SHORT SUMMARY (précis)
Sentence recognition by participants with and without hearing loss was measured in quiet and in babble noise while monitoring two autonomic nervous system measures: heart-rate variability and skin conductance. Heart-rate variability decreased under difficult listening conditions for participants with hearing loss, but not for participants with normal hearing. Skin conductance noise reactivity was greater for those with hearing loss, than for those with normal hearing, but did not vary with the signal-to-noise ratio. Subjective ratings of workload/stress obtained after each listening condition were similar for the two participant groups.
The negative impact of hearing loss on spoken language perception is well documented (Studebaker & Hochberg 1993). There are also indirect effects of hearing loss, such as psychological stress and increased cognitive load that have received more recent attention. For example, hearing loss has been linked to higher levels of chronic stress and job burnout (Hasson et al. 2011). In addition, hearing loss has been associated with anxiety (Mohlman 2009) and with somatic distress symptoms (Eriksson-Mangold & Carlsson 1991).
There is also considerable evidence that hearing loss elicits increased cognitive effort during spoken language processing. For example, persons with hearing loss have poorer performance on word recall during recognition tasks than do their normal-hearing peers, even when speech recognition accuracy is good (McCoy et al. 2005; Rabbitt 1991; Tun et al. 2009). Similar findings have been reported for narrative recall by Piquado et al. (2012). In this study, participants with hearing loss were able to recall fewer narrative elements than those with good hearing. However, the effects of hearing loss disappeared when participants were allowed to self-pace (i.e. slow down) the presentation, implicating processing speed as a mitigating factor. Slower responses for persons with hearing loss have also been reported during a sentence comprehension task even though comprehension accuracy was similar to that of normal-hearing listeners (Tun et al. 2010). These findings support the view that sensory degradation imposed by the hearing loss may lead to a greater consumption of processing resources to maintain speech perception performance (Lunner et al. 2009; Pichora-Fuller 2003).
Cognitive effort may be viewed as one aspect of task-related workload. The term “workload” refers to the actual or perceived energy expended to perform a task. Depending on the task, this may include cognitive, physical, and/or emotional aspects of workload. A task whose demands exceed available resources may result in reduced performance and psychological distress, and may lead to mental and/or physical exhaustion. The cognitive and emotional aspects of communication-related workload are particularly relevant to individuals with hearing loss.
Cognitive effort and psychological distress are often accompanied by physiological reactions. In response to acute stress, the body undergoes a series of neural, endocrine and immunological changes. A prominent aspect of this stress response involves changes in the autonomic nervous system. These may include activation of the sympathetic branch (fight or flight response) and suppression of parasympathetic branch (the branch responsible for rest / recovery) (Lovallo 2005). The autonomic nervous system’s response to stress includes changes in cardiovascular, electrodermal, and pupillary activity. Activation to, and recovery from acute stress is a normal adaptive process. But chronic stress can lead to wear and tear on the body (”allostatic load”) that has long term negative effects on health (McEwen 2008). Given that persons with hearing loss experience greater cognitive load and higher levels of long-term stress than do persons with normal hearing, those with hearing loss may be especially vulnerable to allostatic load.
Chronic psychological stress and the increased cognitive workload imposed by hearing loss during communication may interact synergistically. Psychological stress is known to negatively impact information processing, affecting both working memory and attention (Ashcraft & Kirk 2001; Braunstein-Bercovitz et al. 2001), two processes associated with spoken language perception (Akeroyd 2008). Conversely, increased cognitive load and deficits in speech understanding may lead to psychological distress, particularly for individuals sensitive to the potential for negative evaluation by another person (Dickerson & Kemeny 2004). Thus, stress may arise during communication from increased cognitive load, increased psychological threat, or from an interaction between these factors.
The stressful effects of hearing loss can be assessed using measures of autonomic nervous system activity. For example, hearing-loss effects on psychophysiological reactivity have been reported during speech recognition tasks using the pupil response, considered to be an index of effort (Kramer et al. 1997; Zekveld et al. 2011).
In the current study, electrodermal measures and heart rate variability were used to evaluate the effects of hearing loss and noise level on physiological reactivity during sentence recognition tasks. The electrodermal measure of interest, skin conductance, reflects the amount of moisture excreted from the eccrine glands. Skin conductance is considered to be an index of sympathetic nervous system activity. Specifically, an increase in skin conductance indicates increased sympathetic nervous system arousal (Dawson et al. 2007). Skin conductance has been shown to increase with increased task difficulty for mental and linguistic tasks (Clements & Turpin 1995; Gendolla & Krusken 2001; Kahneman et al. 1969) and in response to psychological distress (Elfering & Grebner 2011; Hofmann et al. 2006). Skin conductance is also sensitive to emotional responses induced by imagery or films (Kreibig 2010).
Task-related changes in skin conductance have also been reported for auditory discrimination paradigms (Greene et al. 1979), and auditory selective-attention tasks (Dawson et al. 1989; Dawson & Schell 1982; Filion et al. 1991). More recently, significant increases in skin conductance level have been reported in normal-hearing listeners with increasing auditory task demand, even when performance was at ceiling level (Mackersie & Cones 2011).
Heart-rate variability (HRV) refers to the moment-to-moment fluctuations in heart rate. These fluctuations reflect activity from both the sympathetic and parasympathetic branches of the autonomic nervous system. Inverse relations between task difficulty and HRV have been observed for both visual attention (Duschek et al. 2009; Moses et al. 2007) and behavioral inhibition tasks (Mathewson et al. 2010). In the auditory modality, there is some evidence of reduced HRV for normal-hearing listeners as noise levels increase during a speech-recognition task (Dorman et al. 2012).
Many investigators use spectral analyses of the varying heart rate to evaluate task-related changes. A fast-Fourier transform is used to convert the interbeat intervals into spectral bands that reflect different rates of change in the heart rate. The low-frequency band (0.04 to 0.015 Hz) reflects activity from both the sympathetic and parasympathetic nervous systems, whereas the high-frequency band (0.15 to 0.4 Hz) reflects mainly parasympathetic activity (Malik 1996). The high-frequency component has been linked to both emotion regulation (Appelhans & Luecken 2006; Geisler et al. 2010) and to executive function (Thayer et al. 2009). Specifically, lower emotional regulation and poorer executive function are associated with lower resting HRV.
Ratings scales have also been used to assess perceived workload during different tasks. The National Aeronautics and Space Administration Task Load Index (NASA-TLX) is one such measure (Hart & Staveland 1988). This widely-used index of perceived workload encompasses several domains, including physical (physical demand), cognitive (effort, mental demand), and emotional (stress/frustration) reactions to a task.
The purpose of the present study was to determine the effects of hearing loss and noise on the high-frequency component of heart-rate variability (HF-HRV), skin conductance, and subjective indices of task load / stress. Based on evidence that persons with hearing loss experience greater cognitive load than do those with normal hearing, we hypothesized that both subjective and psychophysiological reactivity to noise would be greater during speech recognition tasks for participants with hearing loss. Specifically, we predicted higher skin conductance levels (sympathetic nervous system arousal) and lower heart-rate variability (parasympathetic withdrawal) for participants with hearing loss during speech recognition tasks. We also expected higher ratings of effort, mental demand, and stress, and lower ratings of performance for participants with hearing loss. In addition, both psychophysiological and subjective measures of stress were expected to increase as the signal-to-noise ratio worsened, regardless of hearing status.
