Abstract
Objectives
Cervical cancer is increasing but underestimated in developing countries. We calculated the observed and expected incidence of cervical cancer in Lusaka and Southern and Western provinces of Zambia.
Methods/Materials
Data for 2007-2012 was obtained for the 3 provinces. Data included age, residence, year of diagnosis, marital status, occupation, HIV, stage, radiotherapy and chemotherapy. Expected incidence in Southern and Western provinces was calculated based on observed incidence for Lusaka province, adjusting for HIV.
Results
Crude and age-standardized incidence rates (ASR) in Lusaka were 2-4 times higher than incidence in the other 2 provinces. Lusaka had a rate of 54.1/105 and ASR of 82.1/105 in the age group 15-49. The Southern province had a rate of 17.1/105 and ASR of 25.5/105; Western province rate of 12.3/105 and ASR rate of 17.2/105. The observed cervical cancer incidence rates in the Southern and Western provinces were lower than the rate in Lusaka, possibly due to the uncertainty of underreporting/under-diagnosis or actual lower risk for reasons yet unclear. HIV seroprevalence rate in patients from the 3 provinces were 46 – 93% higher than seroprevalence in the respective general populations.
Conclusion
Cervical cancer is significantly underestimated in Zambia and HIV has a significant role in pathogenesis. Future studies should establish methods for case ascertainment and better utilization of hospital- and population-based registries in Zambia and other similar developing countries.
Keywords: Zambia, cervical cancer, incidence, developing countries (LMICs)
INTRODUCTION
Cervical cancer has consistently been among the top 4 most common cancers in women worldwide (1-3). In 2012, there were an estimated 528,000 new cases and 266,000 deaths worldwide (1). Eighty-three percent of all incident cases and 85% of mortality occurs in low-and middle-income countries (LMICs) (1,4,5).
Cervical cancer is the most common cancer and cause of cancer-related death in women in sub-Saharan Africa. It is quite likely that the disease burden in sub-Saharan Africa is underestimated due to the aforementioned reasons in addition to poor cancer registration (5).
In Zambia, the National Cancer Registry (NCR) has been poorly funded and understaffed, adding to limited efficiency of cancer registration. In 2008, the NCR was estimated to capture approximately 10 – 15 % of cancers countrywide (6).
The International Agency for Research on Cancer (IARC) estimates age-standardized incidence rate of cervical cancer in Zambia at 58.0 per 105 and mortality at 36.2 per 105 , making cervical cancer the most common cause of cancer morbidity and mortality in women, and overall in Zambia (1,7). Studies have shown that among the leading factors for cervical cancer in Zambia include the high prevalence of Human Papilloma Virus (HPV) types 16 and 18 (21.6% each), and the high national adult HIV prevalence of 16% (23% in Lusaka) (6-11). Before the Center for Infectious Disease Research in Zambia (CIDRZ) introduced a cervical cancer screening program using visual inspection with acetic acid (VIA) in 2006, there was no screening for cervical cancer in Zambia and all screening was opportunistic (11). Over the course of 7 years (2006 – 2013), the CIDRZ screening program has provided screening to over 100, 000 women (11) and established 24 screening sites — 12 in Lusaka province, and at least 1 in every provincial headquarters (Southern province = 3, Western province =1).
By regulation, all health facilities in Zambia are required to report disease cases to the Ministry of Health (MoH). However, compliance from private hospitals and clinics is poor as very often statistics from private hospitals are conspicuously absent from official statistical health bulletins and reports (12). In late 2006, a new cancer hospital, the Cancer Diseases Hospital (CDH), was opened in Lusaka, for radiotherapy and chemotherapy treatment. Other health facilities in Zambia may diagnose cervical cancer but in order for patients to receive radiotherapy treatment they must be managed at the CDH. Therefore, we conducted this study to calculate incidence of cervical cancer in Lusaka province by pooling data from the main diagnostic and treatment referral sites. The study also aimed to utilize the findings from Lusaka, the region with the best diagnostic and treatment facilities in Zambia, to predict the incidence in the limited-resource settings of Southern and Western provinces. Out of the approximately 13 million individuals and 9 provinces of Zambia, Lusaka province has the largest population of about 2 million individuals and population density100 per square kilometer (13). The province is also the most economically developed with a relative low poverty prevalence of 24% (19). The Southern province is in the middle category with approximately 1.5 million individuals, a density of 19 per square kilometer, and a poverty prevalence of 67% (13, 19). The Western province is one of the two poorest provinces and has an approximate population of 900,000 individuals a population density 7 per square kilometer, and a poverty prevalence of 80% (13, 19).
