Abstract
Background
Hyponatremia in cirrhosis is associated with impaired cognition and poor health-related quality of life(HRQOL). However, the benefit of hyponatremia correction is unclear.
Aim
To evaluate the effect of tolvaptan on serum sodium, cognition, HRQOL, companion burden, and brain MRI (volumetrics, spectroscopy and diffusion tensor imaging) in cirrhotics with hyponatremia.
Methods
Cirrhotics with Na<130meq/l were included for a four-week trial. At screening, patients underwent cognitive and HRQOL testing, serum/urine chemistries and companion burden assessment. Patients then underwent fluid restriction and diuretic withdrawal for two weeks after which cognitive tests were repeated. If Na was still<130meq/L, brain MRI was performed & tolvaptan initiated for 14 days with frequent clinical/laboratory monitoring. After 14 days of tolvaptan, all tests were repeated. Comparisons were made between screen, pre and post-drug periods Na, urine/serum laboratories, cognition, HRQOL and companion burden.
Results
24 cirrhotics were enrolled; seven normalized Na without tolvaptan with improvement in cognition. The remaining 17 received tolvaptan of which 14 completed the study over 13±2 days (age 58±6 yrs, MELD 17, 55%HCV, median 26mg/day of tolvaptan). Serum Na and urine free water clearance increased with tolvaptan without changes in mental status or liver function. Cognitive function, HRQOL and companion burden only improved in these 14 patients after tolvaptan, along with reduced total brain and white matter volume, increase in choline on MRS, and reduced cytotoxic edema.
Conclusions
Short-term tolvaptan therapy is well tolerated in cirrhosis. Hyponatremia correction is associated with cognitive, HRQOL, brain MRI and companion burden improvement.
Keywords: caregiver burden, aquaresis, diffusion tensor imaging, hepatic encephalopathy, magnetic resonance spectroscopy, tolvaptan
Introduction
Hyponatremia, which reflects systemic hemodynamic derangement due to systemic vasodilatation and renal water retention, is associated with poor outcomes in cirrhosis[1]. These adverse outcomes include impaired cognition, reduced health-related quality of life (HRQOL) and a complicated post-transplant course[2-4]. Interventions such as fluid restriction, diuretic withdrawal and aquaretics agents like tolvaptan can improve serum sodium without correcting the underlying hemodynamic derangement[5]. However, it is unclear to what extent the clinically relevant morbidity associated with hyponatremia such as impaired cognition and HRQOL, increased companion burden and brain MR functioning can be improved by correcting serum sodium. Our aim was to study the effect of sodium correction on cognitive function and its effect on the patients (HRQOL and brain MRI) and their companions’ burden.
Methods
We recruited outpatients with cirrhosis[histology, radiology, varices on endoscopy or suggestive laboratory features (platelets<150,000 with an AST/ALT ratio >1 in chronic liver disease] with a serum sodium <130mg/dl within the last 14 days from the McGuire VA and VCU Medical Centers. Patients had to have a companion and able to undergo brain MRI. We included patients with prior HE provided they had a mini-mental status exam (MMSE) >25, their last hepatic encephalopathy (HE) episode was >2 months prior to enrollment and they had suffered <2 episodes within the last 6 months.
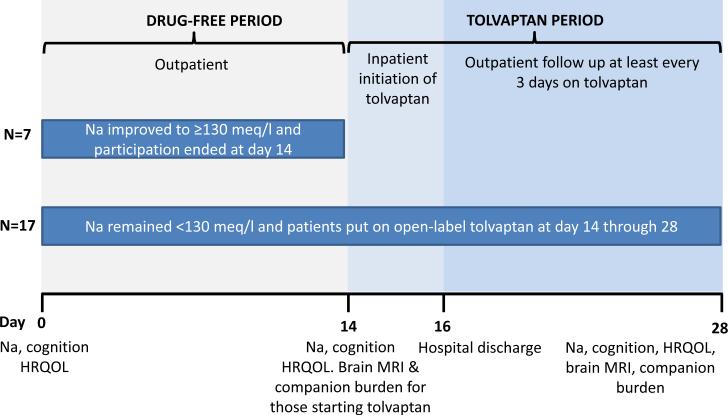
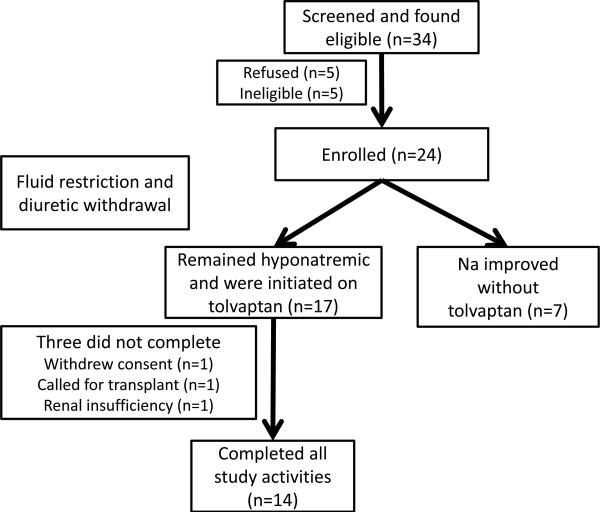
We excluded patients who were abusing alcohol/illicit drugs within the last 3 months, those with psychoactive drug use other than regularly scheduled anti-depressants, other causes of hyponatremia (congestive heart failure, SIADH etc), hypovolemia, TIPS, HIV infection, pregnant patients, those on azole medications, on therapies other than simple fluid restriction or diuretic withdrawal for hyponatremia (vaptan use or hypertonic saline) in the last month and those who were unable to provide consent. The study is registered at www.clinicalstrials.gov NCT0155664 and the overall schema is in figure 1.
