Abstract
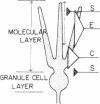
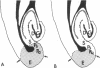
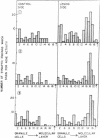
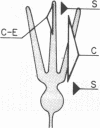
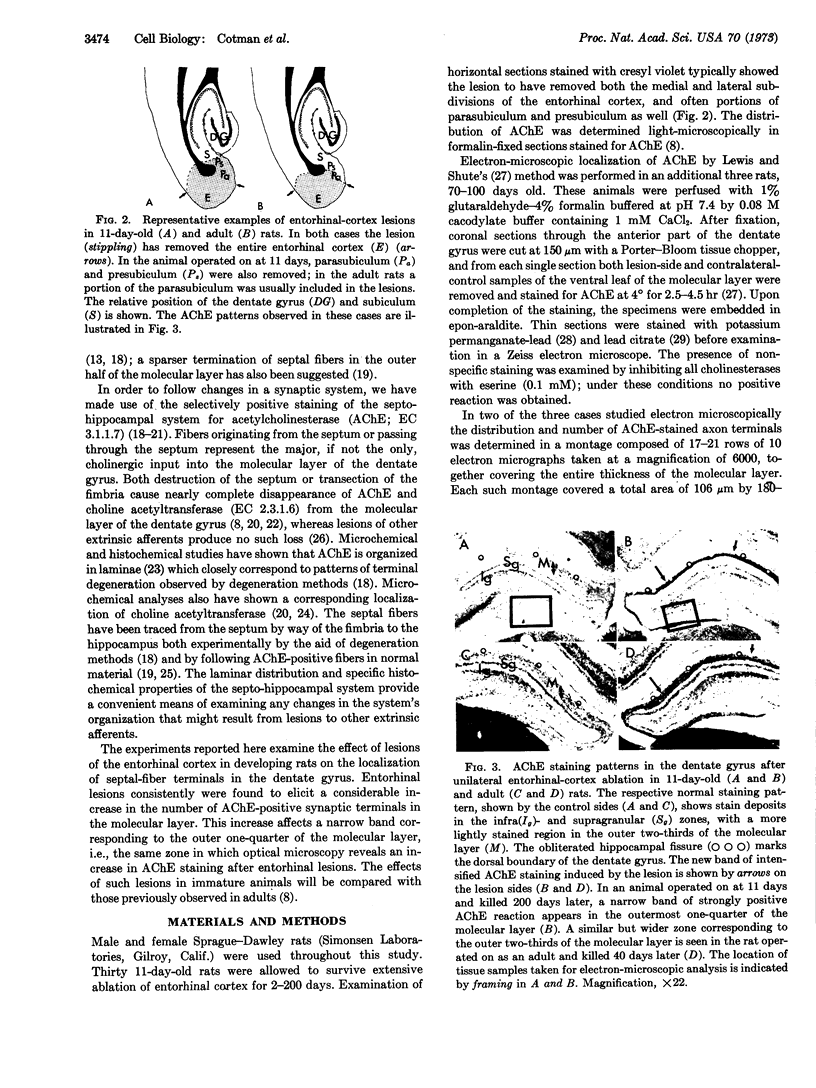
In immature animals, ablation of the entorhinal cortex elicited a rapid intensification of acetylcholinesterase (EC 3.1.1.7) staining in the outer one-quarter of the molecular layer of the dentate gyrus. Subsequent lesions of the septum eliminated this acetylcholinesterase intensification. Electron-microscopic histochemical analysis demonstrated a 30-fold increase in the number of acetylcholinesterase-positive synaptic endings in the intensification zone. The acetylcholinesterase augmentation thus appears attributable, in part at least, to an increase in the number of acetylcholinesterase-rich synaptic endings established by septo-hippocampal fibers. Observations in a comparative study of immature and adult rats point to the animal's developmental state as a major determinant of differences in these lesion-induced neuronal adjustments.