METHODS
Zambia is a country of 753,612 square kilometers geographic area and an estimated population of 13,718,722 in 2011 (13). The country is divided into 9 provincial regions and Lusaka province has the largest population at approximately 2,362,967 individuals, Southern province has a population of approximately 1,642,757 individuals, and Western province 926,478 individuals (13).
Data sources
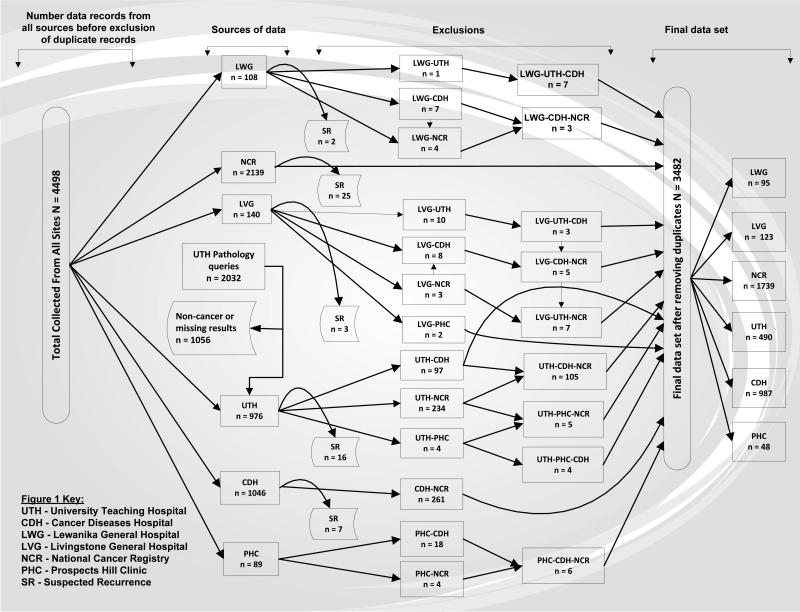
In Lusaka province we obtained data from the following sites: the Cancer Diseases Hospital (CDH, n = 987), the University Teaching Hospital Pathology laboratory (UTH, n = 490), the Prospects Hill Clinic Pathology laboratory (PHC, n = 48), and the National Cancer Registry (NCR, n = 1739). In Southern province, we obtained data from Livingstone General Hospital (LVG, n = 123). In Western province we obtained data from Lewanika General Hospital (LWG, n = 95) (Figure 1).
Figure 1.
Summary of Collected Data
One of the co-authors of this paper (MK) led the data collection process at all research sites in collaboration with the local teams. Data was obtained data from electronic databases, patient medical records, and admission logbooks. The study included data from the Southern and Western provinces from 2007 to 2012, however for the Lusaka province, since the National Cancer Registry is one of the main sources of information from this province and its data was incomplete for 2012; we restricted data for the Lusaka province to the period with complete data from all study sources (2007-2011).
The CDH is a 3rd level hospital and the only center that provides radiotherapy and chemotherapy in Zambia. The UTH is the largest 3rd level hospital in Zambia and its Pathology laboratory receives tissue specimens for processing from all public health facilities in Zambia and some private facilities. Prospects Hill Clinic is a private pathology diagnostic laboratory that serves patients from Lusaka and receives tissue specimens for processing from the UTH and the Cervical Cancer Prevention Program in Zambia (CCPPZ). Livingstone General Hospital is the biggest 2nd level hospital in Southern province and serves part of Western province (Sesheke district) as well. Lewanika General Hospital used to be a 2nd level hospital to cover Western province but due to declining specialist doctor numbers and deteriorating infrastructure, the hospital is now a 1st level. However, Lewanika General Hospital still remains the largest referral point for patients in the Western province. The NCR mainly covers Lusaka province; however, active collection of cases in other provinces is conducted depending on availability of resources.
Data variables
We collected the following patient information from all the sources: patient name, age, year of diagnosis, referral hospital, and province location. Marital status, HIV status, and stage at diagnosis were obtained mainly from the CDH and NCR. Occupation, histology, and radiotherapy and chemotherapy treatment information was only from the CDH. Data from Lewanika General Hospital and Livingstone General Hospital had sparse information on HIV status, marital status, occupation, and stage. This information was found in doctors’ clinical notes and side remarks on patient files and logbooks.
