OxyR regulon in strain 86-028NP. The enhanced survival of the pgdX mutant undergoing oxidative stress must be due to additional regulatory mechanisms. The changes in peroxidase activity in the pgdX mutant, despite no increase in hktE transcripts, suggest that the activity of HktE may be either posttranscriptionally or posttranslationally regulated in strain 86-028NP. Alternatively, there may be a third peroxidase present in strain 86-028NP, as shown by the ΔhktE ΔpgdX mutant still showing resistance to H2O2, as well as the ability to decompose H2O2, albeit at a very low level. Finally, a disruption in the ability of strain 86-028NP to decompose H2O2 may lead to a modulation of nonenzymatic protective mechanisms.
Regulation of free iron is critical in the protection of NTHI from oxidative stress.
The exacerbation of H2O2-induced oxidative stress by the presence of free ferrous iron has been well described. The oxidation of iron from its ferrous to its ferric state results in the generation of hydroxyl radicals that are injurious to DNA and many cellular proteins (3, 28). NTHI, like most bacteria, has an absolute requirement for extracellular sources of iron. NTHI has therefore evolved multiple mechanisms to take up both iron and iron-containing proteins, mechanisms tightly controlled by the ferric uptake regulator, Fur (20). NTHI specifically and bacteria in general must balance the need to take up iron with the requirement that iron is sequestered to prevent driving the Fenton reaction. The effects of a breakdown in this control can be readily observed through the use of strain 86-028NP mutants that lack both the ability to decompose H2O2 and to sequester iron.
The deletion of dps from strain 86-028NP will produce a cell in which the ability to sequester free iron is substantially impaired. Introducing the dps mutation into strains in which either hktE or pgdX was mutated allowed us to determine the effect free iron had in the presence of externally applied H2O2 that cannot be efficiently decomposed. Loss of dps alone did indeed increase the sensitivity of the cells to treatment with H2O2, but the loss of viability was greater when both dps and hktE were absent. The importance of the interplay between the ability to sequester free iron and the decomposition of H2O2 was further demonstrated when a plasmid-encoded copy of hktE (phktE) was introduced into the dps hktE double mutant. When phktE was introduced into the hktE mutant, the complemented strain exhibited significantly increased resistance to H2O2 compared to the resistance of the parent strain. The overexpression of hktE, by virtue of being on a multicopy plasmid, generated enhanced resistance to H2O2. The introduction of phktE into the dps hktE double mutant was similarly protective; the strain could decompose sufficient H2O2 to restore the parental phenotype despite the absence of Dps' ability to chelate iron. The cells are therefore protected from H2O2-induced oxidative stress.
The importance of the ability to sequester iron as protection against H2O2-induced oxidative stress was further demonstrated when iron was chelated as the dps mutant was undergoing treatment with H2O2. We have previously demonstrated that treatment of strain 86-028NP growing exponentially in sBHI with 2,2′-bipyridine induces the expression of genes in the Fur regulon (20). Treatment with 2,2′-bipyridine chelates iron from sBHI, a treatment that induces the expression of genes whose expression is normally repressed by Fur. The loss of endogenous iron binding in the dps mutant is compensated by iron chelation, which minimizes the effect of the Fenton reaction and so protects the dps mutant from the effects of hydroxyl radical formation.
Finally, we showed that increased iron uptake in a mutant that lacks Fur also had a detrimental effect on resistance against H2O2-induced oxidative stress. An initial indication that increased uptake of iron through the loss of Fur was exacerbating sensitivity to H2O2 was the observation that the pgdX fur double mutant would not grow when shaken at 180 rpm. Moreover, the pgdX fur double mutant still exhibited an impaired growth phenotype when shaken at 50 rpm. We suggest that the changes in the growth kinetics of the pgdX fur double mutant relate to the amounts of dissolved oxygen present in the culture medium. Using well-accepted conventions for the setup of shaken liquid cultures, we ensured that the medium never comprised more than 20% of the volume of the 250-ml flask. This allows adequate aeration of the medium with atmospheric gases. Flasks that are shaken at faster speeds have increased dissolved oxygen compared to the levels in those shaken at slower speeds (29, 30). Oxygen diffuses freely across cell membranes. Thus, the oxygen concentration within bacteria is very similar to that within the growth medium (27). We would therefore presume that when the culture is grown at 180 rpm, the increased oxygen in the medium is such that the oxygen-dependent generation of reactive oxygen species within cells occurs at a rate that overwhelms the ability of the double mutants to combat the oxidative stress generated. Reducing the amount of dissolved oxygen in the medium through reduction of the speed the cultures are shaken at minimizes this effect. The exception was the pgdX fur double mutant, which still demonstrated an impaired growth phenotype when grown with shaking at 50 rpm. In H. influenzae strain Rd and H. influenzae type b, PgdX protects against the bacteriostatic effects of endogenously produced H2O2 (31). When the culture was untreated, the loss of pgdX alone in strain 86-028NP had no effect on viability, while the loss of fur alone produced an approximately 2-fold reduction in viability compared to that of the parent. However, the untreated pgdX fur double mutant exhibited viability similar to that of the parent (data not shown). As with cells treated with H2O2, the loss of pgdX in the exponentially growing but untreated pgdX fur double mutant must lead to upregulation of the expression of an additional H2O2-protective mechanism(s). The loss of both pgdX and fur thus has a bacteriostatic effect on exponentially growing 86-028NP in the absence of treatment with exogenous H2O2, as the cells exhibited a much reduced exponential growth rate but had viability similar to that of the parent. Significant killing of the strain 86-028NP pgdX fur double mutant only occurred after treatment with exogenous H2O2. The upregulation of an additional mechanism(s) of H2O2 decomposition in the absence of PgdX cannot compensate for the loss of PgdX in the face of unrestricted iron import. Furthermore, both the hktE fur and the pgdX fur double mutants were killed similarly and more readily than either the hktE or fur single mutants. These data again support our hypothesis that protection against oxidative stress in NTHI is reliant on both decomposition of H2O2 and regulation of free iron concentration.