Fig 1.
Schema of the study design
Screening Visit
If the Na was <130meq/L, the patient was included; procedures performed were (a) history of cirrhosis complications, fluid intake and diuretic use (b) physical examination to exclude overt HE (asterixis, modified-orientation log and MMSE)[6] (c) blood drawn for electrolytes (Na, K, Mg, P), renal function (creatinine, BUN), hepatic function (albumin and MELD score) and venous ammonia (d) cognitive tests and (e) HRQOL: Chronic Liver Disease Questionnaire (CLDQ)[7]. Cognitive tests used were number connection A and B (NCT-A/B), digit symbol (DST), block design (BDT), line tracing (errors and time, LTTe/t), serial dotting(SDT) and inhibitory control tests (ICT lures and target %)[8]. Different versions of paper-pencil tests were used at each visit to reduce learning. Psychometric hepatic encephalopathy score (PHES) was calculated; PHES >-5 standard deviations was considered impaired while the others were considered to have normal cognition[9]. The companions were asked formally about their relationship details with the patient and underwent a validated questionnaire, the perceived caregiver burden (PCB)[10].
At the end of the screening visit, the patients were instructed to restrict their fluid intake to 1.5L/day and their diuretics were withdrawn or reduced as tolerated. Patients were asked to collect their 24 hour urine for the day prior to the pre-drug visit for which they were seen at day 14.
Pre-drug visit
Patients were then seen with the companions and underwent a serum sodium assessment, questioning regarding fluid and diuretic restriction and cognitive testing. If the serum sodium was 130 meq/L, they were not started on tolvaptan and they underwent a Na draw 14 days later. For the remainder, screening visit procedures were repeated, brain MRI performed (details after study design). The 24-hour urinary collection pre-drug was analyzed for volume, osmolality and measured values (Na, K). Companions again completed the PCB questionnaire. Blood was also collected for renin and copeptin measurement (performed at Assaygate, MD).
Inpatient tolvaptan study
Tolvaptan was initiated at 15mg for the first dose. All intake and output was charted, all urine was collected and a 24-hour urinary collection for the electrolytes above was performed daily. Study staff was present with the patient throughout. Serum sodium and mental status were analyzed every 8 hours. Patients were allowed to drink water per thirst. Daily physical examination by study doctors and laboratory evaluation of renal and hepatic function were performed. If the sodium levels rose by 8 meq/L over 24 hours while the patient was at 15mg dosage, it was advanced to 30mg/day which was the maximum dose. After 72 hours, patients were discharged with their assigned tolvaptan doses for a follow-up was within 3 days.
Outpatient drug follow-up
Patients and companions were followed every 3-5 days for at least 4 outpatient visits till the end-of-drug visit. During each visit, tolerability and use of tolvaptan (history/pill count), use of other medications, fluid intake and urine output, and adverse events were inquired, a physical examination was performed and blood electrolytes, venous ammonia, renal and hepatic function were assessed. Patients and companions were then continued in the study till the end-of-drug visit. At any point, if the serum sodium increased to 140meq/L, the drug was held till the next visit. End-of-drug visit At day 14, patients and companions underwent all tests and procedures that were performed at drug initiation. The final pill count was performed and the study participation was terminated.
Statistical analysis and Sample Size
Sample size
The primary endpoint was improvement in PHES. We hypothesized that 15% of patients will improve their cognition between day 0 and 14 indicative of learning which would increase to 50% post-tolvaptan giving us 11 patients to be able to reject the null hypothesis with a power of 80% at an = .05 level. Statistical analysis A time-weighted average (TWA) change and area-under-curve (AUC) in Na post-tolvaptan was calculated. Fisher exact test was used to compare PHES improvement and proportion of those who normalized post-tolvpatan compared to baseline and start of drug values. Paired t-tests were used to compare individual cognitive tests, CLDQ, and burden. Cognitive tests, HRQOL and burden variables were compared between screen and post-drug values, while MR variables were compared between start and end of drug values.