METHODS
Participants
Eighteen adults with hearing loss and 15 adults with clinically-normal hearing were recruited. The sample size was based on power analyses conducted on preliminary heart-rate variability and skin conductance data. These analyses indicated that a minimum sample size of 14 participants per group was needed to detect group differences with a power goal of .80 and an alpha level of .05.
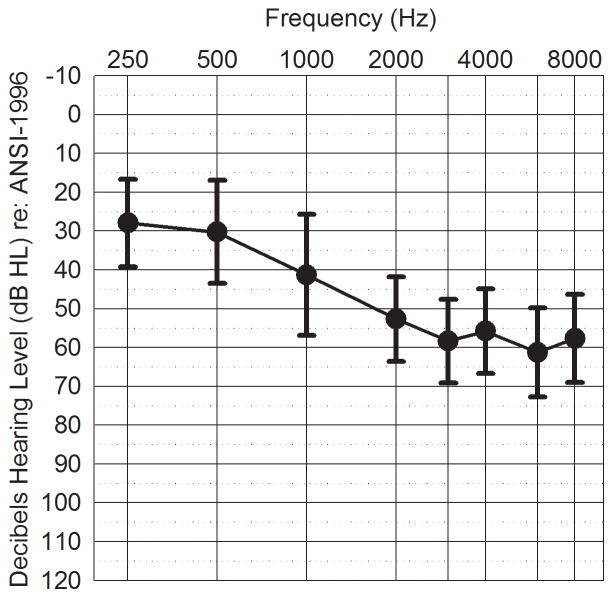
Participants with hearing-loss ranged in age from ages 22 to79 years (mean 58) and consisted of nine men and nine women with sensorineural hearing loss. All but one participant in this group were hearing aid users. Participants had air-bone gaps of 10 dB or less at octave frequencies between 250 and 4000 Hz. Their mean pure-tone thresholds are shown in Figure 1. The test ear was randomly selected for participants with symmetrical hearing. For participants with asymmetrical hearing loss, the ear with the three-frequency average (500, 1000500, 2000 Hz) closet to 45 dB HL and the smallest hearing loss-slope between 500–2000 Hz was selected as these characteristics were most similar to those of the participants with symmetrical hearing loss.
Figure 1.
Mean pure-tone thresholds for participants with hearing loss. Error bars indicate ± 1 SD.
Participants with clinically-normal hearing ranged in age from 24 to 78 years (mean 45 ) and consisted five men and 10 women. Clinically-normal hearing was defined as bilateral pure-tone sensitivity no poorer than 25 dB HL at octave frequencies between 250 and 4000 Hz with no self-reported hearing loss. Eligibility was determined by screening participants at 25 dB HL at octave frequencies between 250 and 8000 Hz. Two participants had mild age-appropriate hearing loss at 6000 and 8000 Hz. For simplicity, we will refer to this group as the “normal-hearing” group throughout the rest of the paper. The test ears were randomly selected in this group.
Stimulus Materials and Measures
Speech and noise stimuli
The speech stimuli consisted of digitized female recordings of the City University of New York (CUNY) Sentences sampled at 22.05 kHz with 16-bit resolution (Boothroyd et al. 1988; Hanin et al. 1988). Each sentence is a statement, command or a question consisting of three to 14-words.
Competing babble was created by mixing five unique CUNY sentences spoken by the same talker into a single channel; two of the sentences were reversed to reduce the intelligibility of the mixture. Different samples of babble were mixed with each sentence to avoid participant adaptation due to frozen (unchanging) noise (Felty et al. 2009). A total of 27 lists were assembled; each list consisted of 12 sentences with approximately 102 words per list. The onset of the target sentence was delayed by 0.750 seconds relative to the onset of the noise.
Subjective measures of task load / stress
Subjective ratings of effort, mental demand, stress, and perceived performance were obtained for each condition using a modified version of the NASA-TLX questionnaire. Two of the original dimensions, physical and temporal demand, were omitted because they are not relevant to simple speech repetition tasks (Mackersie & Cones 2011). A question related to motivation was added to evaluate its influence on the psychophysiological measures; low motivation has been shown to reduce psychophysiological responses (Capa et al. 2008). Motivation was expected to be relatively constant across conditions.
The modified NASA-TLX was administered on a 17″ Planar PT 1700 MU touch-screen computer monitor using FileMaker Pro software. Participants were asked to indicate their answers using a ten-point Likert scale, divided into half-point increments. The NASA TLX questions and their associated categorical anchors appear in Table 1.
Table 1.
Questions and categorical anchors for the modified NASA-TLX questionnaire.
| Category | Question | Categorical Anchor (at 0/ at 10) |
|---|---|---|
| Mental Demand | How mentally demanding was this task? | Not Demanding/ Very Demanding |
| Perceived Performance | How successful were you in accomplishing what you were asked to do? | Failure/Perfect |
| Effort | How hard did you have to work to achieve you level of performance? | Not Very Hard/ Very Hard |
| Stress/ Frustration | How insecure, discouraged, irritated, stressed, and annoyed did you feel during this task? | Very Low/Very High |
| Motivation | How motivated did you feel to work hard and do well on the task? | Not Motivated At All/ Very Motivated |
Psychophysiological measurement
Skin conductance, heart rate, and respiration rate were monitored using a Nexus-10 physiologic monitoring system. The battery-operated recording apparatus was wirelessly connected to a laptop via Bluetooth and remotely controlled via the Biotrace+ software (Mind Media 2008). The Nexus-10 was worn on a shoulder strap and rested at the participant’s side. Recordings were made simultaneously through three separate channels, each with independent 24 bit A–D converters and DC coupled amplifiers.
Skin conductance was measured on the participant’s non-dominant hand using two silver/silver chloride (Ag/AgCl) electrodes attached to cables (NX-GSR1A). The electrodes were placed on opposite sides of the palm over the thenar and hypothenar muscles. During testing, participants were asked to keep their hand facing palm-up to reduce hand movement artifacts. Recordings were made at 32 samples per second in microsiemens.
Electrocardiographic recordings (ECG) were obtained using three Ag/AgCl electrodes attached to snap-on cables (NX-EXG2B). The ground electrode was placed below the left clavicle, the cathode (−) below the right clavicle and the anode (+) was placed on the third rib, in line with the ground. Recordings were made at 256 samples per second in microvolts. ECG recordings and interbeat intervals extracted by the BioTrace+ software were manually inspected for artifacts and premature beats. Segments containing artifacts were excluded and the missing intervals were interpolated from the adjacent interbeat interval values (< 1% interpolated). Spectral analysis of heart-rate variability was calculated by the Biotrace+ software using fast Fourier transforms parsed into frequency bands, given in units of milliseconds squared (ms2). The high-frequency heart-rate variability (HF-HRV) (0.15–0.4 Hz) was used as an index of parasympathetic activity.
HF-HRV can be influenced by respiration, especially when the respiration rate is low (Schipke et al. 1999; Song & Lehrer 2003). Therefore, respiration was monitored to assess the possible influence of respiration rate on HRV measures. Respiration was measured using an elasticized respiration belt (NX-RSP1A) worn over the clothes at the level of the diaphragm. Recordings were sampled at 32 per second and displayed as breaths per minute.