Finally, the most striking data were generated with the dps fur double mutant. When this strain underwent H2O2 treatment, there was a nearly total loss of viability. As the expression levels of both hktE and pgdX were not significantly altered in the absence of Dps (data not shown), we would presume that the dps fur double mutant maintains its ability to efficiently decompose H2O2. It is apparent that the most critical mechanism in the protection of NTHI against oxidative stress is the regulation of free iron. Even in the face of an armament of mechanisms to decompose H2O2, any residual H2O2 left intact can interact with free iron, generate hydroxyl radicals via the Fenton reaction, and kill the cell.
Elucidating the interplay between both enzymes, as well as any additional peroxidase(s), will be critical in understanding how NTHI persists so well within the host. It is clear that NTHI must be able to protect against oxidative stress mediated by peroxide and iron. NTHI must also be able to protect against changes in oxygen concentrations, fluxes that will also generate oxidative stress. NTHI must therefore employ its armament of proteins that can decompose H2O2 and protect against the effects of iron-mediated damage as a critical part of the survival of this important human pathogen within its host.
ACKNOWLEDGMENT
This work was funded by NIAID/NIH grant R01-AI077897 to R.S.M.
REFERENCES
- 1.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imlay JA. 2003. Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 3.Park S, You X, Imlay JA. 2005. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc Natl Acad Sci U S A 102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Brun NE, Crow A, Murphy ME, Mauk AG, Moore GR. 2010. Iron core mineralisation in prokaryotic ferritins. Biochim Biophys Acta 1800:732–744. doi: 10.1016/j.bbagen.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Chiancone E, Ceci P. 2010. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: detoxification of iron and hydrogen peroxide and DNA binding. Biochim Biophys Acta 1800:798–805. doi: 10.1016/j.bbagen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Almiron M, Link AJ, Furlong D, Kolter R. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev 6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun LN, Kwon YM. 2011. Structure, function and regulation of the DNA-binding protein Dps and its role in acid and oxidative stress resistance in Escherichia coli: a review. J Appl Microbiol 110:375–386. doi: 10.1111/j.1365-2672.2010.04890.x. [DOI] [PubMed] [Google Scholar]
- 8.Wong SM, Alugupalli KR, Ram S, Akerley BJ. 2007. The ArcA regulon and oxidative stress resistance in Haemophilus influenzae. Mol Microbiol 64:1375–1390. doi: 10.1111/j.1365-2958.2007.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang B, Hong W, Kock ND, Swords WE. 2012. Dps promotes survival of nontypeable Haemophilus influenzae in biofilm communities in vitro and resistance to clearance in vivo. Front Cell Infect Microbiol 2:58. doi: 10.3389/fcimb.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy TF, Faden H, Bakaletz LO, Kyd JM, Forsgren A, Campos J, Virji M, Pelton SI. 2009. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J 28:43–48. doi: 10.1097/INF.0b013e318184dba2. [DOI] [PubMed] [Google Scholar]
- 11.Harrison A, Bakaletz LO, Munson J, Robert S. 2012. Haemophilus influenzae and oxidative stress. Front Cell Infect Microbiol 2:40. doi: 10.3389/fcimb.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison A, Dyer DW, Gillaspy A, Ray WC, Mungur R, Carson MB, Zhong H, Gipson J, Gipson M, Johnson LS, Lewis L, Bakaletz LO, Munson RS Jr. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J Bacteriol 187:4627–4636. doi: 10.1128/JB.187.13.4627-4636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakaletz LO, Tallan BM, Hoepf T, DeMaria TF, Birck HG, Lim DJ. 1988. Frequency of fimbriation of nontypable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect Immun 56:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes KA, Bakaletz LO. 1997. Adherence of non-typeable Haemophilus influenzae promotes reorganization of the actin cytoskeleton in human or chinchilla epithelial cells in vitro. Microb Pathog 23:157–166. doi: 10.1006/mpat.1997.0145. [DOI] [PubMed] [Google Scholar]