MRI protocol and analysis
(done at pre-drug and end-of drug visits; details in supplementary section). We performed the MRI analysis to evaluate three specific changes: (1) MR Spectroscopy: to evaluate changes in osmolytes that occur as a result of hyponatremia and HE, we measured creatine ratios of choline (Cho), creatine (Cr), myo-inositol (mI) and glutamate+glutamine (Glx) in the right parietal white and posterior gray matter, (2) Diffusion tensor imaging (DTI): to perform analysis of the integrity and edema of white matter (axons of the neurons) that can change in response to fluid shifts, DTI of four major white matter tracts (frontal white matter, anterior/posterior internal capsule, and corpus callosum genu) was performed. This yields the fractional anisotropy (FA), mean diffusivity (MD) and spherical isotropy (CS) and (3) MR morphometry: is a detailed analysis of the structural volume of the brain (total, white matter, gray matter and CSF). These were compared before and after tolvaptan initiation.
Results
Patient course and enrollment
We studied the charts of 47 outpatients, of whom 34 were considered eligible (of the 13 ineligible, 7 had a recent HE episode, 2 had a TIPS and 4 were on anti-seizure/opioid medications). Twenty four of the remaining 34 patients were ultimately consented for the study(five refused, three did not have a companion and two had contraindications to MRI). Of these 24, 7 patients normalized their Na between the screening and pre-drug visit and were not considered for tolvaptan. The remaining 17 patients continued on to receive the drug and 14 completed (one got transplanted, one withdrew consent and another was withdrawn due to increasing creatinine).
Pre-drug period
The baseline characteristics of all patients entered are in Table 1. Those whose sodium improved with reduction of diuretics and fluid restriction were not significantly different from the rest with respect to age (60 vs 57, p=0.23), MELD score (17 vs 18, p=0.8) and albumin (2.7 vs 2.8, p=0.89) but had a non-significant trend towards a higher screening Na (128 vs 125, p=0.06). On 14-day follow up, five of these seven patients (71%) continued to have Na >130meq/l (134±2 meq/l) while 2 again became hyponatremic. No episodes of HE were noted during this follow-up.
Table 1.
Baseline characteristics of all patients who were screened successfully (n=24)
| Variable | Value |
|---|---|
| Age (years) | 58.5±7 |
| Gender (men/women) | 22/2 |
| Etiology of cirrhosis (HCV, Alcohol, HCV+alcohol, NASH, other) | 12/5/3/2/2 |
| MELD score | 18±8 |
| Hepatic encephalopathy | 92% |
| Ascites | 100% |
| Spironolactone dose (mg/day) | 100±106 |
| Furosemide dose (mg/day) | 55±42 |
| Systolic BP (mmHg) | 108±15 |
| Diastolic BP (mmHg) | 63±10 |
| Heart Rate (per minute) | 81 ±15 |
| Laboratory indices | |
| Na (meq/L) | 124.6±3.6 |
| K (meq/L) | 4.3±0.7 |
| P (meq/L) | 3.1±0.8 |
| Mg (meq/L) | 1.9±0.3 |
| Ammonia (μmol/L) | 56.9±25.3 |
| Creatinine (mg/dl) | 1.2±0.5 |
| Total bilirubin (mg/dl) | 4.2±4.1 |
| INR | 1.8±0.6 |
| AST | 90.6±51.4 |
| ALT | 54.5±38.8 |
| Albumin (g/dl) | 2.8±0.6 |
| Cognitive testing | |
| Modified orientation log | 27.8±1.5 |
| Mini-mental status exam | 26.7±0.7 |
| Number Connection-A(s) | 58.2±34.4 |
| Number Connection-B(s) | 190.0±99.8 |
| Digit symbol (raw) | 36.4±15.2 |
| Block design(raw) | 18.8±11.5 |
| Line tracing time (s) | 158.0±58.0 |
| Line tracing errors (no.) | 62.0±53.0 |
| Serial Dotting (s) | 118.8±57.5 |
| ICT lures (no.) | 14.2±8.2 |
| ICT targets (%) | 79.0±22.8 |
| Median PHES score (range) | −12.0 (−18.0-0.0) |
Analysis of patients who completed the study
Fourteen patients had complete data available from baseline till end. The Na TWA post-tolvaptan was significantly higher compared to the pre-tolvaptan levels (126±2 vs. 132±3, p=0.02) and similarly the AUC significantly increased from pre-tolvaptan values (2013±38) to day 1 (2098±39, p<0.0001) and day 14 (2112±65 p<0.0001). The mean TWA increase was 6±2 compared to pre-drug and 7±3 meq/L compared to screening values, while for AUC was 99±60 compared to pre-drug and 101±72 compared to screening. Absolute Na values increased significantly after tolvpatan (Table 1) and the median change between post-tolvaptan absolute Na and pre-tolvaptan was 5.5±3.4 and 6.9±4.0 between post-tolvaptan and screening absolute values. Other laboratory analyses did not show significant changes (Tables 2 and 3) in the group that continued through the inpatient and outpatient arms. There was no significant change in median copeptin (0.9 to 1.0 ng/ml, p=0.78; undetectable in 5 patients before/after therapy) or renin levels (13.0 to 14.05 ng/ml, p=0.56) after tolvaptan.
Table 2.
