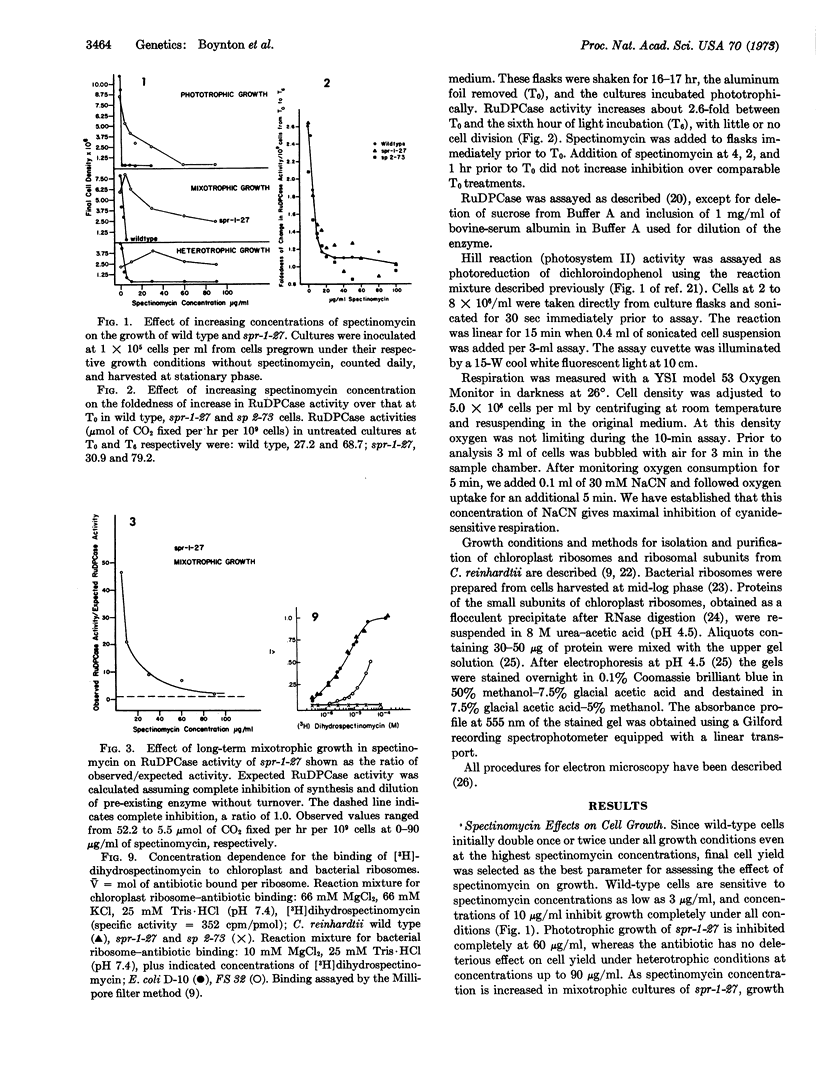
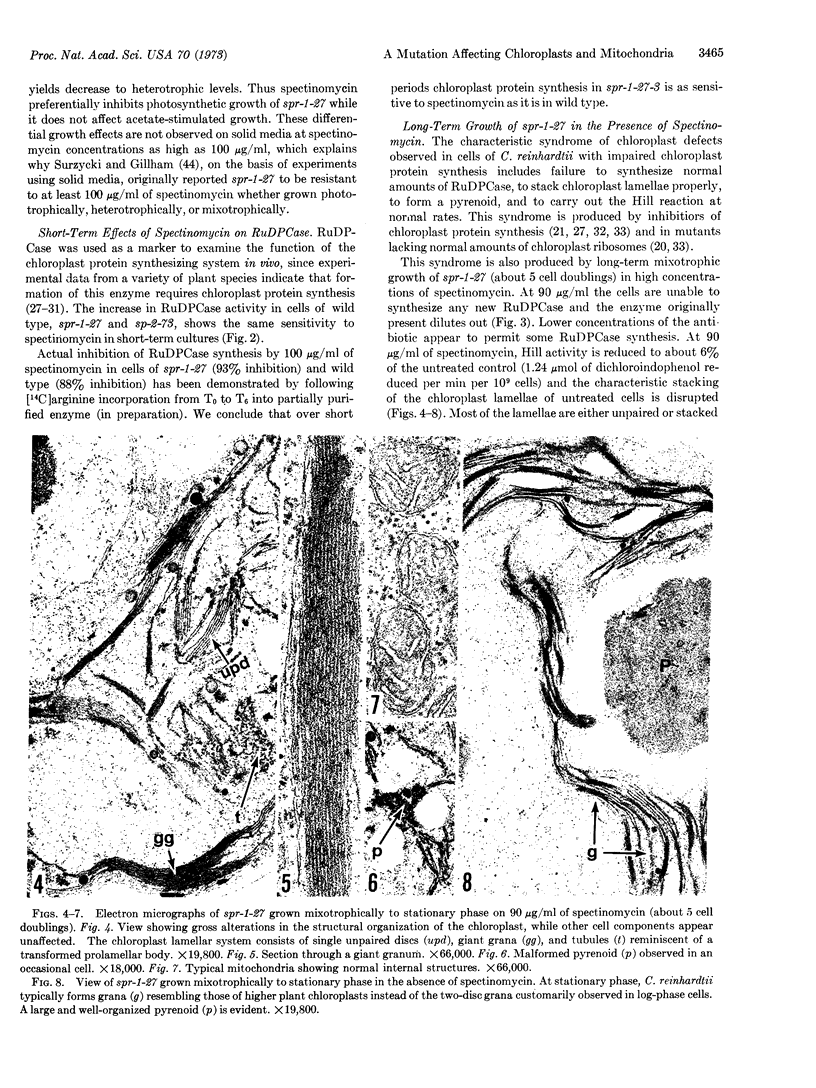
Abstract
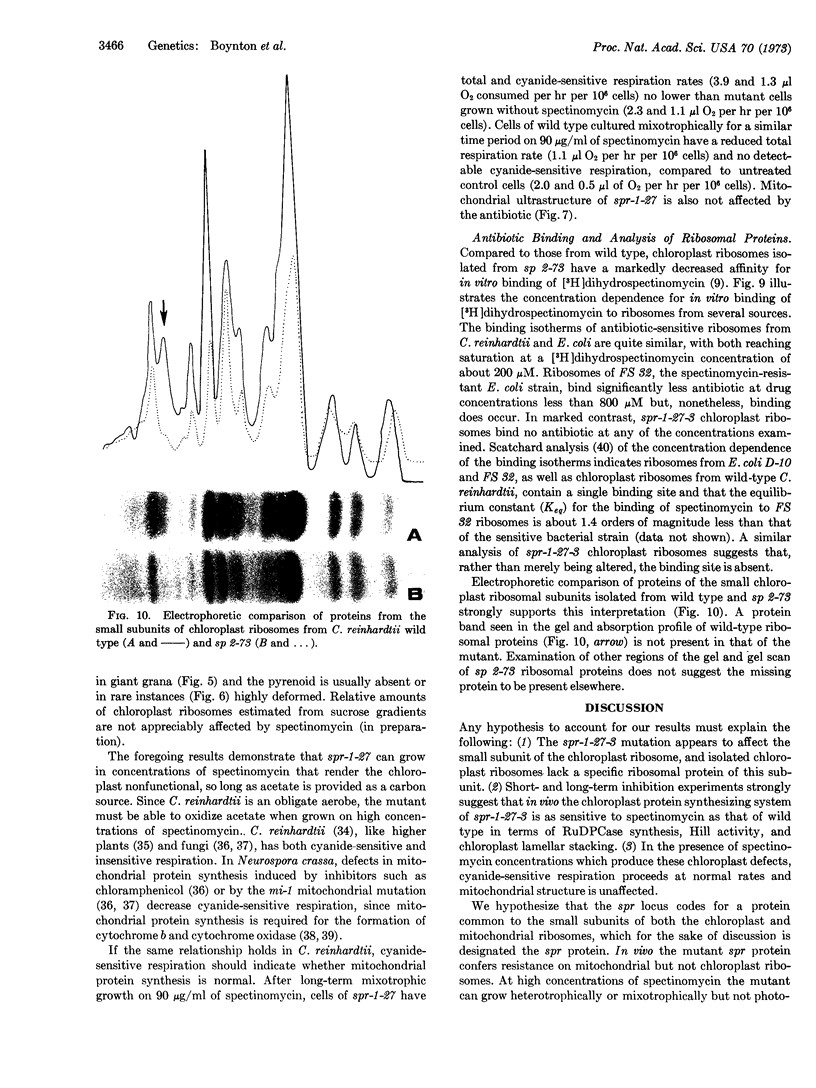
Chloroplast ribosomes isolated from a spectinomycin-resistant mutant (spr-1-27-3) of Chlamydomonas reinhardtii that displays non-Mendelian inheritance fail to bind labeled antibiotic, in contrast to ribosomes from wild-type cells. In vitro resistance of this mutant appears to result from the absence of a specific protein in the small subunit of the chloroplast ribosome. However, chloroplast protein synthesis in the mutant and wild type shows identical sensitivity to spectinomycin in short-term in vivo experiments where ribulosediphosphate carboxylase serves as the marker. Long-term experiments demonstrate that the mutant can grow in the presence of spectinomycin only when acetate is supplied as a carbon source. Mitochondrial structure and function of the mutant are not affected by the antibiotic, whereas chloroplast structure and function are. Apparently, the mitochondrion, rather than the chloroplast, of this mutant is resistant to spectinomycin in vivo. We hypothesize that the gene product of the spr locus is a protein common to both chloroplast and mitochondrial ribosomes. The mutant gene product, in vivo, confers resistance on mitochondrial, but not chloroplast, ribosomes. We suppose that the mutant spr protein loosely attaches to chloroplast ribosomes in vivo so that the antibiotic is bound and blocks protein synthesis, but it dissociates during isolation, resulting in loss of the binding site.
Keywords: antibiotic resistance, protein synthesis, spectinomycin, Chlamydomonas reinhardtii, ribulosediphosphate carboxylase