Procedures
Institutional review board approval was obtained from the San Diego State University Human Research Protections Program before any data were collected. Testing was carried out over two sessions, lasting 90 to 120 minutes each, in a quiet office with an ambient noise level of 35 dBA. Participants were positioned at a 45- degree angle from the tester for the duration of testing. Stimuli were played from a desktop computer, routed into the speech channels of the Grason-Stadler GSI 61 audiometer, and delivered monaurally to an EAR-Tone 3A insert earphone. The non-test ear was occluded with an inactive insert earphone.
Most Comfortable Loudness procedure
To avoid noise aversion effects, the noise level was set at the most-comfortable level (MCL). The MCL was individually determined for participants with hearing loss using categorical loudness ratings. Two- to three-second samples of the female five-talker speech babble were played in ascending 3-dB steps starting at approximately 10 dB above the participant’s pure-tone-average threshold. After each presentation, participants were asked to rate the stimulus on a scale of two to six, (2 = “very soft”; 3 = “soft”; 4 = “comfortable, but slightly soft”; 5 = “comfortable”; 6 = “comfortable, but slightly loud”). Each ascending series was terminated when the participants rated the sound as “6 = comfortable, but slightly loud”. The intensity level rated as a five (“comfortable”) in at least two trials was selected as the MCL. If a range of intensities was rated as “comfortable”, the midpoint of the range was used.
The noise level was fixed at 65 dB SPL for normal-hearing participants. The MCL was confirmed by playing two to three three-second samples of noise at 65 dB SPL and asking listeners to rate the loudness. All participants in the normal-hearing group rated this level as “comfortable”.
Adaptive threshold testing
Adaptive speech-in-noise testing was completed during the first test session. The purpose of this test was to equalize performance across the two participant groups in an attempt to regulate task engagement. Conditions that are too difficult can lead to lower task engagement and lower psychophysiological reactivity (Gendolla 1999; Gendolla & Krusken 2001; Pecchinenda & Smith 1996). After MCL was established, adaptive thresholds were determined using a simple up-down procedure. The sentences were delivered in lists of 20 sentences using custom-written software. The intensity level of the noise remained fixed at the listener’s MCL, while the level of the target sentences was adjusted based on the participant’s response. If the participant correctly identified all of the words in the sentence (substitution of articles “a/the” was considered acceptable) then the intensity of the target was decreased and a new sentence was presented. If words were missed or incorrectly identified, the level of the target was increased. The initial step size was 4 dB for sentences one through four and was reduced to 2 dB for sentences five through twenty.
Adaptive thresholds were based on a minimum of two threshold estimates. Each estimate was based on the mean of the reversals (i.e. transition from ascending to descending or descending to ascending levels) at the minimum step size. If the two adaptive thresholds estimates differed by more than 3 dB, then an additional estimate was obtained. Mean adaptive thresholds were calculated as the average of the two threshold estimates with the smallest difference between them.
For the materials used in this study, this simple up-down procedure based on the entire sentence repeated correctly yielded a words-in-sentences recognition score of approximately 80%. Mean SNR thresholds from the adaptive procedure were used as the 0 dB relative signal-to-noise ratio (relSNR) for the sentence-recognition tests completed during session 2.
Sentence-recognition testing
During the second session, one list was administered in quiet and at relSNRs of −6, −3, 0, and +3 dB using AudioCasper software V4.0 (Boothroyd 2010). For the quiet condition, speech was presented at the listener’s MCL. For noise conditions, the noise level remained fixed at the listener’s MCL and the speech level was adjusted for each relSNR condition. For example, for a 0 dB relSNR, the speech was presented at the level corresponding to the listener’s adaptive threshold; for a +3 relSNR, the speech was presented 3 dB above the level corresponding to the listener’s adaptive threshold.
Order effects were controlled using a randomized Latin-Square design. Lists previously used for adaptive testing were not used for the sentence recognition task. One practice list was administered at 0 dB relSNR before data collection began. Recognition scores for each condition were based on the percentage of correctly repeated words for the entire 12-sentence list.
Psychophysiological and NASA-TLX procedures
A ten-minute baseline recording was obtained before the speech recognition tasks began and a short post-break baseline (three minutes) was obtained after a break at the mid-point of testing. During the baseline recordings, participants were instructed to sit silently and watch the ‘Blue Planet’ DVD on a 17″ monitor. This DVD was chosen for its low emotional content. The sound was muted and closed-captions were enabled. Baseline recordings were included to allow participants to acclimatize to the experimental setting and to allow the resting physiological responses to stabilize.
Physiological measurements were recorded for the duration of each speech recognition task in Session 2. Immediately following each condition, the NASA TLX was administered. Physiological recordings were obtained during 90 second rest (recovery) periods immediately following each administration of the NASA TLX. During rest, participants were asked to sit quietly and relax. The choice of the recovery interval was based on the use of a 60 second interval for the standard stress protocol (mental tests) included with the manufacturer’s software. The duration was increased to 90 seconds to allow additional time for recovery; this inter-condition recovery interval is similar to rest periods used by other investigators (Fournier et al. 1999).
Statistical analysis
Repeated-measures analyses of variance were used to evaluate the differences among conditions and listener groups. Newman-Keuls post-hoc tests were used to examine pair-wise contrasts when significant main effects or interactions were detected. A p-level of less than .05 was considered significant.
RESULTS
Speech recognition
The mean noise levels corresponding to MCL were 72 dB SPL (SD 6.6) for the participants with hearing loss. The mean adaptive thresholds for the participants with and without hearing loss were 5.2 (SD: 0.65) and 3.5 dB (SD: 0.71), respectively. This difference approached statistical significance (t(31) = −1.85, p = .07).
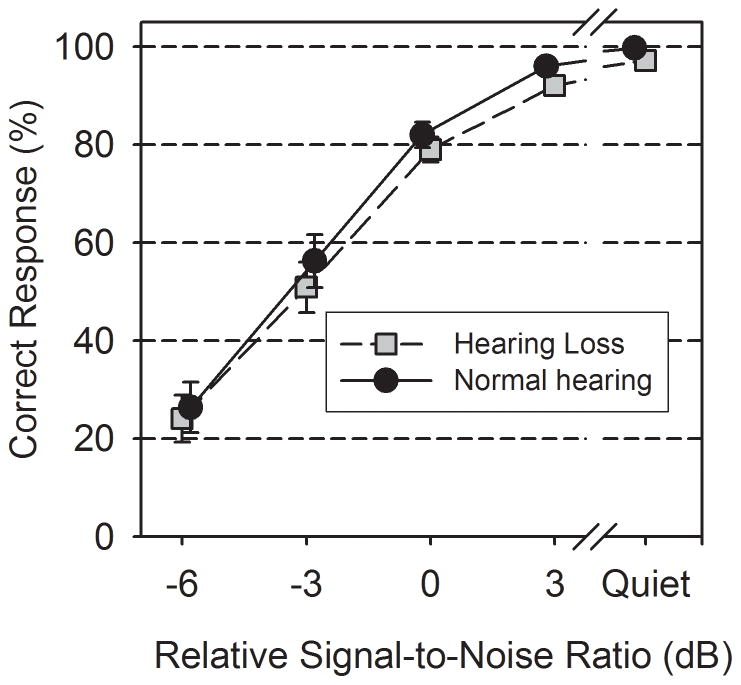
Mean recognition scores for the two groups are shown in Figure 2. Recall that the relSNRs are referenced to adaptive threshold. As expected mean recognition scores were similar for the two groups and performance decreased with a decrease in relSNR. The mean speech presentation level in quiet was 75 dB SPL (SD 6.4) for the participants with hearing loss.