Detailed physical examination and laboratory evaluation of patients who completed the study at each visit (significant changes in bold font)
| Screen (n=24) | Inpatient stay | Outpatient visits (n=14) | End of drug (n=14) | P value compared to start of drug | |||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1: Start of drug (n=17) | Day 2 (n=17) | Day 3 (n=16) | |||||||
| Day number | 0 | 14 | 15 | 16 | 18-19 | 21-22 | 23-25 | 28 | |
| Modified orientation log | 24 | 24 | 24 | 24 | 23 | 24 | 24 | 24 | 0.8 |
| Mini-mental status exam | 29.3 | 29.6 | 29.5 | 29.0 | 29.1 | 29.5 | 29.4 | 29.5 | 0.94 |
| HE development | — | — | — | — | — | — | — | — | |
| Systolic BP | 107.5 | 104.0 | 109 | 106.1 | 108.3 | 102.3 | 106.4 | 104.8 | 0.35 |
| Diastolic BP | 60.8 | 62.0 | 64.9 | 63.2 | 64.7 | 59.8 | 62.7 | 59.7 | 0.56 |
| Heart rate | 80.9 | 80.5 | 81.8 | 79.3 | 79.5 | 84.3 | 79.8 | 78.4 | 0.73 |
| Weight (lb) | 184.3 | 187.7 | 184.4 | 184.7 | 191.7 | 194.9 | 184.6 | 177.3 | 0.03 |
| Tolvaptan dose (mg/day) | -- | 15 | 19.6 | 23 | 24 | 27 | 21 | 22 | |
| Na (meq/L) | 124 | 126 | 130.9 | 131.6 | 132.3 | 132 | 131.1 | 133 | 0.001 |
| K (meq/L) | 4.5 | 4.4 | 4.2 | 4.4 | 4.5 | 4.4 | 4.4 | 4.5 | 0.21 |
| Ammonia (μmol/L) | 63.3 | 63.5 | 65.4 | 54.7 | 49.7 | 49.8 | 46.7 | 44.0 | 0.02 |
| Creatinine (mg/dl) | 1.0 | 1.0 | 1.0 | 1.1 | 1.2 | 1.2 | 1.1 | 1.2 | 0.82 |
| INR | 1.7 | 1.7 | 1.7 | 1.7 | 1.9 | 2.0 | 1.7 | 1.9 | 0.42 |
| AST | 85.4 | 82.1 | 79.8 | 74.2 | 82.6 | 73.1 | 78.1 | 73.4 | 0.56 |
| ALT | 58.6 | 60.1 | 55.5 | 51.2 | 60.5 | 53.8 | 65.7 | 54.5 | 0.78 |
| Total bilirubin (mg/dl) | 4.2 | 4.2 | 4.9 | 6.5 | 6.1 | 5.0 | 5.0 | 0.56 | |
Table 3.
Per-protocol Analysis of the 14 patients who completed the study
| Screening | Drug Initiation | End of drug | |
|---|---|---|---|
| Weight (lb) | 184.3±34.4 | 186.3±35.1 | 177.3±33.6 (p=0.03) |
| Spironolactone dose | 107±111 | 45±103 | 38±50 |
| Furosemide dose | 62±40 | 35±39 | 23±29 |
| Laboratory indices | |||
| Na (meq/L) | 124±3 | 126±2 | 133±4 (p=0.001) |
| K (meq/L) | 4.5±0.7 | 4.4±0.5 | 4.5±0.4 |
| P (meq/L) | 3.1±0,5 | 3.2±0.5 | 3.2±0.7 |
| Mg (meq/L) | 1.9±0.2 | 1.9±0.2 | 1.9±0.4 |
| Ammonia (μmol/L) | 63.3±26.8 | 63.5±24.7 | 44±14* |
| Creatinine (mg/dl) | 1.0±0.3 | 1.0±0.3 | 1.2±0.9 |
| INR | 1.7±0.5 | 1.7±0.5 | 1.9±0.7 |
| AST | 85.4±40.5 | 82.1±45.7 | 73.4±48.9 |
| ALT | 58.6±42.5 | 60.1±44.7 | 54.5±45.9 |
| Albumin (g/dl) | 2.9±0.6 | 2.9±0.7 | 2.8±0.7 |
| Total bilirubin (mg/dl) | 4.2±4.2 | 4.2±3.8 | 5.0±5.6 |
| Hemoglobin(g/dl) | 10.3±1.4 | |10.0±1.3 | 9.8±1.8 |
| WBC count (/mm3) | 6.5±2.9 | 6.1±3.3 | 6.5±3.4 |
| Platelets(/mm3) | 95.6±69.9 | 91.4±72.0 | 97.7±75.2 |
| MELD score | 18±7 | 18±8 | 18±7 |
| Cognitive tests | |||
| Number Connection-A(s) | 59±41 | 64±58 | 48±22 (p=0.02) |
| Number Connection-B(s) | 166±73 | 174±117 | 130±50 (p=0.01) |
| Digit symbol (raw) | 37±15 | 39±15 | 44±18 |
| Block design(raw) | 18±11 | 19±11 | 22±9 |
| Line tracing time (s) | 151 ±61 | 165±127 | 148±102 |
| Line tracing errors (no.) | 54±52 | 55±47 | 45±40 (p=0.001) |
| Serial Dotting (s) | 111±58 | 100±46 | 92±49 |
| ICT lures (no.) | 15±9 | 19±13 | 13±8 (p=0.002) |
| ICT targets (%) | 84±21 | 88±11 | 87±17 |
| Median PHES (range) | −7.0 (−11.0-0.0) | −6.75 (−11.0-0.0) | −2.75 (−9.0-0) |
| % any improvement | - | 0 (0%) | 9 (64%) (p=0.005) |
| Normal cognition | 0 (0%) | 1 (7%) | 7 (50%) (p=0.03) |
All significant p values between drug initiation compared to end-of-drug are in parentheses. None of the comparisons between screening and drug initiation were statistically significant
Tolvaptan was initiated at 15mg, and increased to 30mg/day for eight patients on day 2 (rest remained on 15mg/day) and on day 3 for an additional two patients. Ten patients were discharged home on 30mg/day while four remained on 15 mg/day. These doses continued till the end of the study.
