Abstract
The supervisory attentional system has been proposed to mediate non-routine, goal-oriented behaviour by guiding the selection and maintenance of the goal-relevant task schema. Here, we aimed to delineate the brain regions that mediate these high-level control processes via neuroimaging meta-analysis. In particular, we investigated the core neural correlates of a wide range of tasks requiring supervisory control for the suppression of a routine action in favour of another, non-routine one. Our sample comprised n = 173 experiments employing go/no-go, stop-signal, Stroop or spatial interference tasks. Consistent convergence across all four paradigm classes was restricted to right anterior insula and inferior frontal junction, with anterior midcingulate cortex and pre-supplementary motor area being consistently involved in all but the go/no-go task. Taken together with lesion studies in patients, our findings suggest that the controlled activation and maintenance of adequate task schemata relies, across paradigms, on a right-dominant midcingulo-insular-inferior frontal core network. This also implies that the role of other prefrontal and parietal regions may be less domain-general than previously thought.
Keywords: supervisory attentional system, meta-analysis, go/no-go, stop signal, Stroop, spatial interference, fMRI, PET
1. Introduction
Flexible, adaptive behavior requires continuous balancing between the initiation and inhibition of actions, such as when a prepotent response has to be suppressed in favour of a contextually appropriate one. Cognitive control of action is particularly important in the presence of a changing environment or the up-dating of goals and intentions (cf. Boehler et al., 2010; Schachar et al., 2007; Miller and Cohen, 2001). Norman and Shallice (Norman, 1986) developed a theoretical framework for the implementation of goal-directed, non-routine behaviour against competing pre-dominant, routine responding. According to this framework, automatic or routine actions are based on the activation and implementation of a task schema that represents a learned sequence of input–output rules. Schemata can be activated by triggers, such as sensory input or the outcome of other schemata (Stuss et al., 1995). During well-learned routine behaviours, competition between schemata is controlled by lateral inhibition mechanisms, termed “contention scheduling.” However, the coordination of schemata with higher-level, overarching goals requires the additional employment of a “supervisory attentional system” (SAS), which exerts top-down control by deactivating certain schemata and activating others in the service of higher-order goals (cf. Alexander and Brown, 2010). The implementation of non-routine behaviour against predominant but inadequate response tendencies specifically relies on different sub-processes of the SAS that have been anatomically localized in the frontal cortex. In particular, lesion studies revealed a crucial role of the dorsomedial frontal cortex for energization, the process of initiating and sustaining the currently relevant task schema (cf. Stuss and Alexander, 2007). This sub-process would become necessary whenever a task schema needs to be activated that is not triggered automatically by perceptual and motivational input (cf. Shallice et al., 2008b). In contrast, patients with lesions in the left lateral prefrontal cortex (PFC) show deficits in task-setting, which sets the specific stimulus–response contingencies and is specifically required in the initial stages of learning a task (Shallice et al., 2008a; Shallice et al., 2008b). Right lateral PFC, on the other hand, has been associated with monitoring processes, such as continuously checking the appropriateness of the behavioural output (Stuss, 2006, 2011).
Frequently used tasks that require participants to suppress a predominant response in favour of an appropriate, context-dependent one comprise the Stroop, flanker, Simon, stimulus– response compatibility (SRC), and antisaccade tasks as well as stop-signal and go/no-go tasks (cf. Diamond, 2013; Nee et al., 2007; Sebastian et al., 2013). All these tasks have very often been conceptualized as paradigms that tax inhibitory action control. Poor performance in these tasks has hence been commonly explained as a prefrontally mediated deficit in inhibiting the inappropriate response. However, recent evidence points to a more general role of the PFC in these tasks, being crucial for the active maintenance of task goals as well as the activation of the appropriate behavioural alternative (Everling and Johnston, 2013; Munakata et al., 2011).
In the present study, we aimed at isolating and functionally characterizing brain regions that are essential for the coordination between the inhibition of a predominant, inappropriate response and the activation of the goal-dependent one. We used coordinate-based activation likelihood estimation (ALE) meta-analyses (Eickhoff et al., 2012; Eickhoff et al., 2009; Turkeltaub et al., 2002; Turkeltaub et al., 2012) to integrate results from a diverse range of neuroimaging studies investigating the stop-signal, go/no-go, Stroop, flanker, SRC, antisaccade, and Simon tasks. All of these paradigms require cognitive control over a predominant response tendency and the context-dependent initiation of an appropriate behavioural alternative, that is, either to initiate an alternative, non-dominant response or not respond at all.
In go/no-go and stop-signal tasks, an increased automatic tendency to initiate a particular motor response is induced through a higher frequency of go trials, as compared with inhibition (i.e. no-go or stop) trials. The resulting action bias then has to be suppressed when presented with the inhibition signal during stop or no-go trials, respectively. While in the go/no-go task participants have to withhold a prepotent but not-yet initiated motor response, the stop-signal task requires cancelling an already initiated motor response (cf. Eagle et al., 2008; Schachar et al., 2007). In the other tasks, which can be subsumed under the term “incongruency tasks”, a given stimulus dimension interferes with relevant stimulus and/or response information, thereby affecting responses to the relevant information. According to the dimensional overlap model (Kornblum, 1990, 2002), overlap between a (irrelevant) stimulus dimension and the response dimension results in an automatic translation of the stimulus feature into a response code. During congruent trials, the automatically activated response and the required one are one and the same. In contrast, during incongruent trials, the required response differs from the automatically activated one, thereby leading to an incongruency effect reflected in increased reaction times and error rates. Interestingly, it has been shown that the use of spatial as opposed to non-spatial information may lead to larger (in-)congruency effects in the context of some tasks. For example, Zeischka et al. (2010) investigated the congruency effect in different versions of the flanker task and found increased congruency effects when using arrows as stimuli, as compared to letters or colours. One possible explanation for this finding may be that the use of spatial information produces a simultaneous shift in both (perceptual) spatial attention and (motor) response activation on the ipsilateral side (Cieslik et al., 2010; Notebaert et al., 2001; Stoffer and Yakin, 1994).
Summarizing, we investigated four subcategories of cognitive action control. Action withholding was assessed with the go/no-go task that requires participants to withhold a prepotent but not yet initiated motor response. In contrast, the stop signal task investigates inhibition of an already initiated motor response, which can hence be conceptualized as action cancellation (cf. Eagle et al., 2008; Schachar et al., 2007). Interference control, finally, was investigated by means of congruency tasks that require participants to solve interference between competing response plans, by inhibiting the prepotent response and concurrently initiating the context-appropriate one. The latter were further subdivided into (non-spatial) Stroop versus spatial interference tasks (comprising Simon, SRC, antisaccade and spatial flanker tasks).
In a first step, we tested which brain regions are consistently associated with the four paradigm classes, that is, go/no-go, stop-signal, Stroop and spatial interference tasks. In a second step, we aimed to reveal those regions that are consistently activated whenever the task context requires inhibiting the predominant response and concurrently activating the appropriate task goal for initiating the adequate behaviour. We therefore performed a conjunction analysis across the thresholded ALE maps of all four task types. As all these four paradigm classes require (i) the suppression of actions that are inappropriate in a given context (ii) and the concurrent initiation of the context-appropriate behaviour, this approach should reveal those regions that are critical for the regulatory processes mediated by the SAS.
2. Methods
2.1 Paradigms included
For our meta-analysis, neuroimaging results on the neural correlates of seven different tasks investigating cognitive control of actions were included, namely the Stroop, flanker, Simon, SRC, antisaccade, go/no-go and stop-signal tasks.
Stroop task
In the (standard) colour-word Stroop task (Stroop, 1935), participants are required to suppress a prepotent response, the reading of a word, in favour of a less dominant one, the naming of the ink colour in which the word is written. There are three possible types of trials: (i) congruent trials, in which the colour of the word matches the word’s referent (e.g., the word “red” printed in red); (ii) incongruent trials, in which ink colour and word differ (e.g., the word “blue” printed in red); and (iii) neutral trials, in which the colour of the word and the word’s referent do not provide competing responses (e.g., a series of “X”s printed in red). Other, less common variants such as the numerical Stroop task, where participants have to identify which of two presented numbers is written in a higher font size while ignoring the numerical size, were included as well. In contrast, emotional versions of the Stroop task were not included as there is evidence from behavioural and neuroimaging studies that interference arising from emotional distractors may rely on different processes and neural correlates than interference from non-emotional ones (e.g. Egner et al., 2008; Frings et al., 2010; McKenna and Sharma, 2004).
Flanker task
In the flanker task (Eriksen, 1974), participants are asked to identify and respond to a central target stimulus, such as a letter or an arrow, and to ignore similar distractor stimuli flanking the target. Participants show longer reaction times when the flankers are associated with a different response than the target stimulus (incompatible trial), as compared with trials in which the flankers and the target are associated with the same response (compatible trials) (Eriksen, 1974; Miller, 1991).
Simon task
In the Simon task (Simon, 1990), participants have to respond to a non-spatial stimulus dimension according to an arbitrary rule while ignoring the spatial dimension of the stimulus. Typically, variously coloured stimuli are matched with different responses (say, blue: left-hand response, yellow: right-hand response). When the blue stimulus is presented in the right hemifield (incongruent condition), the irrelevant (but, due to overlearned spatial S-R associations, still influential) spatial placement of the stimulus automatically activates right-hand response codes and interferes with the actually required (i.e., left-hand) response. This, in turn, leads to increased reaction times and error rates, as compared with a spatially congruent condition.
Antisaccade task
In the antisaccade task (Hallett, 1978), participants are instructed to fixate a central position and, after the presentation of a lateralized target stimulus, to perform a saccadic eye-movement (antisaccade) to its mirror-symmetrical position. Participants usually show increased reaction times and error rate when instructed to perform an antisaccade compared to a prosaccade (eye-movement to the lateralized target).
SRC task
Here, participants are required to respond to a stimulus according to its spatial dimension, with the spatial S-R mapping being either compatible or incompatible. A typical example for a congruent mapping would be a left-sided motor response to a left-pointing arrow or a left-lateralized stimulus, while in the incongruent condition, participants would have to respond to these stimuli with their right hand. Incongruent S-R mappings lead to an increase in reaction time and error rate (Fitts and Seeger, 1953).
Go/no-go task
The traditional go/no-go paradigm usually involves two different stimuli, a “go” and a “no-go” stimulus, that are presented in a random sequence. Participants are instructed to rapidly respond to go stimuli, while withholding the response to no-go stimuli. The prepotency towards responding, rather than withholding, is usually achieved by having more go than no-go stimuli in a given block of trials.
Stop-signal task
Here, participants are required to make speeded choice reactions to different target stimuli such as letters. On some trials, a second (e.g. auditory) stimulus is presented shortly after the target, instructing participants to cancel their (often already initiated) response (Logan, 1997).