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. J., Moll B., Surzycki S. J., Levine R. P. Genetic transcription and translation specifying chloroplast components in Chlamydomonas reinhardi. Biochemistry. 1971 Feb 16;10(4):692–701. doi: 10.1021/bi00780a022. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Bourque D. P., Boynton J. E., Gillham N. W. Studies on the structure and cellular location of various ribosome and ribosomal RNA species in the green alga Chlamydomonas reinhardi. J Cell Sci. 1971 Jan;8(1):153–183. doi: 10.1242/jcs.8.1.153. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Burkholder B. Mutations Altering Chloroplast Ribosome Phenotype in Chlamydomonas, II. A New Mendelian Mutation. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1505–1512. doi: 10.1073/pnas.67.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Chabot J. F. Chloroplast ribosome deficient mutants in the green alga Chlamydomonas reinhardi and the question of chloroplast ribosome function. J Cell Sci. 1972 Mar;10(2):267–305. doi: 10.1242/jcs.10.2.267. [DOI] [PubMed] [Google Scholar]
- Criddle R. S., Dau B., Kleinkopf G. E., Huffaker R. C. Differential synthesis of ribulosediphosphate carboxylase subunits. Biochem Biophys Res Commun. 1970 Nov 9;41(3):621–627. doi: 10.1016/0006-291x(70)90058-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Nomura M. The genetics of bacterial ribosomes. Annu Rev Genet. 1972;6:203–234. doi: 10.1146/annurev.ge.06.120172.001223. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Staehelin T. Electrophoretic analysis of proteins from normal and cesium chloride-treated Escherichia coli ribosomes. J Mol Biol. 1967 Mar 14;24(2):149–155. doi: 10.1016/0022-2836(67)90323-3. [DOI] [PubMed] [Google Scholar]
- Gillham N. W., Fifer W. Recombination of nonchromosomal mutations: a 3-point cross in the green alga Chlamydomonas reinhardi. Science. 1968 Nov 8;162(3854):683–684. doi: 10.1126/science.162.3854.683. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W. The effects of inhibitors of RNA and protein synthesis on chloroplast structure and function in wild-type Chlamydomonas reinhardi. J Cell Biol. 1971 Jul;50(1):35–49. doi: 10.1083/jcb.50.