Urine changes
Prior to tolvaptan initiation the mean 24-hour urine volume was 1656±998 ml which significantly increased to 3972±1913 ml at 24 hours and 3095±1909 ml at 48 hours post-tolvaptan (p=0.001). There was a corresponding reduction in urine sodium (38.8±27.4 vs. 18.7±12.1 vs. 19.6±13.7 p=0.004), urine potassium (26.5±12.6 vs. 10.5±5.2 vs. 11.6±7.1) and urine osmolality (416.8±179.3 vs. 222.5±94.0 vs. 285.1±145.7) from baseline to the first two days on the drug. The electrolyte free water clearance was 698±438 ml/day pre-drug which significantly increased to 3019±1561 ml/day in the first 24 hours and 2356±1486 ml/day at 48 hours (p<0.001 compared to pre-drug).
Adverse events
In the pre-drug period, two patients had intermittent nausea, one had hypokalemia and one had intermittent leg cramps. While hospitalized, three patients had electrolyte abnormalities that required correction (three hypokalemia, one hypophosphatemia and one hypomagnesemia). One patient had continued mild muscle cramping and one had continuation of the intermittent nausea from the pre-drug period. None of these required stopping of the study medication. Another patient required initiation of midodrine and octreotide and was withdrawn at day 20 after receiving five drug doses.
Cognitive performance
A significant improvement occurred in those whose sodium had improved after diuretic restriction (NCT-A:64±20 to 60±14, NCT-B:243±123 to 194±98, DST:30±10 to 41±9, LTTt:190±31 to 179±28, LTTe:64±59 to 55±35, SDT:133±52 to 118±60, ICT lures:12±6 to 11±4, target %:62±24 to 85±20; p<0.05 for all but lures/NCT-A/LTTt). However, in those who remained hyponatremic, there was no significant change in cognitive performance (Table 3). In contrast, following tolvaptan, there was a significant improvement in test performance on NCT-A/B, ICT lures and line tracing test errors. There was a significant improvement in the proportion whose PHES score improved and those who returned to normal after the study.
There was a significant correlation between delta sodium (Na TWA minus Na pre-drug) and delta NCT-B (r=-0.6, p=0.02), delta DST (0.4, p=0.05), SDT (0.4, p=0.05) and LTT errors (r=-0.5, p=0.04). This was also found with AUC change and delta NCT-B (r=-0.4, p=0.03), DST (0.36, p=0.05) and LTT errors (r=−0.5,p=0.04).
HRQOL and companion burden
: there was a significant improvement in the CLDQ between pre and end-of-drug period (3.4±1.1 vs 3.8±1.2, p=0.04) but not compared to screening values (3.4±1.2). Most companions (12, 86%) were spouses; rest were children of the subjects. The median relationship duration was 21 years. The screening PCB of the 14 who completed the trial was 78±22 which was unchanged statistically during the pre-drug visit at 80±15. This significantly decreased to 70±12 (p=0.02 compared to pre-drug) at end-of-drug.