Data Management
We applied a strict criterion for removing duplicates and recurrences. The records didn't necessarily have to match exactly on patient name, age, year of diagnosis and location to be considered duplicate. Based on our knowledge of patient navigation, variations in spelling of names, and mismatches in data entry between different points observed under this study, we used our judgment to consider duplicates in some cases.
Data Analysis
We used the Central Statistical Office (CSO) 2010 Census of Population and Housing report data, and the CSO Population and Demographic Projections 2011 – 2035 report data for the population denominators 2010 – 2012. For the years 2007 – 2009 we applied the geometric population projection method based on the 2000 – 2010 provincial annual average population growth rates for the population denominators. The denominator used in all calculations was the female population.
The direct standardization method and the WHO 2000 – 2025 World Standard Population (14) was used to calculate age-standardized rates (ASR).
The Zambia Demographic and Healthy Survey 2007 reported HIV prevalence in the reproductive age 15 – 49 of 20.8% in Lusaka, 14.5% in Southern, and 15.2% in Western province (15). We applied direct proportion adjustment to calculate expected incidence rates in Southern and Western provinces based on observed Lusaka province incidence adjusting for respective provincial HIV prevalence.
T-tests were used to compare mean values between groups and Chi-squared test was used for comparison of proportions between groups. All tests were at 95% significance. SPSS 21.0 was used to perform all statistical analyses.
The study was approved by the institutional review boards of the University of Nebraska and the Ministry of Health of Zambia.
RESULTS
Figure 1 shows a summary of the collected data. At UTH Pathology laboratory, out of 2032 suspected cervical cancer queries, 976 (48%) were histopathologically-confirmed cervical cancers and 1056 (52%) were non-cancers or missing results. Non-cancer specimens comprised cervical intraepithelial neoplasia (n = 364, 34.5%), cervicitis (n = 91, 8.6%), condyloma acuminata (n = 77, 7.3%), other infections (n = 249, 23.6%), normal (n = 160, 15.2%), and missing (n = 115, 10.9%).
Table 1 shows demographic characteristics of patients seen at the study hospitals. The mean age at diagnosis was 46.3 ±15.04, 50 ± 14.96, and 49.1 ± 14.81 years for Lusaka, Southern, and Western province, respectively. We observed a significant difference between age of patients in the 3 provinces (p < 0.001), with patients from the Southern and Western provinces being older compared to Lusaka. Overall, between the 3 provinces about 72% of cervical cancer patients were married.
Table 1.
Demographic characteristics of cervical cancer patients seen at the study hospitals
| Variable | Lusaka | Southern | Western | P-value | |||
|---|---|---|---|---|---|---|---|
| Total No. of Cases | 2407 | 760 | 315 | ||||
| Age in Years2364,743,306 | |||||||
| Mean | 46.3 | 50.0 | 49.1 | < 0.0011 | |||
| Median | 43 | 48 | 47 | ||||
| Std. Deviation | 15.04 | 14.96 | 14.81 | ||||
| Range (Min, Max) | 80 (19,99) | 78 (20,98) | 81 (17,98) | ||||
| Year of Diagnosis 2407,760,315 | No. | % | No. | % | No. | % | |
| 2007 | 306 | 12.7 | 83 | 10.9 | 41 | 13.0 | < 0.0012 |
| 2008 | 381 | 15.8 | 95 | 12.5 | 45 | 14.3 | |
| 2009 | 573 | 23.8 | 145 | 19.1 | 37 | 11.7 | |
| 2010 | 625 | 26.0 | 138 | 18.2 | 44 | 14.0 | |
| 2011 | 522 | 21.7 | 131 | 17.2 | 78 | 24.8 | |
| 2012 | - | - | 168 | 22.1 | 70 | 22.2 | |
| Source 2407,760,315 | |||||||
| Cancer Diseases Hospital | 639 | 26.5 | 256 | 33.7 | 92 | 29.2 | |
| University Teaching Hospital | 386 | 16.0 | 103 | 13.6 | 1 | 0.3 | |