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommersand M. H., Thimann K. V. Terminal Respiration of Vegetative Cells and Zygospores in Chlamydomonas reinhardi. Plant Physiol. 1965 Nov;40(6):1220–1227. doi: 10.1104/pp.40.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K. A major polypeptide of chloroplast membranes of Chlamydomonas reinhardi. Evidence for synthesis in the cytoplasm as a soluble component. J Cell Biol. 1972 Jan;52(1):84–96. doi: 10.1083/jcb.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Kawashima N. Non-synchronous incorporation of C14O2 into amino acids of the two subunits of fraction I protein. Biochem Biophys Res Commun. 1970 Jan 6;38(1):119–124. doi: 10.1016/0006-291x(70)91092-2. [DOI] [PubMed] [Google Scholar]
- Kurland C. G. Structure and function of the bacterial ribosome. Annu Rev Biochem. 1972;41(10):377–408. doi: 10.1146/annurev.bi.41.070172.002113. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. W., Jones R. F. Induction of Mendelian and non-Mendelian streptomycin resistant mutants during the synchronous cell cycle of Chlamydomonas reinhardtii. Mol Gen Genet. 1973 Mar 1;121(2):99–108. doi: 10.1007/BF00277524. [DOI] [PubMed] [Google Scholar]
- Mets L. J., Bogorad L. Mendelian and uniparental alterations in erythromycin binding by plastid ribosomes. Science. 1971 Nov 12;174(4010):707–709. doi: 10.1126/science.174.4010.707. [DOI] [PubMed] [Google Scholar]
- Mets L., Bogorad L. Altered chlorplast ribosomal proteins associated with erythromycin-resistant mutants in two genetic systems of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3779–3783. doi: 10.1073/pnas.69.12.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Sager R., Lane D. Molecular basis of maternal inheritance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2410–2413. doi: 10.1073/pnas.69.9.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. A genetic map of non-Mandelian genes in Chlamydomonas. Proc Natl Acad Sci U S A. 1970 Mar;65(3):593–600. doi: 10.1073/pnas.65.