MRI results
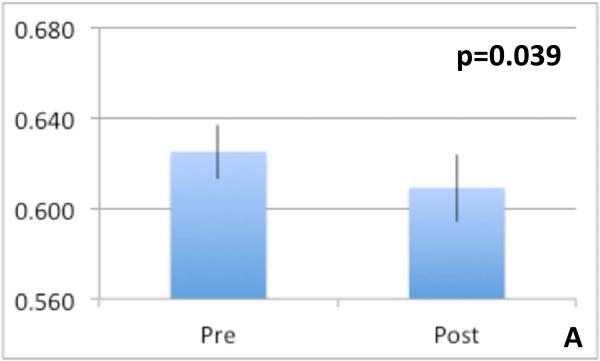
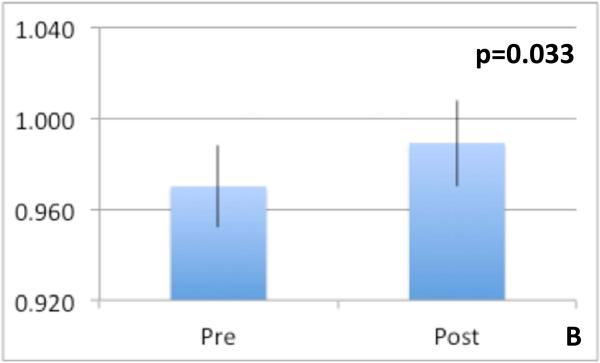
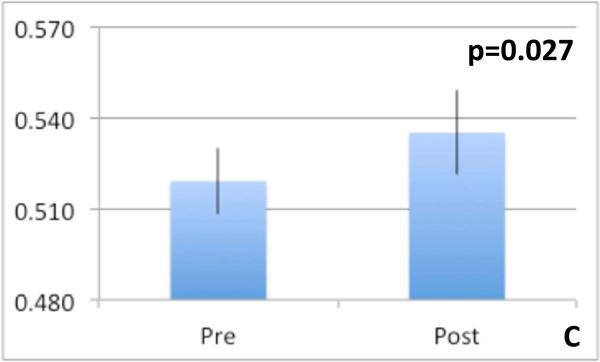
Spectroscopy: We found a significant increase in Cho concentration ratio increased significantly post-tolvaptan treatment in both right parietal white and posterior gray matter (Table 4) without significant change in Glx and mI post-treatment. This indicates an increase in intracellular osmolytes after tolvaptan. DTI: Features potentially representing reduction in cytotoxic edema in the neuronal axons of the genu of the corpus callosum was found [reduced FA (p=0.039), increased MD (p=0.033) and increased CS (p=0.027)] after treatment (Figure 3 A-C). We found no significant differences in FA, MD or CS in any other regions of interest. Morphometry: Final percentage brain volume change was −0.826±0.3% (range = −1.987% to 1.479%) indicating an overall reduction in brain volume post-tolvaptan. When examining segmented brain component volumes separately, we found that post-tolvaptan, total white matter volume decreased (pre 458.47±19.53 cm3; post 451.73±21.34 cm3, p=0.027) with a trend towards increase in total cerebrospinal fluid volume (pre 300.21±14.73 cm3; post 306.87±14.56 cm3, p=0.06) but not significant change in the total gray matter volume (pre 584.77±21.06 cm3; post 583.47±18.16 cm3, p=0.4). Therefore after tolvaptan, total brain and white matter volume decreased.
Table 4.
Magnetic Resonance Spectroscopy Changes after Tolvaptan showing an increase in Choline indicating reduction in astrocytic swelling
| Creatine ratio of metabolites | Right Parietal White Matter | Posterior Gray Matter | ||||
|---|---|---|---|---|---|---|
| Myoinositol | Choline | Glutamate+ Glutamine | Myoinositol | Choline | Glutamate+ Glutamine | |
| Pre-drug | 0.192±0.04 | 0.239±0.01 | 2.4±0.14 | 0.254±0.04 | 0.187±0.01 | 2.375±0.08 |
| After tolvaptan | 0.166±0.04 | 0.285±0.02 | 2.278±0.14 | 0.253±0.03 | 0.213±0.01 | 2.292±0.12 |
| P value | 0.53 | 0.014 | 0.53 | 0.62 | 0.009 | 0.61 |
Fig 3.
Diffusion tensor imaging metrics of the genu of the corpus callosum, a major white matter tract pre and post-tolvaptan showing reduction in cytotoxic edema anisotropy increased after tolvaptan 3A: Fractional anisotropy reduced, 3B: Mean diffusivity increased and 3C: spherical
Discussion
Treatment of hyponatremia in cirrhosis is an important goal because it is associated with a high mortality, risk of falls, progression and recurrence of HE and poor outcomes after transplant[11]. However clinically relevant outcomes that accompany improvement in serum sodium, especially from a cognitive standpoint, have not been completely investigated. Our findings of improved cognition after hyponatremia correction and further improvement of cognition, HRQOL and caregiver burden with tolvaptan, support the hypothesis that chronic systemic hypo-osmolality can contribute to brain dysfunction in cirrhosis, and that correction of hyponatremia may have clinically meaningful benefits. Our results demonstrate that even in patients with cirrhosis, the short-term use of tolvaptan is well tolerated with careful monitoring. As expected, urine volume increased along with electrolyte-free water clearance within the first day of tolvaptan with reduced urine osmolality and increased serum sodium. There were electrolyte abnormalities in patients after tolvaptan therapy that were easily managed and that did not require drug regimen modification. Importantly there was no significant change in ALT or AST levels and no deterioration of liver function at the dose of tolvaptan used.