Keywords: synapse, acetylcholinesterase, septum
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKSTAD T. W. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956 Oct;105(3):417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Topographical and subcellular localization of choline acetyltransferase in rat hippocampal region. J Neurochem. 1970 Jul;17(7):1029–1037. doi: 10.1111/j.1471-4159.1970.tb02256.x. [DOI] [PubMed] [Google Scholar]
- Goodman D. C., Horel J. A. Sprouting of optic tract projections in the brain stem of the rat. J Comp Neurol. 1966 May;127(1):71–88. doi: 10.1002/cne.901270105. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Cowan W. M. Evidence for a temporal factor in the occupation of available synaptic sites during the development of the dentate gyrus. Brain Res. 1972 Jun 22;41(2):452–456. doi: 10.1016/0006-8993(72)90514-8. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Binocular competition in the control of geniculate cell growth. J Comp Neurol. 1972 Jan;144(1):117–129. doi: 10.1002/cne.901440106. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Experiments to determine whether retinogeniculate axons can form translaminar collateral sprouts in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1972 Nov;146(3):407–420. doi: 10.1002/cne.901460306. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A., Jeune B. Origin and termination of the hippocampal perforant path in the rat studied by silver impregnation. J Comp Neurol. 1972 Feb;144(2):215–232. doi: 10.1002/cne.901440206. [DOI] [PubMed] [Google Scholar]
- LIU C. N., CHAMBERS W. W. Intraspinal sprouting of dorsal root axons; development of new collaterals and preterminals following partial denervation of the spinal cord in the cat. AMA Arch Neurol Psychiatry. 1958 Jan;79(1):46–61. [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C., Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967 Jul;191(1):215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain. 1967 Sep;90(3):521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The distribution of cholinesterase in cholinergic neurons demonstrated with the electron microscope. J Cell Sci. 1966 Sep;1(3):381–390. doi: 10.1242/jcs.1.3.381. [DOI] [PubMed] [Google Scholar]
- Lund R. D., Lund J. S. Synaptic adjustment after deafferentation of the superior colliculus of the rat. Science. 1971 Feb 26;171(3973):804–807. doi: 10.1126/science.171.3973.804. [DOI] [PubMed] [Google Scholar]
- Lynch G. S., Mosko S., Parks T., Cotman C. W. Relocation and hyperdevelopment of the dentate gyrus commissural system after entorhinal lesions in immature rats. Brain Res. 1973 Feb 14;50(1):174–178. doi: 10.1016/0006-8993(73)90604-5. [DOI] [PubMed] [Google Scholar]
- Lynch G., Matthews D. A., Mosko S., Parks T., Cotman C. Induced acetylcholinesterase-rich layer in rat dentate gyrus following entorhinal lesions. Brain Res. 1972 Jul 20;42(2):311–318. doi: 10.1016/0006-8993(72)90533-1. [DOI] [PubMed] [Google Scholar]
- Lynch G., Stanfield B., Cotman C. W. Developmental differences in post-lesion axonal growth in the hippocampus. Brain Res. 1973 Sep 14;59:155–168. doi: 10.1016/0006-8993(73)90257-6. [DOI] [PubMed] [Google Scholar]
- MATHISEN J. S., BLACKSTAD T. W. CHOLINESTERASE IN THE HIPPOCAMPAL REGION. DISTRIBUTION AND RELATION TO ARCHITECTONICS AND AFFERENT SYSTEMS. Acta Anat (Basel) 1964;56:216–253. [PubMed] [Google Scholar]
- Moore R. Y., Björklund A., Stenevi U. Plastic changes in the adrenergic innervation of the rat septal area in response to denervation. Brain Res. 1971 Oct 8;33(1):13–35. doi: 10.1016/0006-8993(71)90303-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G. Neuronal plasticity in the septal nuclei of the adult rat. Brain Res. 1969 Jun;14(1):25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- Schneider G. E. Mechanisms of functional recovery following lesions of visual cortex or superior colliculus in neonate and adult hamsters. Brain Behav Evol. 1970;3(1):295–323. doi: 10.1159/000125479. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. Electron microscopy of cholinergic terminals and acetylcholinesterase-containing neurones in the hippocampal formation of the rat. Z Zellforsch Mikrosk Anat. 1966;69:334–343. doi: 10.1007/BF00406286. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Palay S. L. Altered axons and axon terminals in the lateral vestibular nucleus of the rat. Possible example of axonal remodeling. Lab Invest. 1971 Dec;25(6):653–671. [PubMed] [Google Scholar]
- Steward O., Cotman C. W., Lynch G. S. Re-establishment of electrophysiologically functional entorhinal cortical input to the dentate gyrus deafferented by ipsilateral entorhinal lesions: innervation by the contralateral entorhinal cortex. Exp Brain Res. 1973 Nov 29;18(4):396–414. doi: 10.1007/BF00239108. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Glutamate decarboxylase in the rat hippocampal region after lesions of the afferent fibre systems. Evidence that the enzyme is localized in intrinsic neurones. Brain Res. 1972 May 26;40(2):215–235. doi: 10.1016/0006-8993(72)90130-8. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Quantitative histochemistry of acetylcholinesterase in rat hippocampal region correlated to histochemical staining. J Neurochem. 1970 Jun;17(6):739–750. doi: 10.1111/j.1471-4159.1970.tb03344.x. [DOI] [PubMed] [Google Scholar]
- Zimmer J. Ipsilateral afferents to the commissural zone of the fascia dentata, demonstrated in decommissurated rats by silver impregnation. J Comp Neurol. 1971 Aug;142(4):393–416. doi: 10.1002/cne.901420402. [DOI] [PubMed] [Google Scholar]