2.2. Selection criteria for the experiments included in the meta-analyses
Relevant neuroimaging experiments for the meta-analyses were obtained from literature search in PubMed (www.pubmed.com) as well as reference tracing in these retrieved papers and in review articles. Only results from whole-brain group analyses reported as coordinates in a standard reference space (Talairach/Tournoux or Montreal Neurological Institute (MNI)) were included, while results of region-of-interest (ROI) analyses were excluded. Furthermore, only data from healthy adults were included, while data from children and patients were excluded. When patient studies separately reported results from a healthy control group, the results from the healthy group were included. Data from conditions with pharmacological or non-invasive manipulations such as Transcranial Magnetic Stimulation were excluded even if they reported a control group receiving only a placebo condition due to possible anticipation effects that might influence behavioural output as well as underlying neural responses. Moreover, single-subject reports as well as experiments investigating between-group effects pertaining, for example, to handedness, sex, or genotype were excluded. In the present meta-analyses, only contrasts reporting neural correlates of increased demands on cognitive action control contrasted with an “active” control condition were included. Experimental effects compared with a resting baseline and reverse contrasts (i.e. “deactivations”), which are much less consistently reported in the literature, were not considered. Consequently, in the stop-signal and go/no-go task, the critical contrast was inhibition vs. go. However, some go/no-go experiments did not model go trials as separate regressors but included them in the baseline; these experiments were also included as we considered the “baseline” in these experiments an active control condition. In all other tasks, the critical contrast was incongruent vs. congruent, with the Stroop, Simon and flanker tasks also including some experiments contrasting incongruent with neutral conditions (see table S1).
Based on these criteria, 62 experiments investigating the Stroop task, 32 experiments investigating the go/no-go task, 24 experiments investigating the stop signal task, 19 experiments investigating the flanker task (with 11 of these using arrows as stimuli), 15 experiments investigating the Simon task, 11 experiments investigating the SRC task, and 10 experiments investigating the antisaccade task were identified as eligible for inclusion in the meta-analysis. The Simon, flanker (only those 11 using arrows as stimuli), SRC and antisaccade experiments were subsumed under the category “spatial interference task,” given the relatively low number of available experiments for these tasks and, in particular, their common focus on spatially based conflict (cf. Zeischka et al., 2010). To ensure that the results in this mixed group of spatial conflict tasks was not driven by any particular paradigm type, the number of experiments was matched by randomly selecting 10 experiments of every paradigm class. Differences in coordinate space (MNI vs. Talairach space) were accounted for by transforming coordinates reported in Talairach space into MNI coordinates using a linear transformation (Lancaster et al., 2007).
In summary, we investigated four different instances of cognitive action control: 1) action withholding as assessed by the go/no-go task (n = 32), 2) action cancellation as assessed by the stop signal task (n = 24), and 3) and 4) two forms of interference control, as assessed by the Stroop task (n = 62) and by spatial conflict task (n = 40), respectively. Furthermore, minimum conjunction analyses were performed as the key objective of the present study was to isolate those regions that consistently mediate the coordination between the inhibition of a predominant, inappropriate response and the activation of the goal-dependent, behavioural alternative across different task contexts.
2.3 Activation Likelihood Estimation algorithm
All meta-analyses were performed using the revised ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Eickhoff et al., 2012; Eickhoff et al., 2009; Laird et al., 2009a; Laird et al., 2009b; Turkeltaub et al., 2002) according to the standard procedures of our institute (cf. Langner and Eickhoff, 2013; Rottschy et al., 2012). This algorithm aims to identify areas showing a convergence of reported coordinates across experiments that is higher than expected under the assumption of random spatial associations. The key idea behind ALE is to treat the reported foci not as single points but rather as centres for 3-D Gaussian probability distributions capturing the spatial uncertainty associated with each focus. The width of these uncertainty functions was determined based on empirical data on the between-subject and between-template variance, which represent the main components of this uncertainty. Importantly, the applied algorithm weights the between-subject variance by the number of examined subjects per study, accommodating the notion that larger sample sizes should provide more reliable approximations of the ‘true’ activation effect and should therefore be modelled by ‘narrower’ Gaussian distributions (Eickhoff et al., 2009).
The probabilities of all foci reported in a given experiment were then combined for each voxel, resulting in a modelled activation (MA) map (Turkeltaub et al., 2012). Taking the union across these MA maps yielded voxel-wise ALE scores describing the convergence of results across experiments at each particular location of the brain. To distinguish ‘true’ convergence across studies from random convergence (i.e., noise), ALE scores were compared to an empirical null-distribution reflecting a random spatial association between experiments. Hereby, a random-effects inference is invoked, focussing on inference on the above-chance convergence across studies, not clustering of foci within a particular study. Computationally, deriving this null-hypothesis involved sampling a voxel at random from each of the MA maps and taking the union of these values in the same manner as done for the (spatially contingent) voxels in the true analysis, a process which can be solved analytically (Eickhoff et al., 2012). The p-value of the “true” ALE was then given by the proportion of equal or higher values obtained under the null-distribution. The resulting non-parametric p-values for each meta-analysis were then thresholded at a cluster-level corrected threshold of p < 0.05 (cluster-forming threshold at voxel level: p < 0.001, uncorrected) and transformed into z-scores for display. Conjunction analyses were performed by using the conservative minimum statistic (Nichols et al., 2005) to identify voxels where a significant effect was present in all separate analyses.
All results were anatomically labelled by reference to probabilistic cytoarchitectonic maps of the human brain using the SPM Anatomy Toolbox (Eickhoff et al., 2007; Eickhoff et al., 2005). Details on these cytoarchitectonic regions may be found in the following publications on Broca’s region (Amunts et al., 1999), inferior parietal cortex (Caspers et al., 2008; Caspers et al., 2006), superior parietal cortex and intraparietal sulcus (Choi et al., 2006; Scheperjans et al., 2008a; Scheperjans et al., 2008b), motor cortex (Geyer et al., 1996), and the thalamus (connectivity-based regions; Behrens et al., 2003).
3. Results
Meta-analysis of all included experiments
In a first step, we identified those brain regions that showed significant convergence across all 173 experiments. This main-effect revealed consistently increased activity in a bilateral frontoparietal network, consisting of anterior insula (aI) and adjacent inferior frontal gyrus (IFG), dorsolateral prefrontal cortex (DLPFC), dorsal premotor cortex (dPMC), as well as bilateral intraparietal sulcus (IPS) extending into superior parietal lobe (SPL). Moreover, convergent activity was found in the right temporoparietal junction (TPJ) and in the left inferior occipital gyrus as well as in the (pre)-supplementary motor area [(pre)SMA] extending into the anterior midcingulate cortex (aMCC). Subcortical activity was observed in the right thalamus and the right caudate nucleus (Figure 1, Table S2).
Figure 1.
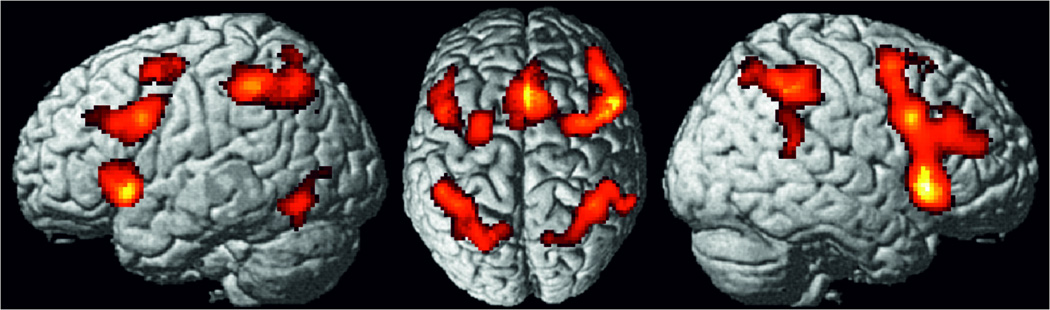
Foci of brain activity showing significant convergence of activity across all experiments included (cluster-level p<0.05, family-wise error-corrected for multiple comparisons, cluster forming threshold p<0.001 at voxel level).
Meta-analyses for the individual task types
Stroop task
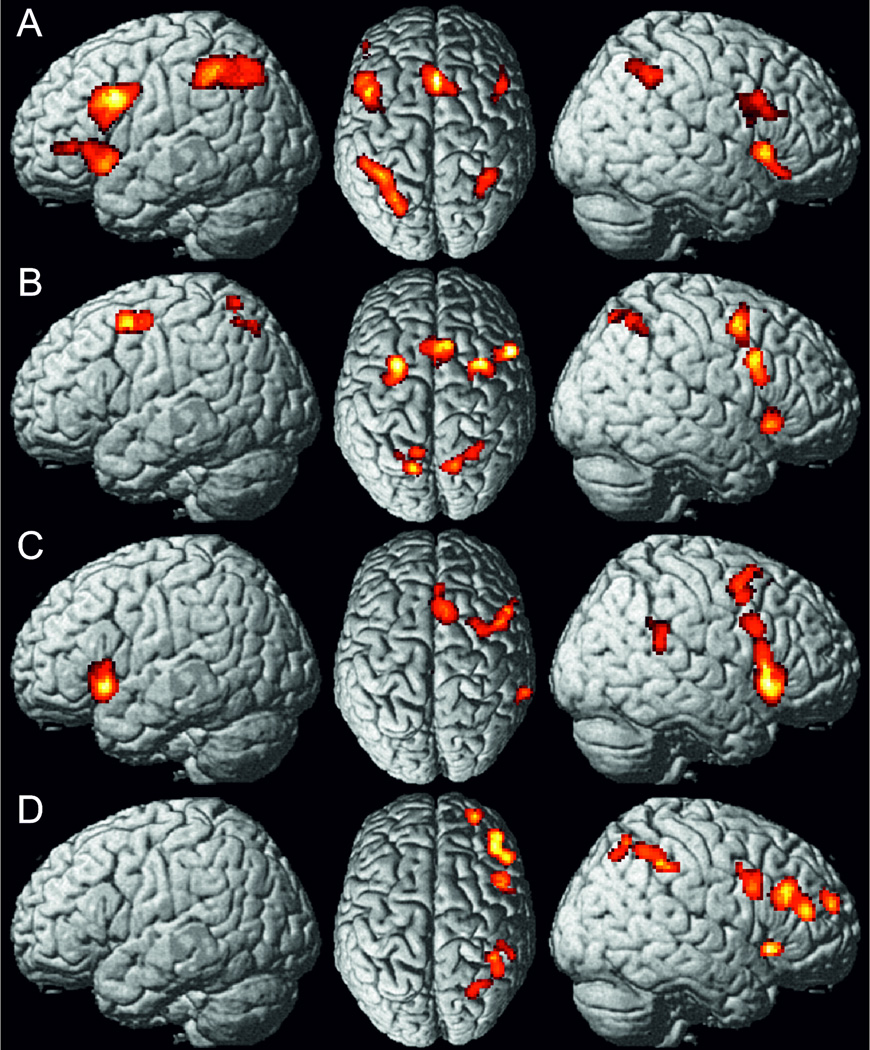
Investigating consistent activation across the experiments using the Stroop task revealed significantly convergent activity in a bilateral network consisting of aI extending into IFG (pars orbitalis/triangularis), the intersection between posterior IFG and the precentral gyrus, i.e., the inferior frontal junction (IFJ), as well as the intraparietal sulcus, with a more wide-spread activity in the left hemisphere that extended into the SPL and IPC. Moreover, significant convergence was found in the preSMA extending into aMCC (Fig. 2A, Table S3). Furthermore, we ran a supplementary meta-analysis that only included those 47 experiments investigating the verbal version, i.e. the colour-word Stroop task. Even though this supplementary meta-analysis revealed slightly smaller cluster of convergence, this additional analysis did not change the overall results, but moreover showed significant convergence in the left inferior occipital gyrus (MNI coordinates: −48 / −59 / −18) (Fig. S1).
