Abstract
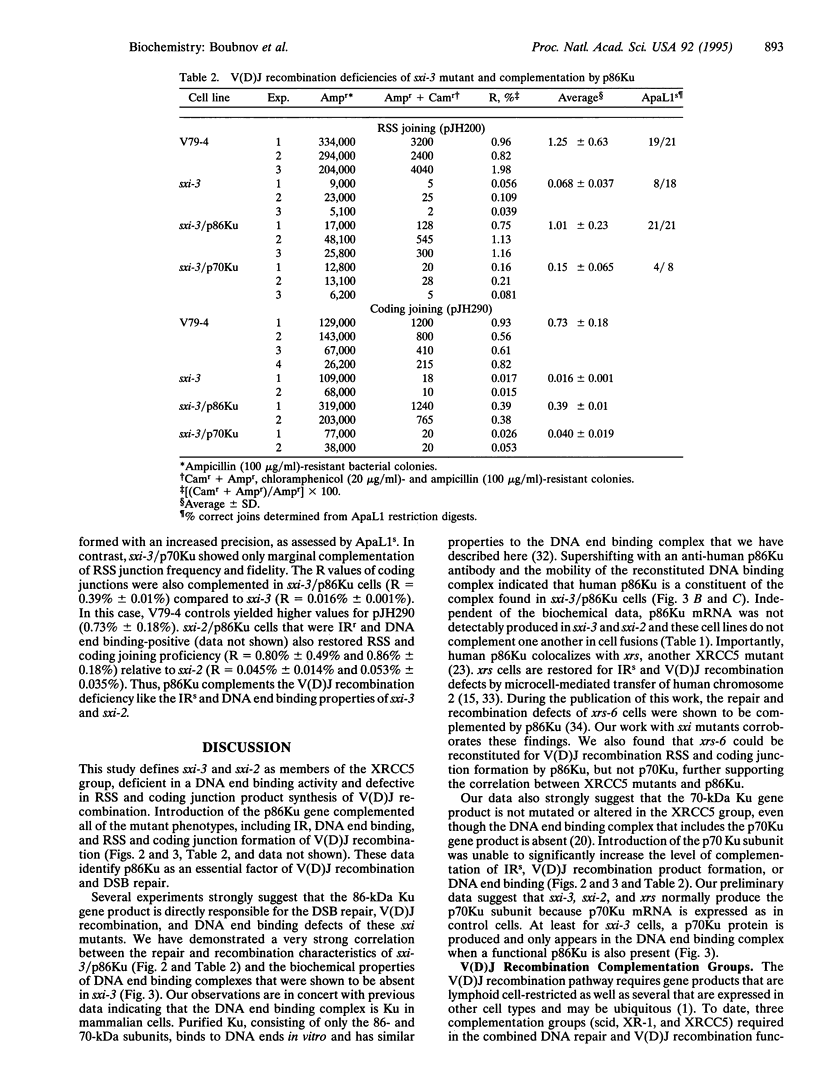
Two ionizing radiation-sensitive (IRs) and DNA double-strand break (DSB) mutants, sxi-3 and sxi-2, were shown to be severely deficient in a DNA end binding activity, similar to a previously described activity of the Ku autoantigen, correlating with the xrs (XRCC5) mutations. Cell fusions with xrs-6, another IRs, DSB repair-deficient cell line, defined these sxi mutants in the XRCC5 group. sxi-3 cells have low expression levels of the p86Ku mRNA. Introduction of the Ku p86 gene, but not the p70 Ku gene, complemented the IRs, DNA end binding, and variable (diversity) joining [V(D)J] recombination signal and coding junction deficiencies of sxi-3. Thus, the p86 Ku gene product is essential for DSB repair and V(D)J recombination.