| Livingstone General Hospital | 0 | 0.0 | 104 | 13.7 | 19 | 6.0 | |
| Lewanika General Hospital | 0 | 0.0 | 0 | 0.0 | 95 | 30.2 | |
| Prospects Laboratory | 47 | 2.0 | 1 | 0.1 | 0 | 0.0 | |
| National Cancer Registry3 | 1335 | 55.5 | 296 | 38.9 | 108 | 34.3 | |
| Marital Status 1902,528,201 | |||||||
| Unmarried | 455 | 23.9 | 141 | 26.7 | 67 | 33.3 | 0.012 |
| Married | 1447 | 76.1 | 387 | 73.3 | 134 | 66.7 | |
| Occupation 443,217,85 | |||||||
| Unemployed Unmarried | 108 | 24.4 | 38 | 17.5 | 24 | 28.2 | < 0.0012 |
| Unemployed Married | 147 | 33.2 | 61 | 28.1 | 26 | 30.6 | |
| Farming / Agriculture | 44 | 9.9 | 75 | 34.6 | 23 | 27.1 | |
| Business | 66 | 14.9 | 14 | 6.5 | 8 | 9.4 | |
| Other | 78 | 17.6 | 29 | 13.4 | 4 | 4.7 | |
| HIV Status 1785,489,184 | |||||||
| Negative | 1243 | 69.6 | 374 | 76.5 | 130 | 70.7 | 0.012 |
| Positive | 542 | 30.4 | 115 | 23.5 | 54 | 29.3 | |
ANOVA comparing Lusaka, Southern, and Western Province
Chi-square comparing Lusaka, Southern, and Western Province
National Cancer Registry Data was not available for 2012.Other superscript values represent total cases in each province for each variable.
Table 2, illustrates the clinical characteristics of patients seen at the study hospitals with clinical data details missing for many cases, high proportion of advanced stages (stage ≥ II) among cases for whom clinical stage is known, and almost three-fourths of cases not receiving any treatment or their treatment status not known and a high proportion with histology proof (an exception in sub-Saharan Africa). In the Lusaka and Southern provinces, 92.5 % of patients for whom clinical stage was known had advanced stage cervical cancer, and in the Western province the proportion was 94.5%. For patients whom histology was clearly indicated, the majority had squamous cell carcinomas; 89.4%, 89.8%, and 92.8% in Lusaka, Southern, and Western province, respectively. The rest of the cases were adenocarcinomas or the histology was not clearly specified.
Table 2.
Clinical characteristics of cervical cancer patients seen at the study hospitals
| Variable | Lusaka | Southern | Western | P-value | |||
|---|---|---|---|---|---|---|---|
| Total number of cases | 2407 | 760 | 315 | ||||
| FIGO Stage | No. | % | No. | % | No. | % | |
| I | 78 | 3.2 | 24 | 3.2 | 8 | 2.5 | 0.061 |
| II | 451 | 18.7 | 112 | 14.7 | 50 | 15.9 | |
| III | 426 | 17.7 | 158 | 20.8 | 75 | 23.8 | |
| IV | 87 | 3.6 | 25 | 3.3 | 13 | 4.1 | |
| Missing | 1365 | 56.7 | 441 | 58.0 | 169 | 53.7 | |
| Treatment Status2 | |||||||
| Radiotherapy & Chemotherapy | 102 | 4.2 | 41 | 5.4 | 26 | 8.3 | < 0.011 |
| Radiotherapy Only | 45 | 1.9 | 41 | 5.4 | 14 | 4.4 | |
| Radiotherapy, Unknown Chemotherapy | 139 | 5.8 | 50 | 6.6 | 14 | 4.4 | |
| Unknown Treatment Status | 2096 | 87.1 | 606 | 79.7 | 255 | 81.0 | |
| Not Received Treatment | 25 | 1.0 | 22 | 2.9 | 6 | 1.9 | |
| Treatment Intent2 | |||||||
| Palliative | 75 | 3.1 | 29 | 3.8 | 9 | 2.9 | < 0.011 |
| Curative | 145 | 6.0 | 94 | 12.4 | 42 | 13.3 | |
| Unknown (treatment status unknown /un-received) | 2187 | 90.9 | 637 | 83.8 | 264 | 83.8 | |
| Histology | |||||||
| Squamous Cell Carcinoma | 1985 | 82.5 | 526 | 69.2 | 167 | 53.0 | < 0.011 |
| Adenocarcinoma | 125 | 5.2 | 46 | 6.1 | 4 | 1.3 | |
| Histopathology Cell Type not Specified | 110 | 4.6 | 14 | 1.8 | 9 | 2.9 | |
| Missing | 187 | 7.8 | 174 | 22.9 | 135 | 42.9 | |
Chi-square comparing Lusaka, Southern, and Western province
Data from CDH only
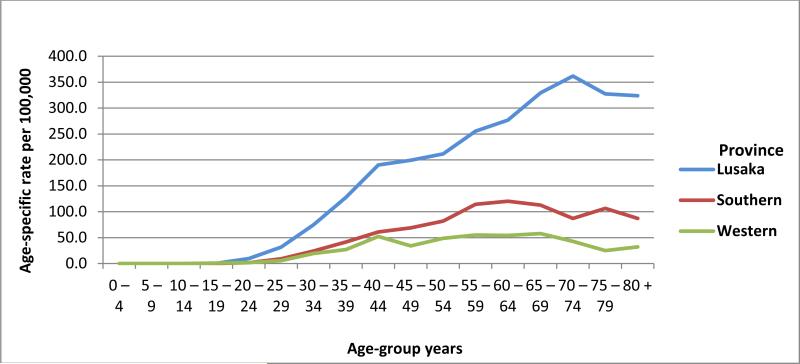
Figure 2 shows age-specific incidence rates among the study population. The majority of cases occurred after age 40. The highest age-specific rates were observed in Lusaka followed by Southern and Western provinces.