Figure 2.
Foci of brain activity showing significant convergence of activity for the four paradigm classes separately, the (A) Stroop, (B) spatial interference, (C) stop-signal and the (D) go/no-go task, (cluster-level p<0.05, family-wise error-corrected for multiple comparisons, cluster forming threshold p<0.001 at voxel level).
Spatial interference task
Investigating consistent activation across the experiments using spatial interference tasks revealed significant convergence of activity in a bilateral dorsal frontoparietal network consisting of dPMC and the superior parietal cortices. Moreover, significant convergence of activity was found in the right IFJ and adjacent IFG (pars opercularis), right aI and the aMCC extending into preSMA (Figure 2B, Table S4).
Stop-Signal task
Performing a meta-analysis across experiments contrasting stop vs go trials in the stop-signal tasks revealed consistent activation in bilateral aI extending into IFG (pars opercularis), the aMCC, the preSMA and the posterior cingulate cortex (PCC). Moreover, right-lateralized activity was found in the IFJ, dPMC and TPJ. Subcortical activity was found in bilateral thalamus (Figure 2C, Table S5).
Go/no-go task
Performing a meta-analysis across experiments contrasting no-go vs go responses in the go/no-go task showed consistent activity in a right-lateralized frontal network consisting of the DLPFC, the IFJ as well as the aI extending into the pars orbitalis of IFG. Moreover, parietal activity was found in the IPS extending into adjacent IPC as well as the SPL (Fig. 2D, Table S6).
Conjunction analyses
To isolate brain activity specifically related to supervisory attentional control, independent of task specifics, we performed a minimum conjunction analysis across the thresholded ALE maps of all four paradigm classes (cf. above). This analysis revealed significant convergence in two clusters: the right aI and right inferior frontal junction (Figure 3, Table S7).
Figure 3.
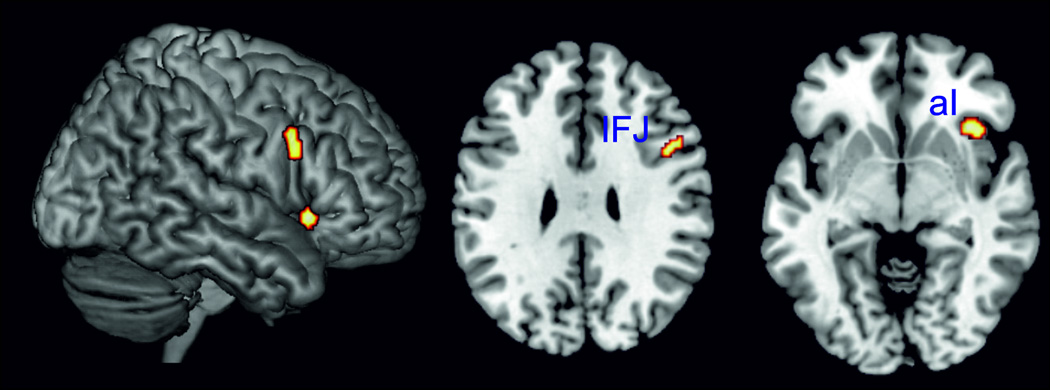
Performing a minimum conjunction analysis across the four paradigm classes (Stroop, spatial interference, stop-signal and go/no-go task) revealed the right anterior insula and the right inferior frontal junction to be the only two regions showing conjoint convergence of activity across all four task types.
Subsequently, conjunction analyses were performed across the thresholded maps for all combinations of three paradigm classes: (i) Stroop AND stop-signal AND spatial interference tasks, (ii) Stroop AND go/no-go AND spatial interference tasks, (iii) go/no-go AND stop-signal AND spatial interference tasks, and (iv) Stroop AND go/no-go AND stop-signal tasks. Thereby it was found that right aI, right IFJ as well as the anterior mid-cingulate cortex and the preSMA were consistently involved in the Stroop, spatial interference and stop signal tasks (Figure 4, Table S7). All other conjunction analyses did not reveal significant convergence of activity in any other region than the right aI and right IFJ, i.e., the full conjunction reported above (Table S7).
Figure 4.
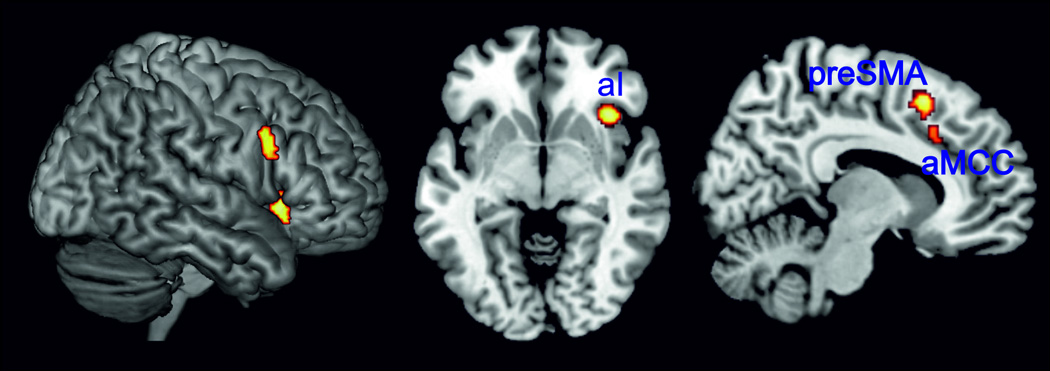
Right aI, right IFJ as well as anterior mid-cingulate cortex and preSMA were conjointly involved in the Stroop, spatial interference and stop-signal tasks as revealed by conjunction analysis.
Differences between the four task types were computed as well and are shown in the Supplementary Material (Figure S2) as the focus of the present meta-analyses was to delineate the core regions consistently recruited across facets of cognitive action control.
4. Discussion
We used coordinate-based ALE meta-analyses to analyze the neural correlates of cognitive action control in different paradigms that all require the suppression of an inappropriate action and the concurrent initiation and execution of the context-appropriate alternative. In total, 173 experiments investigating go/no-go, stop-signal, Stroop and spatial interference paradigms were included. While the main effect across all experiments, independent of task type, revealed a broad bilateral frontoparietal network, the right aI and the right IFJ were the only two regions that showed consistent involvement in all individual paradigm classes, as revealed by a minimum conjunction. Moreover, the aMCC and the preSMA were conjointly recruited by all but the go/no-go task. We hence suggest that the aI and IFJ in the right hemisphere and the aMCC/preSMA play a pivotal role for the regulatory processes mediated by the SAS, while other frontal and parietal regions are associated more specifically with subcomponents of cognitive action control that are differentially engaged in the respective paradigms.
4.1 Main effect of all included experiments
Performing a meta-analysis of all included experiments related to cognitive action control revealed consistent activity in a bilateral frontoparietal network consisting of the aI and adjacent IFG, the DLPFC, dPMC and the IPS extending into the SPL. Moreover, convergent activity was found in the right TPJ and in the left inferior occipital gyrus as well as the aMCC extending into (pre)SMA. Of note, the aMCC has often been labeled “dorsal anterior cingulate cortex” in previous studies according to Brodmann’s well-known nomenclature. However, as there is convincing evidence of a structurally and functionally distinct middle cingulate cortex (cf. Palomero-Gallagher et al., 2009; Vogt, 2004, 2005), we will here use the more precise term “aMCC” instead of the rather widely used but overly broad term “(dorsal) anterior cingulate cortex.” With the exception of the right temporoparietal junction, the present network closely resembles a network that has been found as the main effect of working memory in a recently published meta-analysis study (Rottschy et al., 2012). Another meta-analysis investigating sustained attention (Langner and Eickhoff, 2013) also found consistent involvement in a very similar network, which however showed less extended activity in the dPMC and IPS and was more lateralized to the right hemisphere. As inhibition, working memory as well as sustained and selective attention are regarded as core component factors underlying executive functions (Alvarez and Emory, 2006), this network most likely represents a set of cortical regions that interact with each other during executive control processes across a wide variety of tasks (cf. Müller et al., in press). Along the same lines, a very similar set of regions has been shown to be engaged across a diversity of cognitively demanding tasks and hypothesized to respond in a domain- and process-general manner (Cole and Schneider, 2007; Duncan, 2010; Duncan and Owen, 2000; Fedorenko et al., 2013).
4.2 Conjunction analyses
Conjunction analyses were performed to isolate those brain regions that are consistently associated with the regulatory processes proposed to be mediated by the supervisory attentional system (Norman, 1986; Stuss et al., 1995). All four paradigm classes required the implementation of a non-dominant, context-dependent behaviour against a competing behavioural alternative. Accurate performance in these tasks therefore requires performance monitoring as well as energization of the relevant, nondominant task schema. These processes have been proposed to be mediated by the SAS (Norman, 1986; Stuss et al., 1995), but to date its underlying neural correlates have mainly been investigated using lesion studies. These studies revealed a crucial role of the right lateral prefrontal cortex for monitoring of ongoing performance, with exact location of lesion sites varying from the right lateral to the inferior PFC. In contrast, energization has been strongly associated with the posterior dorsomedial frontal cortex around Brodmann area 24, 9 and 6 (Stuss, 2006, 2011). However, lesion studies have a drawback: the mapping of functions to circumscribed anatomical regions is rather vague. Our conjunction analysis now specifically revealed the right IFJ and the right aI to be the only two regions showing consistent activity across all four paradigm classes investigated. Moreover, all but the go/no-go task revealed consistent involvement of the aMCC and preSMA. Thus, the present results indicate that the aI and IFJ in the right hemisphere as well as the aMCC/preSMA play a central role in supervisory attentional control.
The insula is a functionally heterogeneous region, hypothesized to exert an integrative role between the homeostatic, affective and cognitive systems of the human brain (cf. Craig, 2010; Kurth et al., 2010; Medford and Critchley, 2010; Menon and Uddin, 2010). With respect to its cognitive functions, Dosenbach and colleagues (Dosenbach et al., 2007; Dosenbach et al., 2006) proposed that the aI together with the aMCC/preSMA forms a core network for the implementation and stable maintenance of task sets. This view was based on their finding that this network showed reliable start-cue and sustained activation across a diversity of tasks. Recent studies using different methods support the assumption that especially the right aI plays a critical monitoring role. For example, Sridharan et al. (2008) found that the right aI plays a causal role in activating task-relevant regions while concurrently deactivating other regions, such as the default mode network, that are extraneous to the current task. It has hence been assumed that the right aI acts as an “outflow hub” controlling activity in other brain regions across different tasks and stimulus modalities to initiate and adjust cognitive control mechanisms (cf. Dosenbach et al., 2006; Sridharan et al., 2008). In line with that, functional imaging studies revealed evidence for a specific relation between aI activity and task performance. In particular, an fMRI study of Wager et al. (2005) found a positive correlation of activity in the right aI with task performance across three different tasks that were also investigated within the present meta-analyses (i.e., flanker, SRC, and go/no-go tasks). Bunge et al. (2002) also showed a positive correlation of right aI activity with better task performance (i.e., less interference-related slowing of RTs) in a spatial flanker task. Summarizing, the aI may thus monitor the activation of the relevant task set and, if necessary, sent a control signal to other task-relevant regions, such as medial prefrontal cortex as well as the parietal cortex to enable correct task performance in a moment-to-moment manner (cf. Dosenbach et al., 2008; Holmes et al., 1998; Langner and Eickhoff, 2013; Power and Petersen, 2013).