Figure 2.
Mean recognition scores (words in sentences) of the normal-hearing and hearing loss groups under each listening condition. The relSNR refers to the signal-to-noise ratio referenced to the adaptive threshold (see text). Error bars indicate ± 1 se.
Rationalized arcsine transforms of the sentence recognition scores were used for statistical analysis (Studebaker 1985). A repeated-measures ANOVA using relSNR as a within-subjects factor and hearing status as a between-subjects factor indicated a main effect of relSNR (F(3,93) = 211.13, p< 0.001, ηp2 = 0.87). There was no significant main effect of group (F(1,31) = 1.43, p= 0.24, ηp2 = 0.04) or interaction (F(3,93) = 0.90, p= 0.45, ηp2 = 0.03). These findings confirmed that the mean performance was similar for the two groups.
Psychophysiological Measures
Skin conductance reactivity
Normalized skin conductance reactivity z-scores have been suggested in recent electrodermal measurement guidelines (Boucsein et al. 2012) to reduce the effects of individual differences in raw skin conductance values (Ben-Shakhar 1985). Skin conductance data were normalized for each participant by calculating reactivity indexes (z-scores) referenced to the quiet condition. Statistical features of the BioTrace software were used to extract the means and standard deviations for each condition. Individual z-scores were calculated from the change in skin conductance (noise minus quiet) divided by the standard deviation of the measure. Individual z-scores of greater than 1.96 were considered to show statistically significant reactivity at the .05 level. Raw skin conductance means (μS) for each condition can be found in Supplemental Digital Content 1.
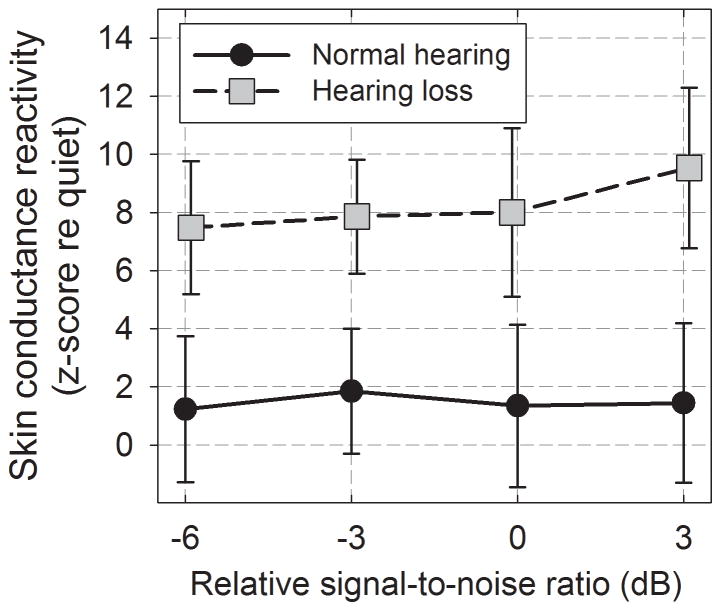
The mean reactivity score averaged across all noise conditions was 1.47 and 8.64 for the participants with normal-hearing and hearing loss, respectively. Figure 3 shows skin conductance reactivity as a function of relSNR. It can be seen that mean reactivity is greater for the group with hearing loss, but there is little change in reactivity across relSNRs for either group. A repeated-measures ANOVA indicated a significant main effect of hearing status (F(1,31) = 5.20, p = 0.03, ηp2 =.14 ), but no effect of relSNR (F(3,93) = 1.30, p = 0.28, ηp2 = .04 or interaction between SNR and hearing status (F(3,93) = 0.05, p = 0.98, ηp2 < .01). Because skin conductance reactivity typically decreases with age (Barontini et al. 1997; Gavazzeni et al. 2008), the analysis was also run using age as a covariate. After accounting for the effect of age, the main effect of hearing status remained significant (F(1,30) = 4.80, p = .03, ηp2 =.14 ).
Figure 3.
Mean skin conductance reactivity indexes (re quiet) for each relative SNR. Error bars indicate ± 1 se.
Heart-rate variability
Heart-rate variability data for one participant with hearing loss was excluded because of ECG artifacts. The remaining data were (natural) log transformed to normalize the positive skew in the data distribution. Mean transformed baseline HF-HRV was 6.42 (SD 1.62) and 5.88 (SD 1.66) for the normal-hearing and hearing-loss groups, respectively.
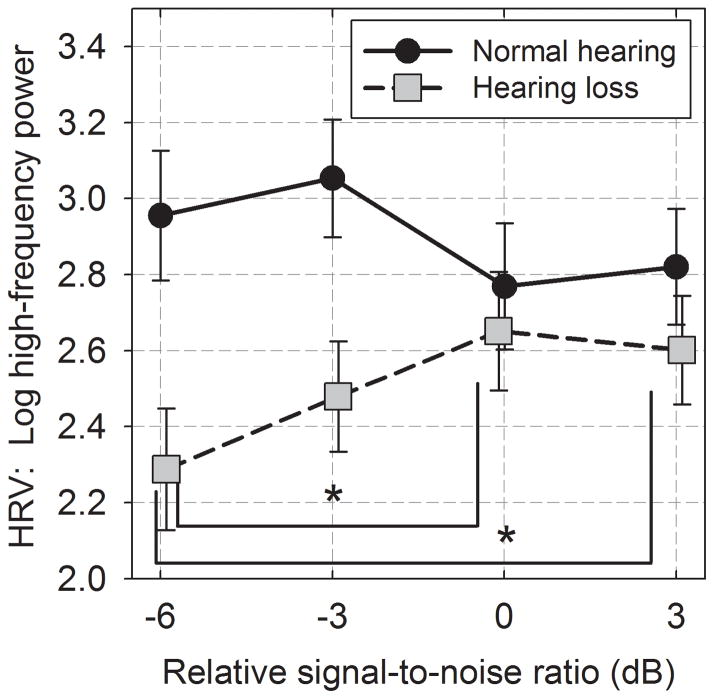
Means for HF-HRV are shown as a function of relSNR in Figure 4 for the two groups of participants. On average, HF-HRV of those with hearing loss decreased systematically as the relSNR decreased. Reduction in HF-HRV was not observed for participants with normal hearing. A repeated-measures ANOVA of the HF-HRV data indicated a significant interaction between hearing status and relSNR (F(3,90) = 3.62, p = 0.016, ηp2 = .11). Because HRV typically decreases with age (Schwartz et al. 1991; Wood et al. 2002), the analysis was also run using age as a covariate to assess possible age-related influences on the results. Age did not contribute significantly to the variance across relSNR (F(3,87)= 0.79 p= 0.50, ηp2 = .03). After accounting for age effects, the interaction between hearing status and relSNR remained significant (F(3,87) = 3.40, p = 0.021, ηp2 = .11). Post-hoc tests indicated that the mean HF-HRV of participants with hearing loss was significantly lower than HF-HRV of those with normal hearing at the two lowest SNRs, but not at the two highest SNRs. For the group with hearing loss, the mean for −6 dB relSNR was significantly lower than the means for 0 and +3 dB relSNR, as denoted by the asterisks in Figure 4.