Figure 2.
Age-specific incidence rates of cervical cancer in Lusaka, Southern, and Western provinces of Zambia (per 100,000)
Table 3 shows crude and adjusted cervical cancer rates for reproductive age 15 – 49 by province for the period 2007-2011. The crude and adjusted cervical cancer rates in Lusaka were about 2 to 4 times higher than the rates in the other 2 provinces. Table 4 shows the observed and expected incidence rates of cervical cancer in the reproductive age 15 – 49 for Southern and Western provinces based on observed incidence rates for Lusaka. Assuming that the Southern and Western provinces had similar risk factors to Lusaka province and adjusting for HIV prevalence, observed cervical cancer incidences in Southern province and Western province were underestimated by about 55% and 69%, respectively.
Table 3.
Crude and adjusted cervical cancer rates for reproductive age 15 – 49 by province for the period 2007-2011
| PROVINCE | |||
|---|---|---|---|
| Lusaka | Southern | Western | |
| Number | 1534 | 310 | 127 |
| Crude rate | 54.1 | 17.1 | 12.3 |
| ASR (W) | 82.1 | 25.5 | 17.2 |
Table 4.
Expected incidence rates in Southern and Western based on Lusaka province observed incidence rates for the period 2007-2011
| PROVINCE | ||
|---|---|---|
| Southern | Western | |
| Observed rate | 17.1 | 12.3 |
| Expected rate | 37.9 | 39.5 |
| Under-estimation | 54.9% | 69.0% |
DISCUSSION
This study identified 5 interesting observations. First, the highest total and age-specific incidence rates of cervical cancer were found in Lusaka province followed by Southern province. The rates found in this study were higher than the IARC Globocan estimates for Zambia and Zimbabwe but similar to estimates for Malawi (1). The higher rate estimates could be due to higher HIV rate in Lusaka compared to the rest of the countries of the Globocan projections (15). Furthermore, not until recently, Lusaka had higher HIV rates than Harare, Zimbabwe, which could be the reason for higher cervical cancer incidence in Lusaka (15). The HIV profile in Malawi is somewhat similar to that of Lusaka and that may have reflected on the similar cervical cancer rates in the 2 countries (15-18).
Second, we found that a little over 1/3 of cervical cancer patients were HIV-positive. Lusaka province has an HIV rate of 20.8 %, Southern province 14.5%, and Western province 15.2% (15). However, there was a significantly higher HIV seroprevalence among cervical cancer patients than the HIV seroprevalence in the general population of each respective province. The HIV seroprevalence in patients from Western province was 93% greater, Southern province 62% greater, and Lusaka province 46% greater than the general population. We also observed that patients in Western and Southern province were more frequently diagnosed with advanced stage cervical cancer (FIGO stage III/IV; Western = 60%, Southern = 57%, Lusaka = 49%). These observed differences may be associated with disparities in access and availability of diagnostic and treatment services as well as socioeconomic factors between the 3 provinces. Poverty prevalence in Lusaka province is 24.4%, Southern province 67.9%, and in the Western province 80.4% (19). It is important to note that the majority of cases seen in Lusaka, Southern, and Western provinces were from the Cancer Diseases Hospital (CDH) and the University Teaching Hospital (UTH).