Full text
PDF
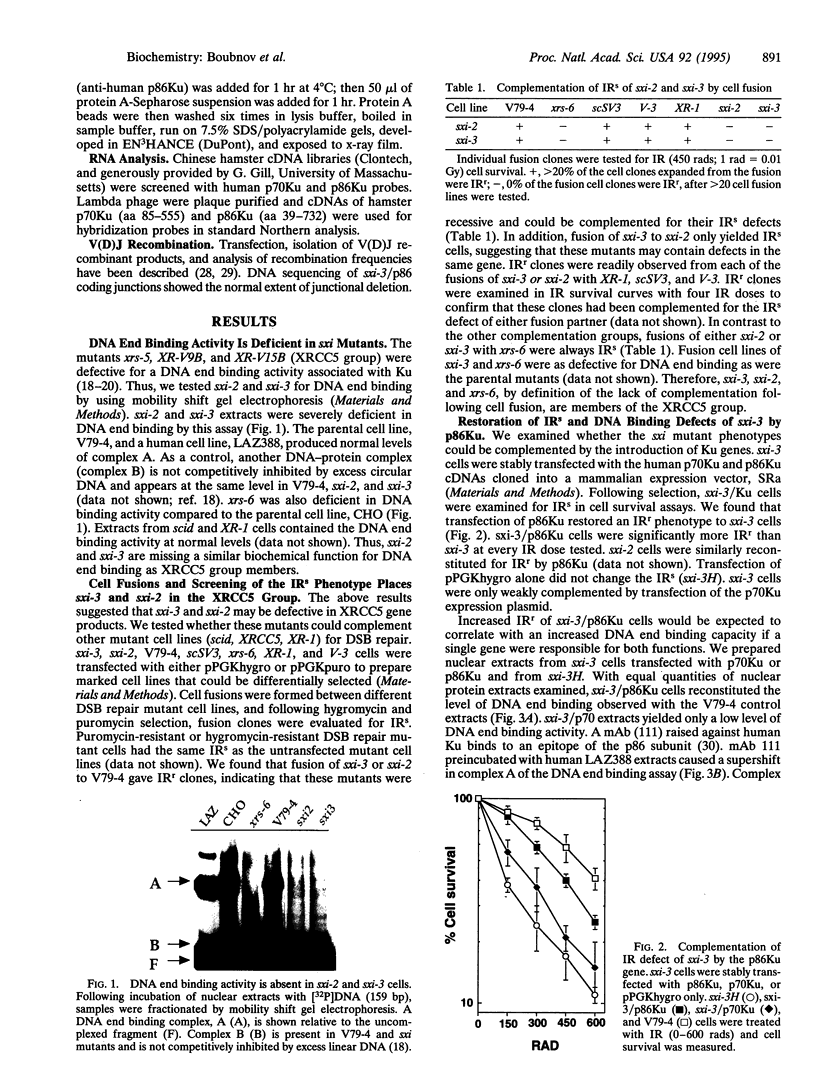

Images in this article
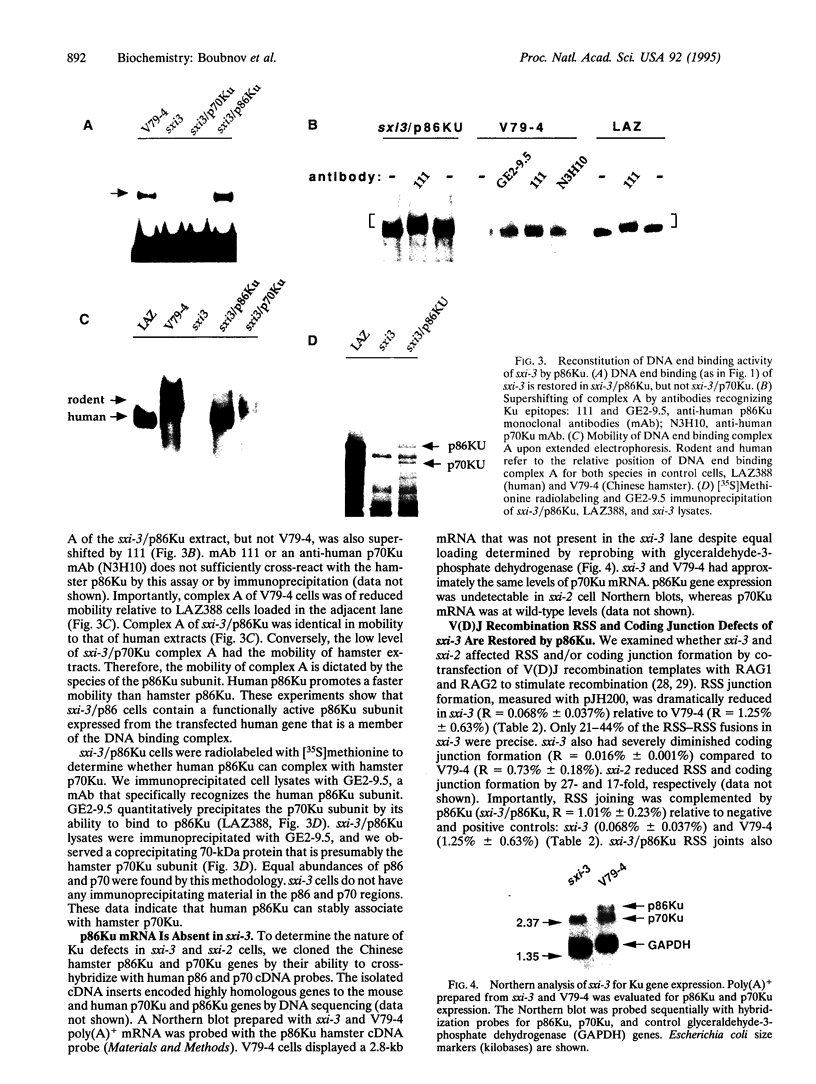
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P. R., Griffith A. J., Craft J., Hardin J. A. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem. 1993 Apr 5;268(10):7594–7601. [PubMed] [Google Scholar]
- Boubnov N. V., Wills Z. P., Weaver D. T. V(D)J recombination coding junction formation without DNA homology: processing of coding termini. Mol Cell Biol. 1993 Nov;13(11):6957–6968. doi: 10.1128/mcb.13.11.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q. Q., Plet A., Imbert J., Lafage-Pochitaloff M., Cerdan C., Blanchard J. M. Chromosomal location and expression of the genes coding for Ku p70 and p80 in human cell lines and normal tissues. Cytogenet Cell Genet. 1994;65(4):221–227. doi: 10.1159/000133635. [DOI] [PubMed] [Google Scholar]
- Chou C. H., Wang J., Knuth M. W., Reeves W. H. Role of a major autoepitope in forming the DNA binding site of the p70 (Ku) antigen. J Exp Med. 1992 Jun 1;175(6):1677–1684. doi: 10.1084/jem.175.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir A., Peterson S. R., Knuth M. W., Lu H., Dynan W. S. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. The nucleotide sequence of a mouse cDNA encoding the 80 kDa subunit of the Ku (p70/p80) autoantigen. Nucleic Acids Res. 1992 Jul 25;20(14):3784–3784. doi: 10.1093/nar/20.14.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Getts R. C., Stamato T. D. Absence of a Ku-like DNA end binding activity in the xrs double-strand DNA repair-deficient mutant. J Biol Chem. 1994 Jun 10;269(23):15981–15984. [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Griffith A. J., Blier P. R., Mimori T., Hardin J. A. Ku polypeptides synthesized in vitro assemble into complexes which recognize ends of double-stranded DNA. J Biol Chem. 1992 Jan 5;267(1):331–338. [PubMed] [Google Scholar]
- Hafezparast M., Kaur G. P., Zdzienicka M., Athwal R. S., Lehmann A. R., Jeggo P. A. Subchromosomal localization of a gene (XRCC5) involved in double strand break repair to the region 2q34-36. Somat Cell Mol Genet. 1993 Sep;19(5):413–421. doi: 10.1007/BF01233246. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson E. A., Schatz D. G., Weaver D. T. The scid gene encodes a trans-acting factor that mediates the rejoining event of Ig gene rearrangement. Genes Dev. 1988 Jul;2(7):817–829. doi: 10.1101/gad.2.7.817. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Schlissel M. S., Weaver D. T. Wild-type V(D)J recombination in scid pre-B cells. Mol Cell Biol. 1990 Oct;10(10):5397–5407. doi: 10.1128/mcb.10.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Hafezparast M., Thompson A. F., Broughton B. C., Kaur G. P., Zdzienicka M. Z., Athwal R. S. Localization of a DNA repair gene (XRCC5) involved in double-strand-break rejoining to human chromosome 2. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6423–6427. doi: 10.1073/pnas.89.14.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Kemp L. M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res. 1983 Dec;112(6):313–327. doi: 10.1016/0167-8817(83)90026-3. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A. Studies on mammalian mutants defective in rejoining double-strand breaks in DNA. Mutat Res. 1990 Jul;239(1):1–16. doi: 10.1016/0165-1110(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Paillard S., Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991 Oct 25;19(20):5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola F., Zdzienicka M. Z., Lieber M. R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993 Jun;13(6):3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell W. K., Chu G. A DNA end-binding factor involved in double-strand break repair and V(D)J recombination. Mol Cell Biol. 1994 Jul;14(7):4741–4748. doi: 10.1128/mcb.14.7.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell W. K., Chu G. Involvement of the Ku autoantigen in the cellular response to DNA double-strand breaks. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7623–7627. doi: 10.1073/pnas.91.16.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W. H. Use of monoclonal antibodies for the characterization of novel DNA-binding proteins recognized by human autoimmune sera. J Exp Med. 1985 Jan 1;161(1):18–39. doi: 10.1084/jem.161.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Chang X. B., Wilson J. H. Comparison of filler DNA at immune, nonimmune, and oncogenic rearrangements suggests multiple mechanisms of formation. Mol Cell Biol. 1989 Jul;9(7):3049–3057. doi: 10.1128/mcb.9.7.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Menetski J. P., Nakajima P. B., Bosma M. J., Gellert M. V(D)J recombination: broken DNA molecules with covalently sealed (hairpin) coding ends in scid mouse thymocytes. Cell. 1992 Sep 18;70(6):983–991. doi: 10.1016/0092-8674(92)90248-b. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Nakajima P. B., Menetski J. P., Bosma M. J., Gellert M. V(D)J recombination in mouse thymocytes: double-strand breaks near T cell receptor delta rearrangement signals. Cell. 1992 Apr 3;69(1):41–53. doi: 10.1016/0092-8674(92)90117-u. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Zhu C., Gellert M. Characterization of broken DNA molecules associated with V(D)J recombination. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10788–10792. doi: 10.1073/pnas.90.22.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M., Constantinescu A., Morrow T., Baxter M., Peng A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5'-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 1993 Dec;7(12B):2520–2532. doi: 10.1101/gad.7.12b.2520. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Weinstein R., Giaccia A., Mackenzie L. Isolation of cell cycle-dependent gamma ray-sensitive Chinese hamster ovary cell. Somatic Cell Genet. 1983 Mar;9(2):165–173. doi: 10.1007/BF01543175. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Cheng H. L., Varghese A. J., Whitmore G., Alt F. W. A DNA repair defect in Chinese hamster ovary cells affects V(D)J recombination similarly to the murine scid mutation. J Biol Chem. 1994 Mar 11;269(10):7439–7442. [PubMed] [Google Scholar]
- Taccioli G. E., Gottlieb T. M., Blunt T., Priestley A., Demengeot J., Mizuta R., Lehmann A. R., Alt F. W., Jackson S. P., Jeggo P. A. Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science. 1994 Sep 2;265(5177):1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993 Apr 9;260(5105):207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. T. V(D)J recombination and double-strand break repair. Adv Immunol. 1995;58:29–85. doi: 10.1016/s0065-2776(08)60619-7. [DOI] [PubMed] [Google Scholar]
- Zhang W. W., Yaneva M. On the mechanisms of Ku protein binding to DNA. Biochem Biophys Res Commun. 1992 Jul 15;186(1):574–579. doi: 10.1016/s0006-291x(05)80847-2. [DOI] [PubMed] [Google Scholar]
- de Vries E., van Driel W., Bergsma W. G., Arnberg A. C., van der Vliet P. C. HeLa nuclear protein recognizing DNA termini and translocating on DNA forming a regular DNA-multimeric protein complex. J Mol Biol. 1989 Jul 5;208(1):65–78. doi: 10.1016/0022-2836(89)90088-0. [DOI] [PubMed] [Google Scholar]