The right IFJ was the second region showing significant convergence of activity across all four paradigm classes. This region is located at the junction of the inferior frontal sulcus and the inferior precentral sulcus (cf. Derrfuss et al., 2005) and has been implicated in task switching and set shifting paradigms but also in the Stroop or n-back task (Derrfuss et al., 2005; Derrfuss et al., 2004). It has hence been discussed to mediate processes concerning the updating of task representations and specification of general task goals (e.g. Brass et al., 2005a; Brass et al., 2005b; Derrfuss et al., 2005; Derrfuss et al., 2004; Kim et al., 2011a). While these studies have consistently shown involvement of particularly the left IFJ, the present meta-analysis revealed consistent involvement of the right IFJ across task types. A recent study by Zheng et al. (2011) found a region located very close to our right IFJ cluster whose activity showed a significant positive correlation with participants´ performance in two different tasks: it showed a positive correlation with the stop-signal reaction time in a stop-signal task and a negative correlation with the false alarm rate, which described the proportion of no-go trials on which participants failed to withhold their response in a go/no-go task.
Even though several other studies have shown activity within the right IFJ during the go/no-go, stop-signal or antisaccade tasks (Aron, 2011; Chikazoe et al., 2009a) as well, the right IFJ has mainly been associated with the detection of changes in stimulus features as well as the detection of infrequent but action-related events (Chikazoe et al., 2009a; Verbruggen et al., 2010). Although the probabilities of inhibition and go stimuli are often unequal in stop-signal and go/no-go tasks (i.e. inhibition trials are usually rarer), this does not necessarily hold for the interference paradigms. Hence, we argue that the right IFJ may not be selectively involved in the detection of infrequent events per se (see also Levy and Wagner, 2011). As the right IFJ was conjointly recruited across all tasks investigated, and participants were always required to integrate a bottom-up stimulus with a non-dominant motor response, we would rather suggest that the function of the right IFJ may go beyond simple detection. Interestingly, a recent meta-analysis investigating vigilant attention (Langner and Eickhoff, 2013) also found consistently stronger involvement in tasks with longer vigilant attention maintenance in the IFJ. Hence, it seems that the IFJ, especially in the right hemisphere, is associated with the continuous reactivating of the non-dominant but relevant S-R mapping against the automatic but inadequate action that has to be inhibited, such as responding to the word meaning in the Stroop task or responding to a left-sided stimulus with the left hand in SRC or Simon tasks (cf. Brass et al., 2005a; Derrfuss et al., 2005). We, therefore, propose that while the aI is associated with the monitoring and general implementation of the relevant task set, the right IFJ is more specifically associated with the continuous reactivation of the relevant task rule that links relevant stimulus features and non-dominant responses in a task-specific manner.
Beside the IFJ and aI, two other regions were rather consistently associated with inhibiting the predominant motor response and activating the context-dependent alternative: aMCC and preSMA were conjointly involved in all but the go/no-go task. Both of these regions have been found jointly involved in many tasks, which make a clear functional dissociation rather difficult. The aMCC has been proposed to mediate the interaction between motor intentions and motivational state (Paus, 2001). In line with this, a recent fMRI study revealed the aMCC to be the key region for volitional action control as this region showed increased activity for internal movement selection compared to reactive movements, with even higher activity when participants could not only choose which hand to use but could also choose the timing, i.e., when to perform the movement (Hoffstaedter et al., 2013). Moreover, the aMCC has been related to performance monitoring by detecting conflicts in information processing and – through interaction with other task-relevant regions – focusing attentional resources on task-relevant information and adapting behavioural plans according to task demands (for a review see (Botvinick et al., 2004)). The adjacent preSMA has been more strongly associated with executive control of motor output, in particular response inhibition as well as selecting the appropriate response between different response alternatives (cf. Barber et al., 2013; Mostofsky and Simmonds, 2008; Nachev et al., 2008). Electrophysiological recordings in non-human primates also demonstrated a specific involvement of the preSMA in switching from an automatic to a controlled response (Isoda and Hikosaka, 2007). Moreover, tracing studies revealed strong anatomical connectivity with anterior premotor areas, area 24c in the cingulum, and the prefrontal cortex (Luppino et al., 1993) supporting the hypothesis that the preSMA is involved in higher cognitive control of motor behaviour. We therefore propose that differences between the aMCC and preSMA are most likely related to the conceptual level of cognitive control: while the aMCC is related to the detection of conflicting response plans on a higher cognitive level such as discerning competing response plans, the preSMA is more strongly associated with monitoring actual motor output by selecting the appropriate motor output (Nachev et al., 2008). The role of the aMCC/preSMA would then correspond to what Stuss and colleagues conceptualized as energization of the appropriate task schema (Stuss et al., 1995). That is, whenever stimuli or the motivational conditions are not optimal for responding, energization of lower-level systems by the posterior dorsomedial frontal cortex is needed (cf. Shallice et al., 2008). This process would come into play whenever the non-dominant task schema, such as responding incongruently in the Stroop task or initiating the stopping of an already started motor response in the stop signal task, needs to be “energized”.
Importantly, we did not find the left lateral PFC to be consistently involved across all four task types despite strong evidence from lesion studies that this region also plays a crucial role for supervisory attentional control, in particular for the sub-process of task-setting (e.g. Shallice et al., 2008a; Shallice et al., 2008b; Stuss and Alexander, 2007). More specifically, it has been shown that patients suffering from left lateral PFC lesions show increased error rates particularly in the initial stages of learning a task (i.e., when new schemata need to be acquired), while performance becomes normal in later trials (Shallice et al., 2008a; Shallice et al., 2008b). In line with that, an fMRI study by Hartstra et al. (2011) found the left lateral PFC including the IFJ to be specifically activated for the implementation of new compared to multiple applied task instructions. Similarly, Ruge and Wolfensteller (2010) found a practice-related activation decrease in the IFJ, with increased BOLD activity present in the first trials of newly instructed S-R mappings and a BOLD signal decrease to above baseline with increasing practice. These findings have been interpreted as showing a specific role of the left lateral PFC in task-setting, that is, setting the specific stimulus–response contingencies that will automatically control behaviour when the tasks becomes routine. In line with this notion, activity within the left lateral PFC, particularly the left IFJ, has also consistently been shown during various forms of task switching where participants have to constantly update and switch the actual relevant task set (Brass and Cramon, 2004; Derrfuss et al., 2005; Kim et al., 2012). As the neuroimaging studies included in our meta-analyses did not examine differences in BOLD fluctuations between the beginning of the a block (i.e. the learning phase) and the phase when participants perform the task in a more automated fashion, this might have driven our finding of no consistent involvement of left lateral PFC across all task types investigated. Interestingly, the Stroop task was the only task that revealed consistent involvement of left lateral PFC including IFJ in the single-task analysis. This is in line with a previous meta-analysis that found consistent involvement of left IFJ in the Stroop task (Derrfuss et al., 2005). A possible reason for this bilateral finding for the Stroop task might be the verbal character of the task, which may lead to stronger recruitment of left-sided prefrontal regions. Another explanation comes from behavioral and neuroimaging studies showing that in the Stroop task two types of conflict play a role: (i) informational (or response) conflict between the contradictory information that arises from the word meaning and the information that arises from the word colour, and (ii) task conflict, i.e. a conflict between the relevant identification of the colour naming task and the irrelevant but automatic reading task (Goldfarb and Henik, 2007; Kalanthroff et al., 2013). As such, the Stroop task requires some form of task-setting or, more particularly, switching between two task sets throughout the task, leading to consistent recruitment of left lateral PFC in this task type.
In summary, we showed that the right aI, IFJ and aMCC/preSMA are consistently found in tasks requiring cognitive control over a predominant response tendency. While previous studies have commonly proposed the PFC to exert specific inhibitory control over other brain regions, we argue that these core regions implement inhibitory control rather indirectly by monitoring the relevant task set and enhancing goal-relevant processing whenever the predominant task schema does not agree with the superordinate behavioural goal (see also Munakata et al., 2011). This interpretation also has implications for patients with frontal lobe lesions or psychiatric disorders such as schizophrenia or attention deficit hyperactivity disorder, where abnormal task performance has commonly been interpreted as a deficit in inhibitory action control (Aron et al., 2003; Broerse et al., 2001; Depue et al., 2010; Rubia et al., 2010). However, it seems that rather than being caused by a failure of directed global inhibition, response errors in these patients may be more strongly related to a failure to monitor and implement the relevant task set (see also Everling and Johnston, 2013; Langner et al., 2014; Munakata et al., 2011).
4.3 Individual task analyses
In addition to the consistently recruited regions discussed above (i.e., right aI, right IFJ, and aMCC/preSMA), several other regions were selectively recruited by distinct paradigm classes. These paradigm-specific foci will be discussed in the following.
4.3.1 Stroop task
For the Stroop task, significant convergence of activity was found bilaterally in aI/IFG, dorsal-posterior IFG, parietal cortex as well as the aMCC/preSMA (Fig. 2A).
Interestingly, a strongly bilateral network was found for the Stroop task, despite the task’s verbal character. It has to be noted that in the present meta-analysis we not only included colour-word Stroop experiments but also slightly different versions such as the numerical Stroop, which may have driven the more bilateral activity. We therefore ran a supplementary meta-analysis across Stroop tasks only including those 47 experiments investigating the classic colour-word version. As can be seen in Figure S1, even though activations were less extended, this analysis did not change the overall results. That is, even when the analysis was restricted to colour-word Stroop tasks, we found a largely symmetric bilateral network. We therefore suggest that performance in the Stroop task relies on a bilateral network, in particular within the prefrontal and parietal cortices.
While most studies have emphasized the role of the frontal cortex and aMCC in the Stroop task as substrates of conflict processing and cognitive control during incongruent responding (cf. Derrfuss et al., 2005; Laird et al., 2005), we also found consistent involvement of parietal regions. Moreover, when only including the colour-word version of the Stroop task, additional convergence of activity was found in the left inferior occipital gyrus. In particular, convergence of activity in the left inferior occipital cortex was located very closely to the visual word form area (McCandliss et al., 2003) that has consistently been related to the perceptual processing of visual letter stimuli (James et al., 2005; Vinckier et al., 2007). We therefore propose that the left inferior occipital cortex is most likely related to a (top-down modulated) increase in the sensory analysis of the stimulus when word meaning and word colour are incongruent.