Figure 4.
Mean high-frequency heart-rate variability as a function of relative SNR. The asterisk indicate significant differences for participants with hearing loss. Error bars indicate ± 1 se.
HF-HRV and Respiration rate
Respiration rate was analyzed to determine if changes in respiration rate reflected the systematic changes observed for the HF-HRV. The mean respiration rates were 17.4 (SD 0.73) and 18.5 (SD 0.67) breaths per min for the normal-hearing and hearing-loss groups, respectively. Across relSNR, breathing rates varied by less than one breath per minute for both groups. A repeated-measures ANOVA confirmed that that there was no significant effect of hearing, relSNR, or interaction between these variables. In addition, a second ANOVA of the HF-HRV data was completed using the change in respiration rate between −6 and 0 dB relSNR as a covariate. After accounting for the effects of respiration, the HF-HRV interaction between hearing status and relSNR remained significant (F(3,87) = 3.95, p = 0.01, ηp2 = .12).
In summary, HF-HRV decreased under the most difficult listening conditions for participants with hearing loss, but not for those with normal hearing. Mean HF-HRV for the hearing-loss group was significantly lower than that of those with normal hearing, but only at the two lowest relSNRs. There was no evidence that age or respiration influenced these findings.
Individual differences in reactivity
Across all SNRs, a significantly larger percentage of listeners with hearing loss had a significant increase in skin conductance with the introduction of noise (61% hearing loss vs 33% normal hearing, χ2 = 5.07, p = 0.02). Excluding the highest relSNR noise level (performance near ceiling level), the percentage of participants who were significantly reactive to noise increased to 72% of those with hearing loss, but remained at 33% for the participants with normal hearing. Nevertheless, approximately one-third of the participants with hearing loss did not show significant changes.
To examine whether the degree of hearing loss or other factors may explain this variability, further analyses were completed. Pearson Product-Moment correlations between skin conductance reactivity and two independent variables, age and pure-tone average, were not significant (age: r(17) = .31, p = .21); PTA: r(17)=.28, p= 0.25). In addition, there was no significant difference between reactivity for men and women participants (t (16)= .72, p = 0.48). Similar patterns were observed for HF-HRV reactivity. Specifically, there was no significant association between HRV reactivity (between −6 and 0 dB relSNR) and either age (r(17) = .15, p= 0.56) or pure-tone average (r(17) = .08, p=0.74).
Baseline HF heart-rate variability and skin conductance reactivity
A correlation analysis between baseline HRV and skin-conductance reactivity was completed. This analysis was motivated by the established link between baseline HF-HRV (i.e. at rest) and stress susceptibility (Thayer et al. 2012). There was a significant negative correlation between baseline HF-HRV (i.e. at rest) and skin conductance reactivity (r (31) = −.41, p = 0.021). This indicates a tendency for individuals with lower baseline parasympathetic activity (lower HF-HRV) to have higher sympathetic nervous system reactivity to noise (increased skin conductance).
Subjective Measures
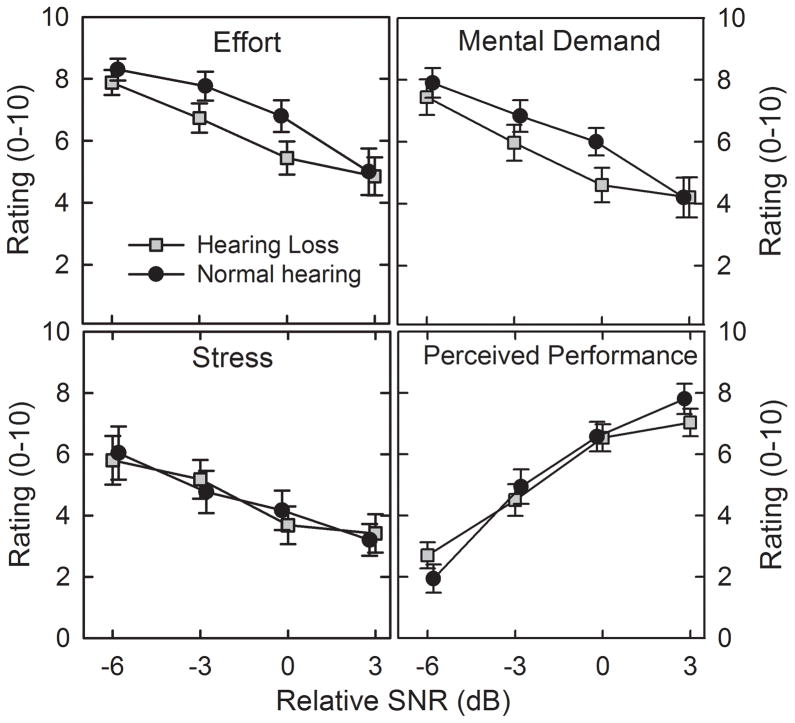
The mean ratings of the effort, mental demand (cognitive domains), stress (emotional), and perceived performance are shown as a function of relSNR in Figure 5. Mean ratings of effort, mental demand, and stress increased as the listening conditions became more difficult, whereas mean ratings of perceived performance decreased. Unlike the psychophysiological measures, however, the ratings were similar for two participant groups.
Figure 5.
Mean ratings of effort, mental demand, stress and perceived performance for each relative SNR. Error bars indicate ± 1 se.
Repeated-measures analyses of variance confirmed a significant main effect of relSNR for each domain (Effort: F(3,93) = 31.88, p < 0.001, ηp2= 0.51; Mental demand: F(3,93) = 31.85, p < 0.001, ηp2= 0.51; Stress / frustration: F(3,93) = 17.88, p < 0.001, ηp2= 0.37; Perceived performance: (F(3,93) = 60.26, p< 0.001, ηp2 = 0.66). There was, however, no significant main effect of group or interaction involving group for any of the four domains. For ratings of effort, mental demand, and perceived performance, the post-hoc tests indicated that means for all noise conditions were significantly different from one another. For the stress ratings, all noise conditions were significantly different from one another except for the two highest relSNRs which did not differ significantly.
The motivation ratings were evaluated separately to determine the possible influence on the results. Motivation ratings changed by 0.17 and 0.03 points across the range of relSNRs for the normal-hearing and hearing loss groups, respectively. A repeated-measures ANOVA confirmed that there were no significant main effects of relSNR or group and no interaction between these variables. In addition, Spearman rank-order correlations between motivation ratings and the psychophysiological variables were not significant. These findings suggest that motivation did not exert a significant influence on the psychophysiological results.
Relations between psychophysiological measures and ratings
Spearman’s rank order correlation coefficients were used to determine the relations between the psychophysiological measures and the subjective ratings of effort, mental demand, stress / frustration, and perceived performance. Dependent variables were the skin conductance z-scores and the HF-HRV corresponding to the adaptive threshold (0 dB SNR). As shown in Table 2, there were no significant correlations between the psychophysiological measures and the four task-load domains.
Table 2.
