Abstract
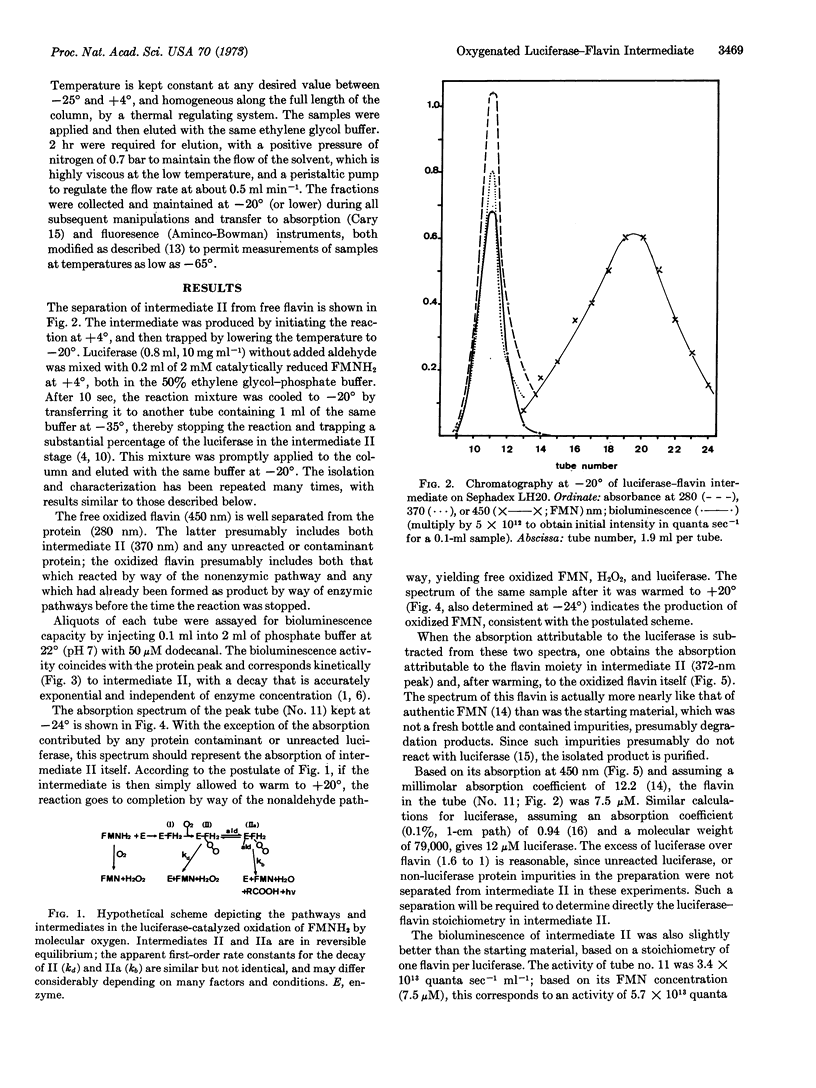
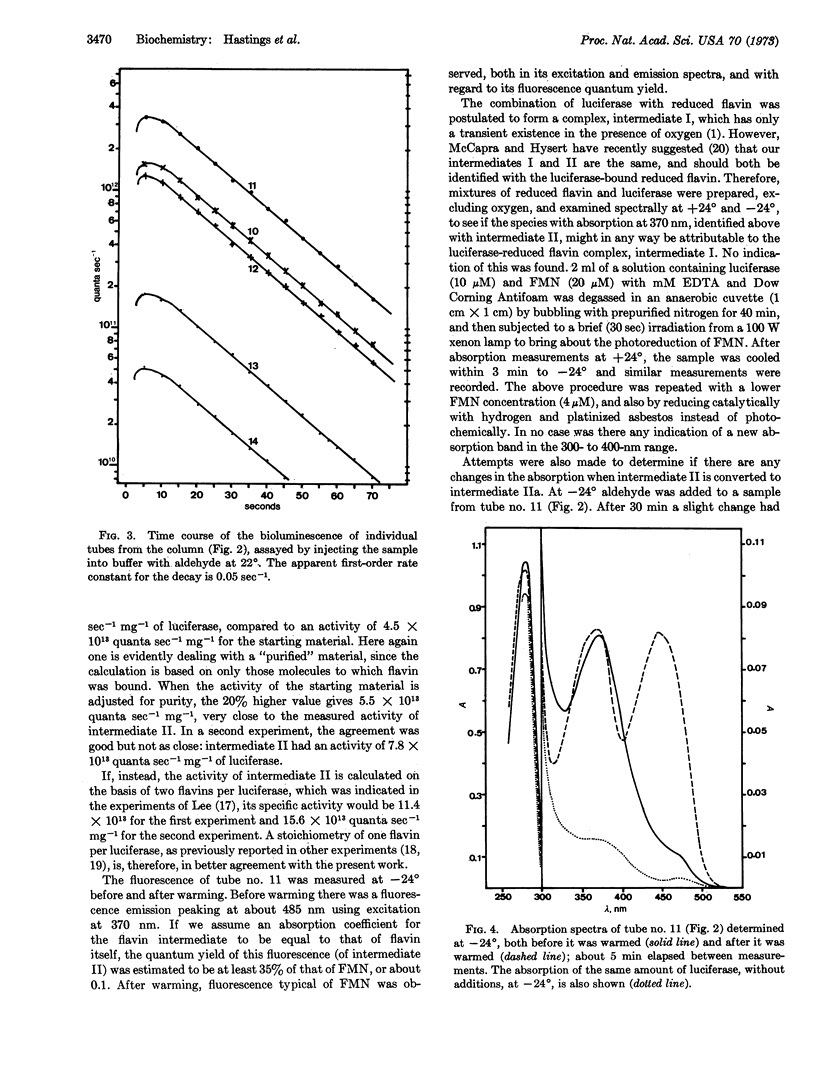
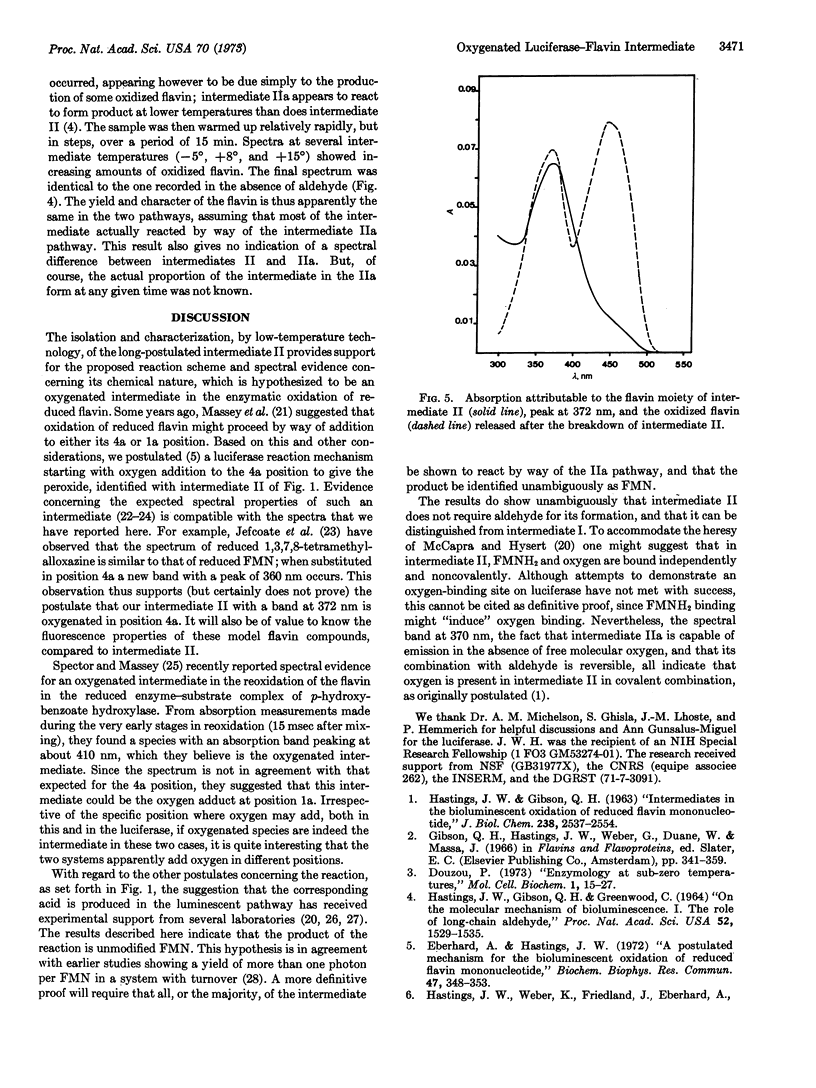
Bacterial luciferase catalyzes the oxidation of reduced flavin mononucleotide by molecular oxygen; long-chain aldehyde is required for light emission. At 20° the bioluminescence has a lifetime of tens of seconds, while excess reduced flavin is removed by way of nonenzymatic autoxidation in less than a second. This observation indicates the existence of a long-lived enzyme intermediate, which has been postulated to be a peroxide of the enzyme-bound reduced flavin. This intermediate was isolated and studied at low temperature (-20°), where it has a lifetime measured in days. It has an absorption with a single band peaking at 372 nm, and fluorescence emission centered at about 485 nm, which might be expected for the postulated flavin peroxide. Upon conversion to product, flavin mononucleotide-like absorption and fluorescence appear, supporting the postulate that flavin turns over in the reaction. Upon injection into buffer at 20° with added aldehyde, bioluminescence occurs. Based on a stoichiometry of one flavin per luciferase molecule, the specific activity of the intermediate is equal to that of pure luciferase.