Parietal foci were located in bilateral medial IPS, in particular area hIP3 (Scheperjans et al., 2008a; Scheperjans et al., 2008b). The medial part of the IPS has been discussed to play a critical role in the selection between competing stimuli as well as different stimulus features (Gillebert et al., 2013a; Gillebert et al., 2013b; Molenberghs et al., 2007; Vandenberghe et al., 2005). In the Stroop task, the automatic tendency to read the word interferes with the task instruction to name the colour of the word. Convergence of activity for the Stroop task in bilateral IPS would thus most likely be related to the selection of the relevant stimulus features when participants are required to focus their attention on the colour of the word while inhibiting the automatic reading. In turn, the IPS may then represent the source of top-down (attentional) signals modulating the processing in earlier visual cortices, i.e., the aforementioned occipital effect.
4.3.2 Spatial interference tasks
In the spatial interference tasks, convergence of activity was found in a bilateral frontoparietal network consisting of the dPMC and SPL as well as the right IFJ and adjacent IFG, right aI, and aMCC/preSMA.
The parietal lobe (around the IPS and SPL) and the dorsal frontal cortex along the precentral sulcus have been proposed to form a dorsal attention network (Corbetta et al., 2008; Corbetta and Shulman, 2002). Increased activity in this network has been shown during spatially incompatible responding (Cieslik et al., 2010; Schumacher et al., 2003) and in relation to attentional reorienting and increased demands of S-R mapping and redirecting of response intentions (e.g. Mars et al., 2007; Rushworth et al., 2003; Sylvester et al., 2003). All paradigms subsumed under the label “spatial interference tasks” in the present study require resolution of spatial conflicts for successful responding. In all these paradigms, an automatic reorientation of attention and response preparation in the direction of the spatial stimulus dimension is induced. Participants then need to reorient their focus of attention to perform adequately and initiate a response to a non-dominant spatial location. Consistent involvement of the dorsal attention network across these tasks should thus reflect an increased need for controlled spatial processing when participants intentionally reorient attention as well as response intentions to adequately respond in a spatially incongruent manner.
Interestingly, the present meta-analysis revealed consistent involvement of area 7A (Scheperjans et al., 2008a; Scheperjans et al., 2008b) in the SPL. This in in line with previous fMRI studies that revealed a crucial role for the SPL in shifting the locus of spatial attention (Capotosto et al., 2013; Vandenberghe and Gillebert, 2013; Vandenberghe et al., 2001) but less involvement of the SPL when stimulus features change without spatial shift (Molenberghs et al., 2007). The present study moreover showed a differential involvement of posterior parietal cortex in the Stroop and spatial interference tasks (Figure S2C). In particular, the medial IPS showed stronger convergence for the Stroop task, while the SPL (area 7A) showed stronger convergence for spatial interference tasks. This finding may well be related to functional imaging studies showing a differentiation within the parietal cortex for attentional shifts. Thereafter, the middle IPS has been associated with the selection between competing stimuli (Gillebert et al., 2013a; Gillebert et al., 2013b; Vandenberghe et al., 2005), and feature attention shifts (Molenberghs et al., 2007). In contrast, the SPL is specifically activated during spatial shifts of attention such as when a spatial displacement occurs in the location of the target or the focus of attention (Capotosto et al., 2013; Molenberghs et al., 2007; Vandenberghe et al., 2001) but less strongly so when stimulus features change without spatial shifts (Molenberghs et al., 2007). Hence, differential parietal involvement when comparing the Stroop and spatial interference tasks in the present meta-analysis might reflect differences in attentional shifting demands, with spatial attention shifts required in spatial interference tasks and feature attention shifts required in the Stroop task.
4.3.3 Stop-signal task
In the stop signal task convergent increased activity for motor response inhibition versus responding was found in the bilateral aI extending into posterior IFG on the right side as well as the right IFJ, dPMC and TPJ. Moreover, the aMCC, preSMA/SMA, posterior cingulate cortex, as well as bilateral thalamus showed significant convergence of activity (Fig. 2C). Interestingly, the right TPJ, posterior cingulate cortex and thalamus were only found for the stop signal tasks but were not consistently associated with any other task type. Conceptually, stop signal tasks differ from the other task types in that they require inhibiting or, rather, cancelling an already initiated motor response, while the go/no-go, Stroop and spatial interference tasks require suppression of a dominant but not yet initiated action. Successful inhibitory control in stop signal tasks requires attentional capture and evaluation of the relevant stop cue and inhibition of the (already initiated) motor response (Logan, 1997). The right TPJ – together with the right frontal cortex – has been suggested to form a ventral attention network that is associated with the (re)direction of attention to behaviorally relevant stimuli outside the current focus of attention (Corbetta et al., 2008; Corbetta and Shulman, 2002; Fox et al., 2006). A recent review, however, proposed a more global role of the right TPJ based on evidence that this area is related to the integration of stimulus information with internal models of task performance and expectations, thereby playing a pivotal role in contextual updating (Geng and Vossel, 2013). In line with this view, the right TPJ has been associated with predictive motor coding, including the updating of action expectations and the comparison of pre-prepared programs with current task requirements (cf. Eickhoff et al., 2011b; Jakobs et al., 2009) as well as updating of temporal predictions of the onset of the next stimulus and its associated response (Langner et al., 2012). With respect to the stop signal task, consistent involvement of the right TPJ would thus most likely be associated with the updating of the context and its associated response when the stop signal is “captured” in the attentional focus, indicating the need to inhibit the already initiated motor response.
In addition, the two other regions that were consistently recruited only by the stop signal tasks, the dorsal PCC and thalamus, have recently been shown to be part of a network specifically associated with the cancellation of already initiated actions (Dambacher et al., 2014). Even though a recent meta-analysis (Swick et al., 2011) also showed consistent involvement of the PCC in the stop signal task, and other authors found stronger involvement of this region in successful compared to failed inhibition (Li et al., 2006), the functional role of this region in the context of response inhibition has rarely been discussed. Rather, the PCC is best known for its central role within the default mode network, a set of brain regions showing strongly correlated activity during the resting state and consistent deactivation during divergent cognitive control tasks (Buckner et al., 2008; Buckner and Carroll, 2007; Raichle et al., 2001; Schilbach et al., 2012). Anatomical evidence and network analyses, however, support the assumption that the PCC is not a homogenous region but can be divided into a dorsal and ventral subregion that differ in their cytoarchitectonic organization as well as their functional properties and network dynamics (Leech et al., 2011; Leech and Sharp, 2014; Vogt et al., 2006). While both subregions show resting state functional connectivity (RS-FC) with the default mode network, the dorsal PCC moreover reveals RS-FC with regions strongly related to cognitive control (Leech et al., 2011). Furthermore, the dorsal PCC has been proposed to play a key role in maintaining a broad attentional focus thereby being able to alter behaviour in response to unexpected changes in the environment that are not part of the current cognitive set (cf. Leech and Sharp, 2014; Pearson et al., 2011). With regard to the present results, this interpretation would be in agreement with dorsal PCC involvement for successful inhibition in the stop signal task when a delayed stimulus in the environment has to be detected. Here it would mediate between internal and external attentional focus to successfully integrate the delayed stop stimulus with the current attentional focus and task set (Leech et al., 2011).
Additionally, the finding of consistent involvement of the thalamus in the stop signal task relates well to other studies showing that the thalamus increases activity during response inhibition (Aron and Poldrack, 2006; Aron et al., 2003; Bellgrove et al., 2004) and suggesting that it plays a key role for successful versus failed inhibition (Li et al., 2006). Moreover, the thalamus seems to be specifically involved in active action cancellation as a recent fMRI study found the thalamus to be more activated when cancelling actions in the stop signal task versus restraining of actions in the go/no-go task (Dambacher et al., 2014). In the present study, consistent activity was specifically found in that part of the thalamus which is anatomically connected with the prefrontal cortex as shown by diffusion data (Behrens et al., 2003), suggesting that this part of the thalamus together with the prefrontal cortex might form a cortical circuit related to the control of response inhibition and specifically comes into play when already initiated motor plans have to be cancelled (see also Aron, 2011; Dambacher et al., 2014).
4.3.4 Go/No-Go task
The go/no-go task was associated with consistent activity in a right-lateralized frontal network consisting of anterior and posterior DLPFC, IFJ, as well as aI extending into the IFG. Moreover, consistent parietal activity was found in the IPS extending into adjacent IPC and SPL (Fig. 2D).
Interestingly, the regions consistently involved in the go/no-go task differed to some extent from the results of the stop signal task even though these two tasks have been discussed very similarly in the literature (cf. Verbruggen and Logan, 2008). The present study, however, provides evidence for differential networks involved in withholding versus cancelling responses (Fig. S2D). In particular, action withholding is consistently linked to activity in the right anterior and posterior DLPFC as well as extended activity in right parietal cortex (covering IPS, SPL and IPC) (Figure 2D). To further characterize lateral prefrontal foci in the go/no-go task, anterior and posterior DLPFC cluster were compared with sub-regions in the dorsolateral frontal cortex recently identified using tractography-based parcellation by Sallet et al. (2013). While our posterior DLPFC cluster is located in cluster 6/Area9/46v as defined by Sallet et al., our anterior cluster was localized in their cluster 7/Area 46.
The (right) DLPFC has been associated with choosing responses based on rules (cf. Ridderinkhof et al., 2004) and the selection of items from working memory (Rowe and Passingham, 2001; Rowe et al., 2000). The DLPFC shows strong anatomical connections with the parietal lobes as well as motor system structures (Bates and Goldman-Rakic, 1993; Cavada and Goldman-Rakic, 1989; Lu et al., 1994; Petrides and Pandya, 1984, 1999; Schmahmann and Pandya, 1997), thereby lying in a perfect position to monitor behaviour by mediating which S-R association has to be implemented in a given context (Miller and Cohen, 2001). The present study reveals that two different parts of the DLPFC – an anterior part and a posterior one – show consistently stronger involvement in no-go compared to go conditions (Fig. 2D). Hierarchical models propose an anterior–posterior gradient in the PFC for executive control processes (Badre and D'Esposito, 2009; Koechlin and Summerfield, 2007), with more anterior parts of lateral PFC involved in the representation of more abstract schemata such as context and future tasks, while more posterior parts of lateral PFC are associated with the implementation of instructions and rules into actual motor output (for a review see (Courtney, 2004)). In line with that, we propose that the more anterior foci in the present study may be related to the representation of contextual information such as task instructions, while the more posterior foci may implement which S-R (or stimulus–stop) association needs to be assessed in the respective condition (cf. Simmonds et al., 2008). This would then be performed conjointly with the parietal cortex, which is assumed to integrate visuo-spatial, motor and memory information into S-R associations under the control of the DLPFC (Corbetta and Shulman, 2002; Gottlieb, 2007) and which showed consistent involvement in go/no-go tasks in the present meta-analysis as well as in previous ones (Simmonds et al., 2008; Swick et al., 2011).