Spearman rho coefficients for psychophysiological measures vs. performance and NASA TLX ratings.
| z- SC | HRV | |
|---|---|---|
| Effort | 0.08 | −0.15 |
| MD | −0.17 | −0.03 |
| Stress | −0.05 | −0.32 |
| Perc Perf | −0.07 | −0.03 |
| %C | −0.29 | 0.25 |
|
| ||
| * p < .05 | ||
Note: MD =mental demand, Perc perf =perceived performance, %C = recognition score at 0 dB relSNR; z-SC = skin conductance z-score.
DISCUSSION
Effects of hearing loss
Psychophysiological measures
The findings generally support the hypothesis that listeners with hearing loss experience greater communication-related psychophysiological stress than their normal-hearing peers, when performance is equated. This conclusion is supported by evidence that skin conductance reactivity to babble noise was higher across the range of SNRs for participants with hearing loss. In addition, high-frequency HRV was lower (consistent with greater task load/stress) for participants with hearing loss at the lower relSNRs, but not the higher relSNRs. These findings complement results of behavioral studies indicating poorer recall and / or slower processing speed during speech recognition by participants with hearing loss, even when speech understanding was good (McCoy et al. 2005; Piquado et al. 2012; Rabbitt 1991; Tun et al. 2009). Together, these studies suggest that listeners with hearing loss show greater stress-related autonomic nervous system activation, and use more cognitive resources to maintain recognition performance than do listeners with normal hearing.
Recall that all but one participant with hearing loss were hearing aid users. Thus, the quality of the speech delivered to the insert earphone was different from the quality they were accustomed to hearing through their hearing aids. Moreover, even though participants were tested at comfortable listening levels and performance was equalized for the two groups, it is likely that high-frequency audibility was somewhat poorer for the group with hearing loss given that frequency shaping (i.e. high-frequency emphasis) was not used. In addition, participants with hearing loss typically have poorer supra-threshold auditory function, such as frequency and temporal resolution, adding further degradation of the neural patterns reaching the brain. Although this degradation was not substantial enough to affect recognition performance once the signal was amplified to a comfortable level, it may have resulted in a greater expenditure of cognitive resources and increased physiological reactivity to noise.
The mean age of the hearing loss group was somewhat higher than the normal-hearing group; however, the psychophysiological effects of hearing loss remained significant after accounting for the effects of age in the analyses. This suggests that group differences in psychophysiological reactivity cannot be explained on the basis of the mean age difference between the two groups.
One must also consider the possibility that the participants with hearing loss entered the experiment with higher levels of stress than did the normal-hearing listeners and were therefore more reactive to experimental manipulations. The groups did not differ significantly in baseline skin conductance levels, however. Moreover, group differences in skin conductance reactivity, while present across SNRs, were not observed in quiet. That is, compared to baseline (no task), reactivity to the speech recognition task in quiet was similar for the two groups of listeners (z-SC =4.36 (NH) vs. 3.00 (HL); t(31) = −.97, p = .34). Thus, an explanation of higher baseline stress or generalized hyper-reactivity for participants with hearing loss seems unlikely.
Subjective ratings of effort / stress
The hypothesis that participants with hearing loss would show higher subjective ratings of task load / stress than those with normal hearing is not supported. In contrast to the psychophysiological markers, participants with hearing loss did not rate the tasks as more demanding or stressful than participants with normal hearing. Rather, listeners with hearing loss seemed unaware of any extra physiological demand placed on the autonomic nervous system during the sentence recognition task in noise.
It is important to note that the majority of participants with hearing loss had considerable experience with speech recognition tasks during the course of clinical treatment and during prior participation in research studies. In contrast, most of the participants with normal hearing were naive to speech-recognition-in-noise tasks and had limited experience with clinical and/or research-based hearing assessments. Therefore, it is possible that greater familiarity with the recognition tasks resulted in lower ratings of effort and stress for those with hearing loss than would have been observed using novel tasks.
The present findings are similar to those of other investigators who have shown group differences between task-related psychophysiological changes, but similar ratings of task difficulty / effort across groups (Contrada et al. 1984; Marfil et al. 1999; Zekveld et al. 2011). Contrada et al. (1984), for example reported greater changes in heart rate during a math task for those with Type A (stress prone) personalities compared to Type B personalities even though there were no significant differences between task difficulty ratings for the groups. Similarly, although Zekveld et al. (2011) reported effects of hearing loss on pupil responses, there were no significant difference between ratings of effort for those with and without hearing loss.
The absence of associations between psychophysiological measures and subjective responses in the present study is consistent with other reports in the literature, including the recent work on pupillometry and hearing loss (Miyake 2001; Zekveld et al. 2011). These results suggest that participants are unaware of the increased resources being activated during the task. Therefore, the psychophysiological consequences of communication demand may be missed if one relies solely on self-report measures of task load/stress.
Effects of signal-to-noise ratios
The hypothesis that both the psychophysiological and subjective measures of stress would increase as the signal-to-noise conditions became more difficult was partially supported. The HF-HRV and skin conductance measures were differentially sensitive to changes in the relSNR. For the group with hearing loss, once activated at a relatively low SNR, skin conductance was uniformly elevated across different relSNRs relative to the quiet condition; however, HF-HRV decreased monotonically at and below relSNRs corresponding to performance levels below 60%. The patterns of sympathetic and parasympathetic activity is known to vary with tasks and individuals (Berntson et al. 1993; Ottaviani et al. 2008). Thus, it is not know whether the patterns observed in this study are specific to the tasks and/or conditions used. The skin conductance findings are qualitatively consistent with previous findings showing that skin conductance increased with a small change in task difficulty, but plateaued when the task became more difficult. (Mackersie & Cones, 2011). Based on these limited data, skin conductance may be more sensitive to task difficulty at the upper end of the performance function or lower end of the effort range. However, further work is needed to confirm this suggestion.
Baseline HRV and skin conductance reactivity
Although skin conductance reactivity was not related to subjective measures, higher skin conductance reactivity was associated with lower baseline HF-HRV. Given that lower HF-HRV has been linked to lower executive function (Thayer et al. 2009), those with lower baseline HF-HRV might be expected to react more strongly to noise to the extent that the speech recognition process engages aspects of executive function (Akeroyd 2008).
This association is consistent with other studies that have shown relations between baseline HF-HRV and other stress indices (Johnsen et al. 2012; Thayer et al. 2009). For example, Johnsen et al. (2012) found that participants with low baseline HF-HRV demonstrated increased cortisol secretion (a stress hormone), after completing cognitive tasks. The authors interpreted this as evidence that low baseline HF-HRV is related to increased susceptibility to stress.
Limitations and Future Work
It was not possible to confirm the source of the differences between reactivity for the participants with and without hearing loss because skin conductance and HRV measures are sensitive to both task load and to emotional reactivity. Therefore, increased psychophysiological reactivity to noise may arise as a result of increased listening effort, increased emotional stress from the testing process, or a combination of these factors. Further work is needed to explore the influence of individual and group differences in emotion regulation and coping strategies on psychophysiological reactivity during speech recognition and communication tasks.