- 15.Bakaletz LO, Leake ER, Billy JM, Kaumaya PT. 1997. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae in the chinchilla. Vaccine 15:955–961. doi: 10.1016/S0264-410X(96)00298-8. [DOI] [PubMed] [Google Scholar]
- 16.Mason KM, Munson RS Jr, Bakaletz LO. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect Immun 71:3454–3462. doi: 10.1128/IAI.71.6.3454-3462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel AR, Szelestey BR, Raffel FK, Sharpe SW, Gearinger RL, Justice SS, Mason KM. 2012. SapF-mediated heme iron utilization enhances persistence and coordinates biofilm architecture of Haemophilus. Front Cell Infect Microbiol 2:42. doi: 10.3389/fcimb.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carruthers MD, Tracy EN, Dickson AC, Ganser KB, Munson RS Jr, Bakaletz LO. 2012. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J Bacteriol 194:1927–1933. doi: 10.1128/JB.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason KM, Bruggeman ME, Munson RS, Bakaletz LO. 2006. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol 62:1357–1372. doi: 10.1111/j.1365-2958.2006.05460.x. [DOI] [PubMed] [Google Scholar]
- 20.Harrison A, Santana EA, Szelestey BR, Newsom DE, White P, Mason KM. 2013. Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae. Infect Immun 81:1221–1233. doi: 10.1128/IAI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison A, Ray WC, Baker BD, Armbruster DW, Bakaletz LO, Munson RS Jr. 2007. The OxyR regulon in nontypeable Haemophilus influenzae. J Bacteriol 189:1004–1012. doi: 10.1128/JB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauwels F, Vergauwen B, Van Beeumen JJ. 2004. Physiological characterization of Haemophilus influenzae Rd deficient in its glutathione-dependent peroxidase PGdx. J Biol Chem 279:12163–12170. doi: 10.1074/jbc.M312037200. [DOI] [PubMed] [Google Scholar]
- 23.Imlay JA, Linn S. 1988. DNA damage and oxygen radical toxicity. Science 240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 24.Craig JE, Cliffe A, Garnett K, High NJ. 2001. Survival of nontypeable Haemophilus influenzae in macrophages. FEMS Microbiol Lett 203:55–61. doi: 10.1111/j.1574-6968.2001.tb10820.x. [DOI] [PubMed] [Google Scholar]
- 25.Naylor EJ, Bakstad D, Biffen M, Thong B, Calverley P, Scott S, Hart CA, Moots RJ, Edwards SW. 2007. Haemophilus influenzae induces neutrophil necrosis: a role in chronic obstructive pulmonary disease? Am J Respir Cell Mol Biol 37:135–143. doi: 10.1165/rcmb.2006-0375OC. [DOI] [PubMed] [Google Scholar]
- 26.Pericone CD, Overweg K, Hermans PW, Weiser JN. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun 68:3990–3997. doi: 10.1128/IAI.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anjem A, Imlay JA. 2012. Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem 287:15544–15556. doi: 10.1074/jbc.M111.330365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittmann C, Kim HM, John G, Heinzle E. 2003. Characterization and application of an optical sensor for quantification of dissolved O2 in shake-flasks. Biotechnol Lett 25:377–380. doi: 10.1023/A:1022402212537. [DOI] [PubMed] [Google Scholar]
- 30.Schiefelbein S, Frohlich A, John GT, Beutler F, Wittmann C, Becker J. 2013. Oxygen supply in disposable shake-flasks: prediction of oxygen transfer rate, oxygen saturation and maximum cell concentration during aerobic growth. Biotechnol Lett 35:1223–1230. doi: 10.1007/s10529-013-1203-9. [DOI] [PubMed] [Google Scholar]
- 31.Vergauwen B, Herbert M, Van Beeumen JJ. 2006. Hydrogen peroxide scavenging is not a virulence determinant in the pathogenesis of Haemophilus influenzae type b strain Eagan. BMC Microbiol 6:3. doi: 10.1186/1471-2180-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