Interestingly, the go/no-go task was the only task that did not reveal a consistent involvement of the aMCC and preSMA in cognitive action control. In contrast, two previous meta-analyses did find consistent recruitment of the preSMA for the go/no-go task (Simmonds et al., 2008; Swick et al., 2011). This discrepancy might be driven by different factors. For example, the two other meta-analyses included a smaller number of studies (11 and 21 experiments respectively) and used less conservative thresholds. More importantly, however, selection criteria differed between those studies and our study: as both previous meta-analyses also included experiments using “no-go vs. baseline” or “no-go vs. fixation” contrasts, respectively, while in the present meta-analysis only “no-go vs. go” contrasts were included. Hence, given that “go”-conditions also recruit the aMCC/preSMA, the probability to find consistent activity in aMCC/preSMA was much higher in the two previous studies. Our results reveal that the aMCC and preSMA are not consistently more strongly involved in no-go versus go responses. This might be explained by the fact that in the no-go condition participants have to withhold a motor response without initiating an alternative action. Integrating our results with those from the two previous meta-analyses (Simmonds et al., 2008; Swick et al., 2011) we suggest that response withholding indeed does consistently recruit the aMCC/preSMA, but not to a stronger degree than does the initiation of a go-response.
5. Conclusion
In this study, we investigated the neural substrates of cognitive action control via coordinate-based ALE meta-analyses of brain activity reported for Stroop, spatial interference, stop-signal, and go/no-go tasks. Our study provides evidence for a pivotal role of the right aI and right IFJ in supervisory attentional control, because these were the only two regions consistently involved in all four paradigm classes, as revealed by a minimum conjunction analysis. Furthermore, aMCC and preSMA were commonly recruited by all but the go/no-go task. In contrast to previous studies that proposed the PFC to exert specific inhibitory control over other brain regions, we argue that these core regions implement inhibitory control rather indirectly by monitoring the relevant task set and exciting goal-relevant processing areas whenever the predominant task schema does not agree with the superordinate behavioural goal. Following the conceptual model of Stuss, Shallice and colleagues (Stuss, 2011; Stuss et al., 1995), we would argue that the aI and IFJ in the right hemisphere support schema activation monitoring processes, with the aI playing a crucial role in monitoring and general implementation of the relevant task set, while the right IFJ is more specifically associated with the continuous reactivation of the relevant S-R rule. The aMCC/preSMA, on the other hand, is thought to mediate energization of the adequate (general) task schema.
Supplementary Material
Highlights.
We delineated the key regions mediating supervisory attentional control
Right anterior insula plays a central role in task set monitoring
Right inferior frontal junction continuously reactivates the relevant task rule
Posterior dorsomedial frontal cortex mediates energization of task schemata
Acknowledgements
We thank all contacted authors who contributed results of relevant contrasts not explicitly reported in the original publications, and we apologize to all authors whose eligible papers we might have missed.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/4-1; EI 816/6-1 and LA 3071/3-1.), the National Institute of Mental Health (R01-MH074457) and the European EFT program (Human Brain Project).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. J Neurosci. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Aichert DS, Williams SC, Moller HJ, Kumari V, Ettinger U. Functional neural correlates of psychometric schizotypy: an fMRI study of antisaccades. Psychophysiology. 2012;49:345–356. doi: 10.1111/j.1469-8986.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Topics in cognitive science. 2010;2:658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: cytoarchitecture and intersubject variability. The Journal of comparative neurology. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington’s disease. Brain : a journal of neurology. 2003;126:713–723. doi: 10.1093/brain/awg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neurosciece. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: a fMRI study. European archives of psychiatry and clinical neuroscience. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Jacobson BL, Webb A, Wszalek T, Cohen NJ, Kramer AF. Attentional selection and the processing of task-irrelevant information: insights from fMRI examinations of the Stroop task. Progress in brain research. 2001;134:459–470. doi: 10.1016/s0079-6123(01)34030-x. [DOI] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Effects of working memory demand on neural mechanisms of motor response selection and control. Journal of cognitive neuroscience. 2013;25:1235–1248. doi: 10.1162/jocn_a_00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry research. 2011;194:111–118. doi: 10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Basten U, Stelzel C, Fiebach CJ. Trait anxiety modulates the neural efficiency of inhibitory control. Journal of cognitive neuroscience. 2011;23:3132–3145. doi: 10.1162/jocn_a_00003. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. The Journal of comparative neurology. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Becker TM, Kerns JG, Macdonald AW, 3rd, Carter CS. Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a Stroop task. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2619–2625. doi: 10.1038/sj.npp.1301673. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, Dolan RJ. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31:907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain--conjunction analyses of the Stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends in cognitive sciences. 2005a;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, von Cramon DY. The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia. 2005b;43:89–98. doi: 10.1016/j.neuropsychologia.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task preparation with functional magnetic resonance imaging. Journal of cognitive neuroscience. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia. 2001;39:742–756. doi: 10.1016/s0028-3932(00)00155-x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends in cognitive sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Leung HC. Cortical activity during manual response inhibition guided by color and orientation cues. Brain research. 2009;1261:20–28. doi: 10.1016/j.brainres.2008.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Leung HC. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. Plos One. 2011;6:e20840. doi: 10.1371/journal.pone.0020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capotosto P, Tosoni A, Spadone S, Sestieri C, Perrucci MG, Romani GL, Della Penna S, Corbetta M. Anatomical segregation of visual selection mechanisms in human parietal cortex. J Neurosci. 2013;33:6225–6229. doi: 10.1523/JNEUROSCI.4983-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain structure & function. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. The Journal of comparative neurology. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009a;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci. 2009b;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. Journal of cognitive neuroscience. 2007;19:69–80. doi: 10.1162/jocn.2007.19.1.69. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. The Journal of comparative neurology. 2006;495:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen TA, Lockwood JL, Almryde KR, Plante E. Neural substrates of attentive listening assessed with a novel auditory Stroop task. Frontiers in human neuroscience. 2011;4:236. doi: 10.3389/fnhum.2010.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Kurth F, Eickhoff SB. Dissociating bottom-up and top-down processes in a manual stimulus-response compatibility task. Journal of neurophysiology. 2010;104:1472–1483. doi: 10.1152/jn.00261.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre EL, Filippi CG, Newhouse PA, Dumas JA. The Stroop effect in kana and kanji scripts in native Japanese speakers: an fMRI study. Brain and language. 2008;107:124–132. doi: 10.1016/j.bandl.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courtney SM. Attention and cognitive control as emergent properties of information representation in working memory. Cognitive, affective & behavioral neuroscience. 2004;4:501–516. doi: 10.3758/cabn.4.4.501. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain structure & function. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T. A network approach to response inhibition: dissociating functional connectivity of neural components involved in action restraint and action cancellation. The European journal of neuroscience. 2014;39:821–831. doi: 10.1111/ejn.12425. [DOI] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Erik Willcutt EG, Ruzic L, Banich MT. Inhibitory control of memory etrieval and motor procssing associated with the right lateral prefrontal cortex: evidence from deficits in individuals with ADHD. Neuopsychologia. 2010;48:3909–3917. doi: 10.1016/j.neuropsychologia.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive Functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doricchi F, Perani D, Incoccia C, Grassi F, Cappa SF, Bettinardi V, Galati G, Pizzamiglio L, Fazio F. Neural control of fast-regular saccades and antisaccades: an investigation using positron emission tomography. Experimental brain research. 1997;116:50–62. doi: 10.1007/pl00005744. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in cognitive sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HSC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in cognitive sciences. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology. 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distractors. Cerebral Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage. 2011a;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Pomjanski W, Jakobs O, Zilles K, Langner R. Neural correlates of developing and adapting behavioral biases in speeded choice reactions--an fMRI study on predictive motor coding. Cereb Cortex. 2011b;21:1178–1191. doi: 10.1093/cercor/bhq188. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a non-search task. Perception Psychophysics. 1974;16:143–149. [Google Scholar]
- Ettinger U, Williams SC, Patel D, Michel TM, Nwaigwe A, Caceres A, Mehta MA, Anilkumar AP, Kumari V. Effects of acute nicotine on brain function in healthy smokers and non-smokers: estimation of inter-individual response heterogeneity. Neuroimage. 2009;45:549–561. doi: 10.1016/j.neuroimage.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Everling S, Johnston K. Control of the superior colliculus by the lateral prefrontal cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368:20130068. doi: 10.1098/rstb.2013.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD. Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J Neurosci. 2007;27:2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain research. Cognitive brain research. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fauth-Buhler M, de Rover M, Rubia K, Garavan H, Abbott S, Clark L, Vollstadt-Klein S, Mann K, Schumann G, Robbins TW. Brain networks subserving fixed versus performance-adjusted delay stop trials in a stop signal task. Behav Brain Res. 2012;235:89–97. doi: 10.1016/j.bbr.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013;110:16616–16621. doi: 10.1073/pnas.1315235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts PM, Seeger CM. S-R compatibility: spatial characteristics of stimulus and response codes. Journal of experimental psychology. 1953;46:199–210. doi: 10.1037/h0062827. [DOI] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. Journal of neurophysiology. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, van den Wildenberg WP, Ridderinkhof KR. Neural mechanisms, temporal dynamics, and individual differences in interference control. J. Cogn. Neurosci. 2008;20:1854–1865. doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings C, Englert J, Wenture D, Bermeitinger C. Decomposing the emotional stroop effect. Q J Exp Psychol. 2010;63:42–49. doi: 10.1080/17470210903156594. [DOI] [PubMed] [Google Scholar]
- Fruhholz S, Godde B, Finke M, Herrmann M. Spatio-temporal brain dynamics in a combined stimulus-stimulus and stimulus-response conflict task. Neuroimage. 2011;54:622–634. doi: 10.1016/j.neuroimage.2010.07.071. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Ruff CC, Driver J. Saccades to a remembered location elicit spatially specific activation in human retinotopic visual cortex. Journal of cognitive neuroscience. 2009;21:230–245. doi: 10.1162/jocn.2008.21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Vossel S. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci Biobehav Rev. 2013;37:2608–2620. doi: 10.1016/j.neubiorev.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post RM. Regional brain activity when selecting a response despite interference: An H2 (15) O PET study of the stroop and an emotional stroop. Hum. Brain Mapp. 1994;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop) The Journal of neuropsychiatry and clinical neurosciences. 1997;9:55–63. doi: 10.1176/jnp.9.1.55. [DOI] [PubMed] [Google Scholar]