Interestingly, and unexpectedly, we observed a significant reduction in venous ammonia in patients after tolvaptan that began on day 3 and progressed throughout day 14. There were no changes in the patients’ mental status throughout and no specific modifications of lactulose or rifaximin were instituted. Since tolvaptan does not have an effect of the gut ecology, it is unlikely that gut-associated ammonia production would be affected. Studies have shown that the kidneys become a net producer of ammonia in cirrhosis and it could be hypothesized that a higher diffusion of ammonia across the renal tubular membrane due to the significantly increased urine flow could be a mechanism behind this ammonia reduction[12-14]. This change in ammonia was also accompanied by a significant improvement in selected cognitive tests and brain imaging parameters. A significant proportion of patients who were hyponatremic after fluid restriction experienced cognitive improvement after tolvaptan compared to their screening and pre-drug change. The lack of improvement between screening and pre-drug testing replicates prior studies which show that patients with prior HE have impaired learning and argues against the subsequent cognitive improvement being due to learning alone[15, 16]. While there was a trend towards improvement in all tests only after tolvaptan, it was predominantly among tests of executive functions (NCT-B, ICT lures and line tracing errors) and not as marked in those that rely on psychomotor speed (digit symbol, serial dotting, line tracing time)[17] which is similar to a prior abstract that showed NCT-B improvement after vaptan therapy[18]. This profile of cognitive change after tolvaptan points towards changes in the frontal lobe functioning, although MR spectroscopy showed a generalized improvement. This was supported by the similar cognitive improvements in those whose sodium improved without tolvaptan.
The pathogenesis of cognitive improvement could be related to the re-building of the active osmolytes that are diminished when HE and hyponatremia co-exist[19]. We found evidence of this in the significant increase in choline/creatine ratios in anterior white and posterior gray matter. HE alone, likely through ammonia, increases Glx while hyponatremia decreases it in the brain; therefore the similar Glx after tolvaptan argues against the ammonia reduction as the primary reason behind the MRS changes[20]. We also found a reduction in total brain volume and white matter accompanied by a pattern suggestive of cytotoxic edema improvement (reduced fractional anisotropy and increased mean diffusivity and spherical isotropy) in one of the purest white matter regions, the genu of the corpus callosum[21]. This is interesting because it was initially believed that astrocytes were the predominant site for brain edema in cirrhosis but recent evidence also shows white matter edema[22]. This pattern of multi-modal MR change indicates that this cognitive improvement after sodium correction is potentially mediated by restoration of intracellular osmolytes, reduction in total brain volume due to primarily decreased white matter volume, which is associated with movement of water from inside the axon to the interstitial space. This decrease in brain volume after tolvaptan has been shown to improve cognition in non-cirrhotic animal models but we have extended it to humans[23].
We found that this improvement in cognition was accompanied by reduction in companion burden and HRQOL improvement post-tolvaptan. Companions have been shown in several studies to be personally and financially affected by the disease process. Hyponatremia, with its incident falls and predisposition to HE development may be a major contributor to this burden[10, 24-26]. HRQOL results reflect prior similar studies with hyponatremia correction[2, 5]. These specific results could have been influenced by the open-label nature of this study. It is difficult to blind the use of tolvpatan given the immense aquaresis and the need to monitor serum sodium change for safety reasons by the investigators. However it is unlikely that the open-label design would have impacted the Na levels, cognitive testing and brain MR results. The first part of the study replicated the standard of care, i.e. fluid restriction and diuretic withdrawal resulted in cognitive improvement in a subset in most of whom the Na improvement was sustained at 14 days, but not in those who remained hyponatremic. We also did not find a change in copeptin or renin changes after tolvaptan use which is probably due to inherent variations in these compounds as has been noted before[27]. However, we found that the correction of hyponatremia, regardless of modality employed, is associated with improvement in clinically relevant outcomes.
This study only shows a short-term proof of principle of the safety and potential efficacy of tolvaptan without change in liver function or mental status. Further long-term results focusing on cognitive outcomes are needed. Since the use of tolvaptan can be associated with dehydration and rapid changes in Na, requires careful inpatient initiation, input/output monitoring and slow liberalization of fluid intake, it should only be used in appropriately selected patients under expert supervision[28].
We conclude that hyponatremia correction improves cognition and tolvaptan treatment is associated with improved companion burden, HRQOL and cognition with reduction in brain volume and edema on multi-modal MR imaging.
Supplementary Material
Fig 2.
Progress of subjects through the study
Acknowledgments
Financial support: NIAAA NIH RO1AA020203, NIDDK NIH RO1DK087913, McGuire Research Institute and an investigator-initiated grant from Otsuka Pharmaceuticals. None of the funders had any role in the study design, protocol development, analysis of results and decision to publish.
Abbreviations
- HRQOL
health-related quality of life
- HE
hepatic encephalopathy
- SIADH
syndrome of inappropriate ADH secretion
- MMSE
mini-mental status exam
- NCT-A/B
number connection test A/B
- DST
digit symbol test
- BDT
block design test
- SDT
serial dotting test
- LTT
line tracing test
- ICT
inhibitory control test
- PHES
Psychometric hepatic encephalopathy score
- CLDQ
chronic liver disease questionnaire
- PCB
perceived caregiver burden
- TWA
time-weighted average
- AUC
area under the curve
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- CS
spherical isotropy
- MD
mean diffusivity
- Cho
choline
- Cr
creatine
- mI
myo-inositol
- Glx
glutamate+glutamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr Bajaj has served as a consultant to Otsuka Pharmaceuticals.