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlanger G., Sager R., Ramanis Z. Mutation of a cytoplasmic gene in Chlamydomonas alters chlorplast ribosome function. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3551–3555. doi: 10.1073/pnas.69.12.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Weiss H., Jackl G. Inhibition of the assembly of cytochrome oxidase in Neurospora crassa by chloramphenicol. Eur J Biochem. 1972 Nov 7;30(3):413–417. doi: 10.1111/j.1432-1033.1972.tb02112.x. [DOI] [PubMed] [Google Scholar]
- Sparling P. F., Modolell J., Takeda Y., Davis B. D. Ribosomes from Escherichia coli merodiplods heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968 Nov 14;37(3):407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzycki S. J., Gillham N. W. Organelle mutations and their expression in Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1301–1306. doi: 10.1073/pnas.68.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surzycki S. J., Goodenough U. W., Levine R. P., Armstrong J. J. Nuclear and chloroplast control of chloroplast structure and function in Chlamydomonas reinhardi. Symp Soc Exp Biol. 1970;24:13–37. [PubMed] [Google Scholar]
- Togasaki R. K., Levine R. P. Chloroplast structure and function in ac-20, a mutant strain of Chlamydomonas reinhardi. I. CO2 fixation and ribulose-1,5-diphosphate carboxylase synthesis. J Cell Biol. 1970 Mar;44(3):531–539. doi: 10.1083/jcb.44.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss H. Cytochrome b in Neurospora crassa mitochondria. A membrane protein containing subunits of cytoplasmic and mitochondrial origin. Eur J Biochem. 1972 Nov 7;30(3):469–478. doi: 10.1111/j.1432-1033.1972.tb02119.x. [DOI] [PubMed] [Google Scholar]
- von Jagow G., Weiss H., Klingenberg M. Comparison of the respiratory chain of Neurospora crassa wild type and the mi-mutants mi-1 and mi-3. Eur J Biochem. 1973 Feb 15;33(1):140–157. doi: 10.1111/j.1432-1033.1973.tb02665.x. [DOI] [PubMed] [Google Scholar]