- Georgiou-Karistianis N, Akhlaghi H, Corben LA, Delatycki MB, Storey E, Bradshaw JL, Egan GF. Decreased functional brain activation in Friedreich ataxia using the Simon effect task. Brain Cogn. 2012;79:200–208. doi: 10.1016/j.bandc.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- Gillebert CR, Caspari N, Wagemans J, Peeters R, Dupont P, Vandenberghe R. Spatial stimulus configuration and attentional selection: extrastriate and superior parietal interactions. Cereb Cortex. 2013a;23:2840–2854. doi: 10.1093/cercor/bhs263. [DOI] [PubMed] [Google Scholar]
- Gillebert CR, Mantini D, Peeters R, Dupont P, Vandenberghe R. Cytoarchitectonic mapping of attentional selection and reorienting in parietal cortex. Neuroimage. 2013b;67:257–272. doi: 10.1016/j.neuroimage.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Goldfarb L, Henik A. Evidence for task conflict in the Stroop effect. J Exp Psychol Human. 2007;33:1170–1176. doi: 10.1037/0096-1523.33.5.1170. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Grandjean J, D’Ostilio K, Fias W, Phillips C, Balteau E, Degueldre C, Luxen A, Maquet P, Salmon E, Collette F. Exploration of the mechanisms underlying the ISPC effect: evidence from behavioral and neuroimaging data. Neuropsychologia. 2013;51:1040–1049. doi: 10.1016/j.neuropsychologia.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Grandjean J, D’Ostilio K, Phillips C, Balteau E, Degueldre C, Luxen A, Maquet P, Salmon E, Collette F. Modulation of brain activity during a Stroop inhibitory task by the kind of cognitive control required. Plos One. 2012;7:e41513. doi: 10.1371/journal.pone.0041513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hart SJ, Green SR, Casp M, Belger A. Emotional priming effects during Stroop task performance. Neuroimage. 2010;49:2662–2670. doi: 10.1016/j.neuroimage.2009.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstra E, Kuhn S, Verguts T, Brass M. The implementation of verbal instructions: an fMRI study. Hum Brain Mapp. 2011;32:1811–1824. doi: 10.1002/hbm.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JD. Material-dependent and material-independent selection processes in the frontal and parietal lobes: an event-related fMRI investigation of response competition. Neuropsychologia. 2003;41:1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. Journal of cognitive neuroscience. 2000;2(12 Suppl):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Hendrick OM, Ide JS, Luo X, Li CS. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. Plos One. 2010;5:e13155. doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Murphy K, Garavan H. Beyond common resources: the cortical basis for resolving task interference. Neuroimage. 2004a;23:202–212. doi: 10.1016/j.neuroimage.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: patterns of cortical activation and deactivation prior to response inhibition. Journal of cognitive neuroscience. 2004b;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Hirose S, Chikazoe J, Watanabe T, Jimura K, Kunimatsu A, Abe O, Ohtomo K, Miyashita Y, Konishi S. Efficiency of go/no-go task performance implemented in the left hemisphere. J Neurosci. 2012;32:9059–9065. doi: 10.1523/JNEUROSCI.0540-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. The "What" and "When" of Self-Initiated Movements. Cereb Cortex. 2013;23:520–530. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of computer assisted tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Fulham WR, Johnston PJ, Michie PT. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biological psychology. 2012;89:220–231. doi: 10.1016/j.biopsycho.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Mazziotta JC. Brain-behavior relationships: evidence from practice effects in spatial stimulus-response compatibility. Journal of neurophysiology. 1996;76:321–331. doi: 10.1152/jn.1996.76.1.321. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nature neuroscience. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, Eickhoff SB. Effects of timing and movement uncertainty implicate the temporo-parietal junction in the prediction of forthcoming motor actions. Neuroimage. 2009;47:667–677. doi: 10.1016/j.neuroimage.2009.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong AC, Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cognitive, affective & behavioral neuroscience. 2005;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophr Res. 2007;97:184–193. doi: 10.1016/j.schres.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Anton JL, Mazzola-Pomietto P. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar disorders. 2009b;11:530–538. doi: 10.1111/j.1399-5618.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Mazzola-Pomietto P. Reduced brain activation in euthymic bipolar patients during response inhibition: an event-related fMRI study. Psychiatry research. 2009a;173:45–51. doi: 10.1016/j.pscychresns.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Kalanthroff E, Goldfarb L, Usher M, Henik A. Stop interfering: Stroop task conflict independence from informational conflict and interference. Q J Exp Psychol. 2013;66:1356–1367. doi: 10.1080/17470218.2012.741606. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. The European journal of neuroscience. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Kenner NM, Mumford JA, Hommer RE, Skup M, Leibenluft E, Poldrack RA. Inhibitory motor control in response stopping and response switching. J Neurosci. 2010;30:8512–8518. doi: 10.1523/JNEUROSCI.1096-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am. J. Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kim C, Chung C, Kim J. Multiple cognitive control mechanisms associated with the nature of conflict. Neuroscience letters. 2010;476:156–160. doi: 10.1016/j.neulet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Cilles SE, Gold BT. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J Neurosci. 2011a;31:4771–4779. doi: 10.1523/JNEUROSCI.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Kroger JK, Kim J. A functional dissociation of conflict processing within anterior cingulate cortex. Hum Brain Mapp. 2011b;32:304–312. doi: 10.1002/hbm.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: A Meta-Analysis. Hum Brain Mapp. 2012a;33:130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Gold BT. Common and distinct neural mechanisms of attentional switching and response conflict. Brain research. 2012b;1469:92–102. doi: 10.1016/j.brainres.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Korb FM, Egner T. Priming of control: implicit contextual cuing of top-down attentional set. J Neurosci. 2012;32:8192–8200. doi: 10.1523/JNEUROSCI.0934-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends in cognitive sciences. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: Cognitive basis for stimulus-response compatibility - A Model and taxonomy. Psychological Review. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Stevens G. Sequential effects of dimensional overlap: findings and issues. In: Prinz W, Homme B, editors. Common mechanisms in perception and action. Attention and Performance XIX. Cambridge: MIT Press; 2002. pp. 9–54. [Google Scholar]
- Kronhaus DM, Lawrence NS, Williams AM, Frangou S, Brammer MJ, Williams SC, Andrew CM, Phillips ML. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar disorders. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain structure & function. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE Meta-Analysis Workflows Via the Brainmap Database: Progress Towards A Probabilistic Functional Brain Atlas. Frontiers in neuroinformatics. 2009a;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009b;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Cieslik EC, Behrwind SD, Roski C, Caspers S, Amunts K, Eickhoff SB. Aging and response conflict solution: behavioural and functional connectivity changes. Brain structure & function. 2014 doi: 10.1007/s00429-014-0758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychological bulletin. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Eickhoff SB, Boers F, Chatterjee A, Willmes K, Sturm W. Staying responsive to the world: Modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum Brain Mapp. 2012;33:398–418. doi: 10.1002/hbm.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Dolan RJ, Critchley HD. Controlling emotional expression: behavioral and neural correlates of nonimitative emotional responses. Cereb. Cortex. 2008;18:104–113. doi: 10.1093/cercor/bhm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cereb Cortex. 2006;16:827–834. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock RT. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. The Journal of comparative neurology. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Dale CL, Hough MG. Preparatory allocation of attention and adjustments in conflict processing. Neuroimage. 2007;35:949–958. doi: 10.1016/j.neuroimage.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. The Journal of comparative neurology. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Maclin EL, Gratton G, Fabiani M. Visual spatial localization conflict: an fMRI study. Neuroreport. 2001;12:3633–3636. doi: 10.1097/00001756-200111160-00051. [DOI] [PubMed] [Google Scholar]
- Maguire RP, Broerse A, de Jong BM, Cornelissen FW, Meiners LC, Leenders KL, den Boer JA. Evidence of enhancement of spatial attention during inhibition of a visuo-motor response. Neuroimage. 2003;20:1339–1345. doi: 10.1016/S1053-8119(03)00402-6. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Cain MS, Polli FE, Edelman JA, Fischl B, Barton JJ. Neural activity is modulated by trial history: a functional magnetic resonance imaging study of the effects of a previous antisaccade. J Neurosci. 2007;27:1791–1798. doi: 10.1523/JNEUROSCI.3662-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallarés J, Camara E, Münte TF, Rodríguez-Fornells A. Neural mechanisms underlying adaptive actions after slips. J. Cogn. Neurosci. 2008;20:1595–1610. doi: 10.1162/jocn.2008.20117. [DOI] [PubMed] [Google Scholar]
- Mars RB, Piekema C, Coles MG, Hulstijn W, Toni I. On the programming and reprogramming of actions. Cereb Cortex. 2007;17:2972–2979. doi: 10.1093/cercor/bhm022. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, Taira M, Kojima T. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry research. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Matsumoto E, Misaki M, Miyauchi S. Neural mechanisms of spatial stimulus-response compatibility: the effect of crossed-hand position. Experimental brain research. 2004;158:9–17. doi: 10.1007/s00221-004-1872-7. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage. 2004;22:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Kaladjian A, Azorin JM, Anton JL, Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. J Psychiatr Res. 2009;43:432–441. doi: 10.1016/j.jpsychires.2008.05.004. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in cognitive sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McKenna FP, Sharma D. Reversing the emotional stroop effect reveals that it is not what it seems: the role of fast and slow components. J Exp Psychol Learn Mem Cogn. 2004;30:382–392. doi: 10.1037/0278-7393.30.2.382. [DOI] [PubMed] [Google Scholar]
- McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: an fMRI, within-subjects investigation. Neuropsychologia. 2008;46:2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Mead LA, Mayer AR, Bobholz JA, Woodley SJ, Cunningham JM, Hammeke TA, Rao SM. Neural basis of the Stroop interference task: response competition or selective attention? Journal of the International Neuropsychological Society : JINS. 2002;8:735–742. doi: 10.1017/s1355617702860015. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain structure & function. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher T, Gruber O. Oddball and incongruity effects during Stroop task performance: a comparative fMRI study on selective attention. Brain research. 2006;1121:136–149. doi: 10.1016/j.brainres.2006.08.120. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain research. Cognitive brain research. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller J. The flanker compatibility effect as a function of visual angle, attentional focus, visual transients, and perceptual load: a search for boundary conditions. Perception & psychophysics. 1991;49:270–288. doi: 10.3758/bf03214311. [DOI] [PubMed] [Google Scholar]
- Mitchell RL. The BOLD response during Stroop task-like inhibition paradigms: Effects of task difficulty and task-relevant modality. Brain Cogn. 2005;59:23–37. doi: 10.1016/j.bandc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RR. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex. 2007;17:2703–2712. doi: 10.1093/cercor/bhl179. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. Journal of cognitive neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Struct Funct. doi: 10.1007/s00429-014-0797-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in cognitive sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nagamatsu LS, Hsu CL, Handy TC, Liu-Ambrose T. Functional neural correlates of reduced physiological falls risk. Behavioral and brain functions : BBF. 2011;7:37. doi: 10.1186/1744-9081-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry research. 2005;139:101–114. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, affective & behavioral neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to action: Willed and automatic control of behaviour., in. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self regulation. New York, NY: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Norris DG, Zysset S, Mildner T, Wiggins CJ. An investigation of the value of spin-echo-based fMRI using a Stroop color-word matching task and EPI at 3 T. Neuroimage. 2002;15:719–726. doi: 10.1006/nimg.2001.1005. [DOI] [PubMed] [Google Scholar]