Author contributions: JSB was involved in all aspects, DMH, GF, JBW helped conceptualize and analyze the results, LRT performed statistical analyses, HCG, AU, MBW were involved in patient recruitment
Presentation: Portions of this study were presented in abstract form at the Liver Meeting in Washington, DC in 2013.
References
- 1.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahluwalia V, Wade JB, Thacker L, Kraft KA, Sterling RK, Stravitz RT, et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J Hepatol. 2013;59:467–473. doi: 10.1016/j.jhep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 4.Hackworth WA, Heuman DM, Sanyal AJ, Fisher RA, Sterling RK, Luketic VA, et al. Effect of hyponatraemia on outcomes following orthotopic liver transplantation. Liver Int. 2009;29:1071–1077. doi: 10.1111/j.1478-3231.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas A, Gines P, Marotta P, Czerwiec F, Oyuang J, Guevara M, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571–578. doi: 10.1016/j.jhep.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Salam M, Matherly S, Farooq IS, Stravitz RT, Sterling RK, Sanyal AJ, et al. Modified-orientation log to assess hepatic encephalopathy. Aliment Pharmacol Ther. 2012;35:913–920. doi: 10.1111/j.1365-2036.2012.05038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600. e1591. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–773. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–1653. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyer TD. Tolvaptan and hyponatremia in a patient with cirrhosis. Hepatology. 2010;51:699–702. doi: 10.1002/hep.23522. [DOI] [PubMed] [Google Scholar]
- 12.Olde Damink SW, Jalan R, Deutz NE, Redhead DN, Dejong CH, Hynd P, et al. The kidney plays a major role in the hyperammonemia seen after simulated or actual GI bleeding in patients with cirrhosis. Hepatology. 2003;37:1277–1285. doi: 10.1053/jhep.2003.50221. [DOI] [PubMed] [Google Scholar]
- 13.Jalan R, Kapoor D. Enhanced renal ammonia excretion following volume expansion in patients with well compensated cirrhosis of the liver. Gut. 2003;52:1041–1045. doi: 10.1136/gut.52.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiroyama M, Aoyagi T, Fujiwara Y, Oshikawa S, Sanbe A, Endo F, et al. Hyperammonaemia in V1a vasopressin receptor knockout mice caused by the promoted proteolysis and reduced intrahepatic blood volume. J Physiol. 2007;581:1183–1192. doi: 10.1113/jphysiol.2007.129569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riggio O, Nardelli S, Moscucci F, Pasquale C, Ridola L, Merli M. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16:133–146. doi: 10.1016/j.cld.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50:2014–2021. doi: 10.1002/hep.23216. [DOI] [PubMed] [Google Scholar]
- 18.Cordoba J, Guevara M, Watson HR, Le Guennec S. P. G. Improvement of hyponatremia in cirrhosis increases speed of complex information processing (Abstract). Hepatology. 2009;4:452A. [Google Scholar]
- 19.Cordoba J, Garcia-Martinez R, Simon-Talero M. Hyponatremic and hepatic encephalopathies: similarities, differences and coexistence. Metab Brain Dis. 2010;25:73–80. doi: 10.1007/s11011-010-9172-3. [DOI] [PubMed] [Google Scholar]
- 20.Heins J, Zwingmann C. Organic osmolytes in hyponatremia and ammonia toxicity. Metab Brain Dis. 2010;25:81–89. doi: 10.1007/s11011-010-9170-5. [DOI] [PubMed] [Google Scholar]
- 21.Sarma MK, Huda A, Nagarajan R, Hinkin CH, Wilson N, Gupta RK, et al. Multidimensional MR spectroscopy: towards a better understanding of hepatic encephalopathy. Metab Brain Dis. 2011;26:173–184. doi: 10.1007/s11011-011-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keiding S, Pavese N. Brain metabolism in patients with hepatic encephalopathy studied by PET and MR. Arch Biochem Biophys. 2013;536:131–142. doi: 10.1016/j.abb.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki T, Ohmoto K, Hirose T, Fujiki H. Chronic hyponatremia impairs memory in rats: effects of vasopressin antagonist tolvaptan. J Endocrinol. 2010;206:105–111. doi: 10.1677/JOE-10-0050. [DOI] [PubMed] [Google Scholar]
- 24.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71, e71–78. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 25.Rakoski MO, McCammon RJ, Piette JD, Iwashyna TJ, Marrero JA, Lok AS, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology. 2012;55:184–191. doi: 10.1002/hep.24616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano G, Roman E, Cordoba J, Torrens M, Poca M, Torras X, et al. Cognitive dysfunction in cirrhosis is associated with falls: a prospective study. Hepatology. 2012;55:1922–1930. doi: 10.1002/hep.25554. [DOI] [PubMed] [Google Scholar]
- 27.Sola E, Moreira R, Rodriguez E, Barreto R, Cela R, Morales M, et al. PLASMA COPEPTIN LEVELS ARE INCREASED IN CIRRHOSIS AND CORRELATE WITH HYPONATREMIA AND CIRCULATORY DYSFUNCTION (abstract). Hepatology. 2013;58:S229. [Google Scholar]
- 28. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022275s007lbl.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.