Keywords: bioluminescence, enzyme intermediates, oxygen
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORMIER M. J., TOTTER J. R. Quantum efficiency determinations on components of the bacterial luminescence system. Biochim Biophys Acta. 1957 Aug;25(2):229–237. doi: 10.1016/0006-3002(57)90463-8. [DOI] [PubMed] [Google Scholar]
- Douzou P. Enzymology at sub-zero temperatures. Mol Cell Biochem. 1973 May 11;1(1):15–27. doi: 10.1007/BF01659935. [DOI] [PubMed] [Google Scholar]
- Eberhard A., Hastings J. W. A postulated mechanism for the bioluminescent oxidation of reduced flavin mononucleotide. Biochem Biophys Res Commun. 1972 Apr 28;47(2):348–353. doi: 10.1016/0006-291x(72)90719-x. [DOI] [PubMed] [Google Scholar]
- Gunsalus-Miguel A., Meighen E. A., Nicoli M. Z., Nealson K. H., Hastings J. W. Purification and properties of bacterial luciferases. J Biol Chem. 1972 Jan 25;247(2):398–404. [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H., GREENWOOD C. ON THE MOLECULAR MECHANISM OF BIOLUMINESCENCE. I. THE ROLE OF LONG-CHAIN ALDEHYDE. Proc Natl Acad Sci U S A. 1964 Dec;52:1529–1535. doi: 10.1073/pnas.52.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem. 1963 Jul;238:2537–2554. [PubMed] [Google Scholar]
- Hastings J. W., Weber K., Friedland J., Eberhard A., Mitchell G. W., Gunsalus A. Structurally distinct bacterial luciferases. Biochemistry. 1969 Dec;8(12):4681–4689. doi: 10.1021/bi00840a004. [DOI] [PubMed] [Google Scholar]
- Hui-Bon-Hoa G., Douzou P. Ionic strength and protonic activity of supercooled solutions used in experiments with enzyme systems. J Biol Chem. 1973 Jul 10;248(13):4649–4654. [PubMed] [Google Scholar]
- Lavelle F., Henry J. P., Michelson A. M. Etude en milieu fluide et à basse température de la réaction d'émission de bioluminescence de Photobacterium Leiognathi. C R Acad Sci Hebd Seances Acad Sci D. 1970 Apr 27;270(17):2126–2129. [PubMed] [Google Scholar]
- Massey V., Müller F., Feldberg R., Schuman M., Sullivan P. A., Howell L. G., Mayhew S. G., Matthews R. G., Foust G. P. The reactivity of flavoproteins with sulfite. Possible relevance to the problem of oxygen reactivity. J Biol Chem. 1969 Aug 10;244(15):3999–4006. [PubMed] [Google Scholar]
- McCapra F., Hysert D. W. Bacterial bioluminescence-identification of fatty acid as product, its quantum yield and a suggested mechanism. Biochem Biophys Res Commun. 1973 May 1;52(1):298–304. doi: 10.1016/0006-291x(73)90987-x. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Hastings J. W. Binding site determination from kinetic data. Reduced flavin mononucleotide binding to bacterial luciferase. J Biol Chem. 1971 Dec 25;246(24):7666–7674. [PubMed] [Google Scholar]
- Meighen E. A., Nicoli M. Z., Hastings J. W. Hybridization of bacterial luciferase with a variant produced by chemical modification. Biochemistry. 1971 Oct 26;10(22):4062–4068. doi: 10.1021/bi00798a008. [DOI] [PubMed] [Google Scholar]
- Mitchell G. W., Hastings J. W. A stable, inexpensive, solid-state photomultiplier photometer. Anal Biochem. 1971 Jan;39(1):243–250. doi: 10.1016/0003-2697(71)90481-7. [DOI] [PubMed] [Google Scholar]
- Mitchell G., Hastings J. W. The effect of flavin isomers and analogues upon the color of bacterial bioluminescence. J Biol Chem. 1969 May 25;244(10):2572–2576. [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Kohama Y. Reactions involved in bioluminescence systems of limpet (Latia neritoides) and luminous bacteria. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2086–2089. doi: 10.1073/pnas.69.8.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Massey V. p-Hydroxybenzoate hydroxylase from Pseudomonas fluorescens. Evidence for an oxygenated flavin intermediate. J Biol Chem. 1972 Sep 10;247(17):5632–5636. [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. H., Hemmerich P., Massey V. Reduktive Photoalkylierung des Flavinkerns und Flavinkatalysierte Photodecarboxylierung von Phenylacetat. Helv Chim Acta. 1967 Dec 11;50(8):2269–2279. doi: 10.1002/hlca.19670500812. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Nakamura T. Studies on luciferase from Photobacterium phosphoreum. II. Substrate specificity and stoichiometry of the reaction in vitro. J Biochem. 1972 Sep;72(3):647–653. doi: 10.1093/oxfordjournals.jbchem.a129942. [DOI] [PubMed] [Google Scholar]