- Notebaert W, Soetens E, Melis A. Sequential analysis of a Simon task--evidence for an attention-shift account. Psychological research. 2001;65:170–184. doi: 10.1007/s004260000054. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci U S A. 1995;92:925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD. Neural systems supporting the control of affective and cognitive conflicts. Journal of cognitive neuroscience. 2009;21:1842–1855. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page LA, Rubia K, Deeley Q, Daly E, Toal F, Mataix-Cols D, Giampietro V, Schmitz N, Murphy DG. A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry research. 2009;174:202–209. doi: 10.1016/j.pscychresns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp. 2009;30:2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. Journal of neurophysiology. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends in cognitive sciences. 2011;15:143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. The Journal of comparative neurology. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. The European journal of neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Polk TA, Drake RM, Jonides JJ, Smith MR, Smith EE. Attention enhances the neural processing of relevant features and suppresses the processing of irrelevant features in humans: a functional magnetic resonance imaging study of the Stroop task. J. Neurosci. 2008;17:13786–13792. doi: 10.1523/JNEUROSCI.1026-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. The American journal of psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Power JD, Petersen SE. Control-related systems in the human brain. Current opinion in neurobiology. 2013;23:223–228. doi: 10.1016/j.conb.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Erickson KI, Colcombe SJ, Kim JS, Voss MW, Kramer AF. Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn. 2009;71:328–335. doi: 10.1016/j.bandc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahm C, Liberg B, Wiberg-Kristoffersen M, Aspelin P, Msghina M. Rostro-caudal and dorso-ventral gradients in medial and lateral prefrontal cortex during cognitive control of affective and cognitive interference. Scandinavian journal of psychology. 2013;54:66–71. doi: 10.1111/sjop.12023. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Rosenberg R, Gjedde A, Gade A. Putative tests of frontal lobe function: a PET-study of brain activation during Stroop’s Test and verbal fluency. Journal of clinical and experimental neuropsychology. 2002;24:534–547. doi: 10.1076/jcen.24.4.534.1033. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Roelofs A, van Turennout M, Coles MG. Anterior cingulate cortex activity can be independent of response conflict in Stroop-like tasks. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13884–13889. doi: 10.1073/pnas.0606265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Koven NS, Randolph JJ, Flashman LA, Pixley HS, Ricketts SM, Wishart HA, Saykin AJ. Functional magnetic resonance imaging of executive control in bipolar disorder. Neuroreport. 2006;17:1085–1089. doi: 10.1097/01.wnr.0000227979.06013.57. [DOI] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol. Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Rothmayr C, Sodian B, Hajak G, Dohnel K, Meinhardt J, Sommer M. Common and distinct neural networks for false-belief reasoning and inhibitory control. Neuroimage. 2011;56:1705–1713. doi: 10.1016/j.neuroimage.2010.12.052. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ. Disorder-specific dysfunction on right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge H, Wolfensteller U. Rapid formation of pragmatic rule representations in the human brain during instruction-based learning. Cereb Cortex. 2010;20:1656–1667. doi: 10.1093/cercor/bhp228. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Woodward TS, Laurens KR, Liddle PF. The role of the anterior cingulate cortex in conflict processing: evidence from reverse stroop interference. Neuroimage. 2001;14:1150–1158. doi: 10.1006/nimg.2001.0893. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20(Suppl 1):S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Schwartz S, Vuilleumier P. Fear and stop: a role for the amygdala in motor inhibition by emotional signals. Neuroimage. 2011;55:1825–1835. doi: 10.1016/j.neuroimage.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Neubert FX, Jbabdi S, O’Reilly JX, Filippini N, Thomas AG, Rushworth MF. The organization of dorsal frontal cortex in humans and macaques. J Neurosci. 2013;33:12255–12274. doi: 10.1523/JNEUROSCI.5108-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of abnormal child psychology. 2007;35:229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K. Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex. 2008a;18:2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008b;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. Plos One. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci. 1997;17:438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Vinco S, Hoeft F, Pfefferbaum A, Sullivan EV. Double dissociation between action-driven and perception-driven conflict resolution invoking anterior versus posterior brain systems. Neuroimage. 2009;48:381–390. doi: 10.1016/j.neuroimage.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Cole MW, D'Esposito M. Selection and maintenance of stimulus-response rules during preparation and performance of a spatial choice-reaction task. Brain Res. 2007;1136:77–87. doi: 10.1016/j.brainres.2006.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D’Esposito M. Neural evidence for representation-specific response selection. Journal of cognitive neuroscience. 2003;15:1111–1121. doi: 10.1162/089892903322598085. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Gerdes B, Feige B, Kloppel S, Lange T, Philipsen A, Tebartz van Elst L, Lieb K, Tuscher O. Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry research. 2012;202:132–141. doi: 10.1016/j.pscychresns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Pohl MF, Kloppel S, Feige B, Lange T, Stahl C, Voss A, Klauer KC, Lieb K, Tuscher O. Disentangling common and specific neural subprocesses of response inhibition. Neuroimage. 2013;64:601–615. doi: 10.1016/j.neuroimage.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Seok Jeong B, Kwon JS, Yoon Kim S, Lee C, Youn T, Moon CH, Yoon Kim C. Functional imaging evidence of the relationship between recurrent psychotic episodes and neurodegenerative course in schizophrenia. Psychiatry Res. 2005;139:219–228. doi: 10.1016/j.pscychresns.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Multiple effects of prefrontal lesions on task-switching. Frontiers in human neuroscience. 2008a;1 doi: 10.3389/neuro.09.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Picton TW, Alexander MP, Gillingham S. Mapping task switching in frontal cortex through neuropsychological group studies. Front Neurosci. 2008b;2:79–85. doi: 10.3389/neuro.01.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Spielberg JM, Edgar JC, Sass SM, Stewart JL, Sutton BP, Banich MT, Miller GA. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR. The effects of an irrelevant directional cue on human information processing. In: Proctor RW, Reeve TG, editors. Stimulus-response compatibility: An integrated perspective. North Holland: Amsterdam, The Netherlands; 1990. pp. 31–86. [Google Scholar]
- Sommer M, Hajak G, Dohnel K, Meinhardt J, Muller JL. Emotion-dependent modulation of interference processes: an fMRI study. Acta neurobiologiae experimentalis. 2008;68:193–203. doi: 10.55782/ane-2008-1688. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. P Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel C, Haworth EJ, Peters E, Hemsley DR, Sharma T, Gray JA, Pickering A, Gregory L, Simmons A, Bullmore ET, Williams SC. Neuroimaging correlates of negative priming. Neuroreport. 2001;12:3619–3624. doi: 10.1097/00001756-200111160-00049. [DOI] [PubMed] [Google Scholar]
- Stoffer TH, Yakin AR. The functional role of attention for spatial coding in the Simon effect. Psychological research. 1994;56:151–162. doi: 10.1007/BF00419702. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of experimental psychology. 1935;18:643–662. [Google Scholar]
- Stuss DT. Frontal lobes and attention: processes and networks, fractionation and integration. Journal of the International Neuropsychological Society : JINS. 2006;12:261–271. doi: 10.1017/S1355617706060358. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos T R Soc B. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. Journal of the International Neuropsychological Society : JINS. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Annals of the New York Academy of Sciences. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Tabu H, Mima T, Aso T, Takahashi R, Fukuyama H. Functional relevance of pre-supplementary motor areas for the choice to stop during Stop signal task. Neuroscience research. 2011;70:277–284. doi: 10.1016/j.neures.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Tabu H, Mima T, Aso T, Takahashi R, Fukuyama H. Common inhibitory prefrontal activation during inhibition of hand and foot responses. Neuroimage. 2012;59:3373–3378. doi: 10.1016/j.neuroimage.2011.10.092. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Minoshima S, Oliver LM, Koeppe RA. Changes in medial cortical blood flow with a stimulus-response compatibility task. Neuropsychologia. 1994;32:249–255. doi: 10.1016/0028-3932(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Geeraerts S, Molenberghs P, Lafosse C, Vandenbulcke M, Peeters K, Peeters R, Van Hecke P, Orban GA. Attentional responses to unattended stimuli in human parietal cortex. Brain : a journal of neurology. 2005;128:2843–2857. doi: 10.1093/brain/awh522. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Gillebert CR. Dissociations between spatial-attentional processes within parietal cortex: insights from hybrid spatial cueing and change detection paradigms. Frontiers in human neuroscience. 2013;7:366. doi: 10.3389/fnhum.2013.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM. Functional specificity of superior parietal mediation of spatial shifting. Neuroimage. 2001;14:661–673. doi: 10.1006/nimg.2001.0860. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci U S A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. Journal of experimental psychology. General. 2008;137:649–672. doi: 10.1037/a0013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Gammelgaard L, Egander A, Clemmensen K, Rasmussen NA, Gjedde A, Rosenberg R. The Danish PET/depression project: performance on Stroop’s test linked to white matter lesions in the brain. Psychiatry research. 2004;130:117–130. doi: 10.1016/j.pscychresns.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Vogt LJ. Cingulate Gyrus. In: Paxinos G, Mai JK, editors. The Human Nervous System. Amsterdam: Elsevier; 2004. pp. 915–949. [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Walther S, Goya-Maldonado R, Stippich C, Weisbrod M, Kaiser S. A supramodal network for response inhibition. Neuroreport. 2010;21:191–195. doi: 10.1097/WNR.0b013e328335640f. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Wittfoth M, Buck D, Fahle M, Herrmann M. Comparison of two Simon tasks: neuronal correlates of conflict resolution based on coherent motion perception. Neuroimage. 2006;32:921–929. doi: 10.1016/j.neuroimage.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Wittfoth M, Kustermann E, Fahle M, Herrmann M. The influence of response conflict on error processing: evidence from event-related fMRI. Brain research. 2008;1194:118–129. doi: 10.1016/j.brainres.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- Ye Z, Zhou X. Conflict control during sentence comprehension: fMRI evidence. Neuroimage. 2009;48:280–290. doi: 10.1016/j.neuroimage.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Zeischka P, Deroost N, Maetens K, Soetens E. Reduced congruency effects only for repeated spatial irrelevant information. Eur J Cogn Psychol. 2010;22:1137–1167. [Google Scholar]
- Zheng D, Oka T, Bokura H, Yamaguchi S. The key locus of common response inhibition network for no-go and stop signals. Journal of cognitive neuroscience. 2008;20:1434–1442. doi: 10.1162/jocn.2008.20100. [DOI] [PubMed] [Google Scholar]
- Zhu DC, Zacks RT, Slade JM. Brain activation during interference resolution in young and older adults: an fMRI study. Neuroimage. 2010;50:810–817. doi: 10.1016/j.neuroimage.2009.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Feng G, Zhang JX, Li G, Li H, Wang S. The role of the left prefrontal cortex in sentence-level semantic integration. Neuroimage. 2013;76:325–331. doi: 10.1016/j.neuroimage.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Zoccatelli G, Beltramello A, Alessandrini F, Pizzini FB, Tassinari G. Word and position interference in stroop tasks: a behavioral and fMRI study. Experimental brain research. 2010;207:139–147. doi: 10.1007/s00221-010-2433-x. [DOI] [PubMed] [Google Scholar]
- Zurawska Vel Grajewska B, Sim EJ, Hoenig K, Herrnberger B, Kiefer M. Mechanisms underlying flexible adaptation of cognitive control: behavioral and neuroimaging evidence in a flanker task. Brain research. 2011;1421:52–65. doi: 10.1016/j.brainres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Zysset S, Muller K, Lohmann G, von Cramon DY. Color-word matching stroop task: separating interference and response conflict. Neuroimage. 2001;13:29–36. doi: 10.1006/nimg.2000.0665. [DOI] [PubMed] [Google Scholar]
- Zysset S, Schroeter ML, Neumann J, von Cramon DY. Stroop interference, hemodynamic response and aging: an event-related fMRI study. Neurobiology of aging. 2007;28:937–946. doi: 10.1016/j.neurobiolaging.2006.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.