The noise used in this study was relatively simple five-talker babble presented through an earphone to one ear. Further work is needed to examine the effects of different noise types. It is possible that more acoustically complex noise that includes spatial separation would result in increased skin conductance sensitivity to changes in SNR. In addition, although the design controlled for group difference in recognition performance and noise loudness, it did not account for potential differences in the acceptability of the signal-to-noise ratios used. The influence of acceptable noise levels on psychophysiological reactivity merits further study.
The current study employed a conventional speech repetition task. While this procedure is convenient and is widely used in both hearing research and clinical applications, the task does not entail the same level of cognitive or social demand as do true communication interactions. As such, this task may underestimate the level of real-world communication stress. There is a need to develop ecologically valid communication paradigms that entail more realistic cognitive and social demands.
Although it is difficult to directly generalize the laboratory findings of the current study to the problems experienced by persons with hearing loss in everyday life, it is possible that the increased prevalence of chronic stress and fatigue reported in population studies of persons with hearing loss may be linked to communication-related stress. This communication-related stress may lead to chronic over-activation of the physiological stress response under unfavorable listening situations. Further work is needed to examine the associations among these factors.
Conclusions
On average, participants with hearing loss showed greater sympathetic nervous system arousal (evidenced by increased skin conductance reactivity) and greater parasympathetic suppression (evidenced by decreased HF-HRV) during speech recognition in babble noise than did participants with normal hearing. The effects of hearing loss on HF-HRV were observed only for the two lowest relSNRs, whereas the effects were constant across relSNRs for skin conductance. Heart-rate variability was more sensitive to changes in signal-to-noise ratio than skin conductance for the speech repetition task and five-talker babble competition used in this study.
In contrast to the psychophysiological findings, there were no effects of hearing loss on subjective ratings. For both groups, perceived effort, mental demand, stress increased with increased task difficulty and perceived performance decreased with task difficulty. Findings suggest that compared to persons with normal-hearing listeners, persons with hearing loss may have increased autonomic nervous-system stress responses in noisy environments, even when recognition accuracy and self-reported task load/stress are similar.
Supplementary Material
Supplemental Digital Content 1. Table of raw skin conductance means (in μSiemens) for each group and condition. Standard deviations are indicated in parentheses.
References
- Akeroyd MA. Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol. 2008;47(Suppl 2):S53–S71. doi: 10.1080/14992020802301142. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. 2006;10:229–240. [Google Scholar]
- Ashcraft MH, Kirk EP. The relationships among working memory, math anxiety, and performance. J Exp Psychol Gen. 2001;130:224–237. doi: 10.1037//0096-3445.130.2.224. [DOI] [PubMed] [Google Scholar]
- Barontini M, Lazzari JO, Levin G, Armando I, Basso SJ. Age-related changes in sympathetic activity: biochemical measurements and target organ responses. Arch Gerontol Geriatr. 1997;25:175–186. doi: 10.1016/s0167-4943(97)00008-3. [DOI] [PubMed] [Google Scholar]
- Ben-Shakhar G. Standardization within individuals: A simple method to neutralize individual differences in psychophysiological responsivity. Psychophysiology. 1985;22:292–299. doi: 10.1111/j.1469-8986.1985.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. [Review] Psychol Bull. 1993;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- Boothroyd A. AudioCasper (v4.0): Computer-assisted Auditory Speech Perception training. San Diego: 2010. [Google Scholar]
- Boothroyd A, Hnath-Chisolm T, Hanin L, Kishon-Rabin L. Voice fundamental frequency as an auditory supplement to the speechreading of sentences. Ear Hear. 1988;9:306–312. doi: 10.1097/00003446-198812000-00006. [DOI] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth W, Dawson ME, Filion DL. Publication recommendations for electrodermal measurements. Psychophysiology. 2012;49:1017–1034. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- Braunstein-Bercovitz H, Dimentman-Ashkenazi I, Lubow RE. Stress affects the selection or relevant from irrelevant stimuli. Emotion. 2001;1:182–192. doi: 10.1037/1528-3542.1.2.182. [DOI] [PubMed] [Google Scholar]
- Capa RL, Audiffren M, Ragot S. The effects of achievement motivation, task difficulty, and goal difficulty on physiological, behavioral, and subjective effort. Psychophysiology. 2008;45:859–868. doi: 10.1111/j.1469-8986.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- Clements K, Turpin G. Effects of feedback and task difficulty on electrodermal activity and heart rate: An examination of Fowles’ three arousal model. Journal of Psychophysiology. 1995;9:231–242. [Google Scholar]
- Contrada RJ, Wright RA, Glass DC. Task difficulty, Type A behavior pattern, and cardiovascular response. Psychophysiology. 1984;21:638–646. doi: 10.1111/j.1469-8986.1984.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Filion DL, Schell AM. Is elicitation of the autonomic orienting response associated with allocation of processing resources. Psychophysiology. 1989;26:560–572. doi: 10.1111/j.1469-8986.1989.tb00710.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM. Electrodermal responses to attended and nonattended significant stimuli during dichotic listening. J Exp Psychol Hum Percept Perform. 1982;8:315–324. doi: 10.1037//0096-1523.8.2.315. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal response. In: Cacioppo JT, Tassinary LG, editors. The Handbook of Psychophysiology. Cambridge: Cambridge University Press; 2007. pp. 159–181. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Spahr A, Gifford RH, Cook S, Zhang T, Loiselle L, Schramm D. Current research with cochlear omplants at Arizona State University. J Am Acad Audiol. 2012;23:385–395. doi: 10.3766/jaaa.23.6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek S, Muckenthaler M, Werner N, Reyes del Paso GA. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol. 2009;81:110–117. doi: 10.1016/j.biopsycho.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Elfering A, Grebner S. Ambulatory assessment of skin conductivity during first thesis presentation: Lower self-confidence predicts prolonged stress response. Appl Psychophysiol Biofeedback. 2011;36:93–99. doi: 10.1007/s10484-011-9152-3. [DOI] [PubMed] [Google Scholar]
- Eriksson-Mangold M, Carlsson SG. Psychological and somatic distress in relation to perceived hearing disability, hearing handicap, and hearing measurements. J Psychosom Res. 1991;35:729–740. doi: 10.1016/0022-3999(91)90124-7. [DOI] [PubMed] [Google Scholar]
- Felty RA, Buchwald A, Pisoni DB. Adaptation to frozen babble in spoken word recognition. J Acoust Soc Am. 2009;125:EL93–97. doi: 10.1121/1.3073733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM, Hazlett EA. The relationship between skin conductance orienting and the allocation of processing resources. Psychophysiology. 1991;28:410–424. doi: 10.1111/j.1469-8986.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Fournier LR, Wilson GF, Swain CR. Electrophysiological, behavioral, and subjective indexes of workload when performing multiple tasks: Manipulations of task difficulty and training. Int J Psychophysiol. 1999;31:129–145. doi: 10.1016/s0167-8760(98)00049-x. [DOI] [PubMed] [Google Scholar]
- Gavazzeni J, Wiens S, Fischer H. Age effects to negative arousal differ for self-report and electrodermal activity. Psychophysiology. 2008;45:148–151. doi: 10.1111/j.1469-8986.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- Geisler FCM, Vennewald N, Kubiak T, Weber H. The impact of heart rate variability on subjective well-being is mediated by emotion regulation. Pers Individ Dif. 2010;49:723–728. [Google Scholar]
- Gendolla GH. Self-relevance of performance, task difficulty, and task engagement assessed as cardiovascular response. Motiv Emot. 1999;23:45–66. [Google Scholar]
- Gendolla GH, Krusken J. The joint impact of mood state and task difficulty on cardiovascular and electrodermal reactivity in active coping. Psychophysiology. 2001;38:548–556. doi: 10.1017/s0048577201000622. [DOI] [PubMed] [Google Scholar]
- Greene RL, Kille SE, Hogan FA. Electrodermal measures of attention and effort to stimulus onset and offset for intramodal and intermodal tasks. Percept Mot Skills. 1979;48:411–418. doi: 10.2466/pms.1979.48.2.411. [DOI] [PubMed] [Google Scholar]
- Hanin L, Boothroyd A, Hnath-Chisolm T. Tactile presentation of voice fundamental frequency as an aid to the speechreading of sentences. Ear Hear. 1988;9:335–341. doi: 10.1097/00003446-198812000-00010. [DOI] [PubMed] [Google Scholar]
- Hart S, Staveland L. Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. In: Hancock P, Meshkat N, editors. Human Mental Workload. North-Holland Elsevier Science; 1988. pp. 139–183. [Google Scholar]
- Hasson D, Theorell T, Wallén MB, Leineweber C, Canlon B. Stress and prevalence of hearing problems in the Swedish working population. BMC Public Health. 2011;11:130–130. doi: 10.1186/1471-2458-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Moscovitch DA, Kim HJ. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiol. 2006;61:134–142. doi: 10.1016/j.ijpsycho.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Johnsen BR, Hansen AL, Murison R, Eid J, Thayer JF. Heart rate variability and cortisol responses during attentional and working memory tasks in naval cadets. Int Marit Health. 2012;63:181–187. [PubMed] [Google Scholar]
- Kahneman D, Tursky B, Shapiro D, Crider A. Pupillary, heart rate, and skin resistance changes during a mental task. J Exp Psychol. 1969;79:164–167. doi: 10.1037/h0026952. [DOI] [PubMed] [Google Scholar]
- Kramer SE, Kapteyn TS, Festen JM, Kuik DJ. Assessing aspects of hearing handicap by means of pupil dilation. Audiol Neurootol. 1997;36:155–164. doi: 10.3109/00206099709071969. [DOI] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biol Psychol. 2010;84:394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Stress and Health. 2. Thousand Oaks, CA: Sage Publications; 2005. [Google Scholar]
- Lunner T, Rudner M, Rönnberg J. Cognition and hearing aids. Scand J Psychol. 2009;50:395–403. doi: 10.1111/j.1467-9450.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- Mackersie CL, Cones H. Subjective and psychophysiological indexes of listening effort in a competing-talker task. J Am Acad Audiol. 2011;22:113–122. doi: 10.3766/jaaa.22.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik MM. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. [Practice Guideline] Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Marfil MN, Santaella MDF, Leon AG, Turpin G, Castellar JV. Individual differences associated with cardiac defence response: Psychophysiological and personality variables. Psychology in Spain. 1999;3:54–62. [Google Scholar]
- Mathewson KJ, Jetha MK, Drmic IE, Bryson SE, Goldberg JO, Hall GB, Schmidt LA. Autonomic predictors of stroop performance in young and middle-aged adults. Int J Psychophysiol. 2010;76:123–129. doi: 10.1016/j.ijpsycho.2010.02.007. [DOI] [PubMed] [Google Scholar]
- McCoy SL, Tun PA, Cox LC, Colangelo M, Stewart RA, Wingfield A. Hearing loss and perceptual effort: downstream effects on older adults’ memory for speech. Q J Exp Psychol A. 2005;58:22–33. doi: 10.1080/02724980443000151. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mind Media, B. V. Biotrace+ for Nexus-10 (version 2008a) [computer software] Roermond-Herten; Netherlands: 2008. [Google Scholar]
- Miyake S. Multivariate workload evaluation combining physiological and subjective measures. Int J Psychophysiol. 2001;40:233–238. doi: 10.1016/s0167-8760(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Mohlman J. Cognitive self-consciousness—a predictor of increased anxiety following first-time diagnosis of age-related hearing loss. Aging Ment Health. 2009;13:246–254. doi: 10.1080/13607860802428026. [DOI] [PubMed] [Google Scholar]
- Moses ZB, Luecken LJ, Eason JC. Measuring task-related changes in heart rate variability. Conference Proceedings: IEEE Engineering in Medicine and Biology Society. 2007:644–647. doi: 10.1109/IEMBS.2007.4352372. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Shapiro D, Davydov DM, Goldstein IB. Autonomic stress response: Modes and ambulatory heart rate level and variability. Journal of Psychophysiology. 2008;22(1):28–40. [Google Scholar]
- Pecchinenda A, Smith CA. The affective significance of skin conductance activity during a difficult problem-solving task. Cognition & Emotion. 1996;10:481–503. [Google Scholar]
- Pichora-Fuller MK. Cognitive aging and auditory information processing. Int J Audiol. 2003;42(Suppl 2):2S26–32. [PubMed] [Google Scholar]
- Piquado T, Benichov J, Brownell H, Wingfield A. The hidden effect of hearing acuity on speech recall, and compensatory effects of self-paced listening. Int J Audiol. 2012;51:576–583. doi: 10.3109/14992027.2012.684403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt PMA. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol Suppl. 1991;476:167–176. doi: 10.3109/00016489109127274. [DOI] [PubMed] [Google Scholar]
- Schipke JD, Pelzer M, Arnold G. Effect of respiration rate on short-term heart rate variability. J Clin Basic Cardiol. 1999;2:92–94. [Google Scholar]
- Schwartz JB, Gibb WJ, Tran T. Aging effects on heart rate variation. J Gerontol. 1991;46:M99–106. doi: 10.1093/geronj/46.3.m99. [DOI] [PubMed] [Google Scholar]
- Song HS, Lehrer PM. The effects of specific respiratory rates on heart rate and heart rate variability. Appl Psychophysiol Biofeedback. 2003;28:13–23. doi: 10.1023/a:1022312815649. [DOI] [PubMed] [Google Scholar]
- Studebaker GA. A “rationalized” arcsine transform. J Speech Hear Res. 1985;28:455–462. doi: 10.1044/jshr.2803.455. [DOI] [PubMed] [Google Scholar]
- Studebaker GA, Hochberg I. Acoustical Factors Affecting Hearing Aid Performance. 2. Boston: Allyn Bacon; 1993. [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Tun PA, Benichov J, Wingfield A. Response latencies in auditory sentence comprehension: Effects of linguistic versus perceptual challenge. Psychol Aging. 2010;25:730–735. doi: 10.1037/a0019300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24:761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R, Maraj B, Lee CM, Reyes R. Short-term heart rate variability during a cognitive challenge in young and older adults. Age Ageing. 2002;31:131–135. doi: 10.1093/ageing/31.2.131. [DOI] [PubMed] [Google Scholar]
- Zekveld AA, Kramer SE, Festen JM. Cognitive load during speech perception in noise: the influence of age, hearing loss, and cognition on the pupil response. Ear Hear. 2011;32:498–510. doi: 10.1097/AUD.0b013e31820512bb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table of raw skin conductance means (in μSiemens) for each group and condition. Standard deviations are indicated in parentheses.