Third, we found a significant under-reporting of cervical cancer incidence in Southern and Western provinces. The under-reporting could be due to a lack of cancer registration in the provinces and/or the lack of or incomplete documentation of diagnosis in medical records. Furthermore, it is possible that patients die without being diagnosed with cervical cancer or reported as a case. The limited documentation of mortality makes it difficult to assess the magnitude of cervical cancer in peripheral regions in Zambia.
It is possible that some patients from different provinces in Zambia may report addresses of relatives/friends who reside in Lusaka. While the patients are asked about their permanent and temporary address during their first visit to the hospital in Lusaka, it cannot be confirmed that all patients report their actual permanent addresses. Among the factors that may contribute to this inflation is reporting by relatives or friends on behalf of the patients or lack of patient appreciation of the importance of the permanent address. There is no immediate method to account for this possibility but it should be considered and further explored in future studies.
Furthermore, 52% of patients from Lusaka, 40% of patients from the Southern province, and 35% of patients from the Western province had unknown treatment information reported in the medical records. The high proportion of patients with unknown treatment could be attributed to incomplete documentation of treatment information, lack of record linkage and data integration systems, and perhaps lack of treatment. Whereas chemotherapy and radiotherapy treatment is offered free of charge at the CDH, the indirect expenses associated with treatment are unaffordable by most patients and could be a barrier in receiving or completing free treatment. Indirect costs and expenses that patients are required to provide include laboratory fees for investigations (approximately $200), if needed. In addition patients from outside Lusaka must pay for transportation and food expenses for themselves and accompanying relatives who are required to nurse them especially in advanced. Studies in limited-resource settings have shown that financial expenses for cancer care beyond the cost of medications, surgery, and related treatment are significant barriers to availing cancer treatment. Out-of-pocket expenses for transportation, food, and lost income have been shown to inhibit and reduce patients’ willingness to start treatment for chronic illnesses (20-23).
The shortage in clinics, health professionals, and awareness about cervical cancer and its early detection, diagnosis, and treatment are more prominent in the 2 provinces included in this study than in Lusaka. For example, Lusaka has 6 hospitals compared to 2 hospitals in the Southern province, and none in the Western province. Also, Lusaka has significantly higher concentration of health care workers than the Southern and Western Provinces (24).
Fourth, when comparing whether patients received curative or palliative treatment, more proportions of patients from Southern and Western provinces had curative treatment than patients from Lusaka. This may have been due to the fact that beside under-documentation of treatment information, patients from distant locations who needed palliative treatment did not see a reason for paying for expenses of travel to the CDH for terminal conditions. A significant proportion of cervical cancer patients referred from these hospitals fail to make it to UTH or CDH. Failure of referral is usually due to patients’ preferences of seeking palliative care or management by local traditional healers and lack of financial resources for transportation and accommodation in Lusaka.
Fifth, the UTH Pathology results showed no evidence of malignancy in approximately 50% of tissue specimens suspected of cervical cancer. This may be due to high rate of referrals of early detection cases in Zambia or limited clinical skills and experience of primary care professionals who refer cases of infections as suspected cancers. Processing and examining half the workload of the main pathology department of the country for non-malignant lesions lead to long wait time for processing malignant lesions and delaying treatment of cervical cancer patients in the country.
As highlighted in the Introduction section, Zambia has made significant emphasis and achievements for the diagnosis of cervical cancer since 2006 through establishments of screening sites. Zambia also has succeeded in reducing the HIV infection rate, a major factor in increasing cervical cancer risk. The United Nations World AIDS’ Day report for 2012 emphasized that the rate of new HIV infections in Zamia has declined between 2001 and 2011 by 68% and 80% of HIV patients received treatment (25). Zambia has established the first cancer center in Lusaka and more efforts are needed for treating cancer patients and emphasizing patient, public, and professional cancer education efforts.
In conclusion, this study demonstrated the burden, late stage, and underestimation of cervical cancer in 3 Zambian provinces. The study also highlighted the importance of HIV in cervical cancer pathogenesis and the challenges encountered in diagnosis and case ascertainment in Zambia and possibly other similar low-income countries. With increasing screening facilities in Zambia and longer survival for HIV patients who receive anti-retroviral treatment, the incidence of cervical cancer is likely to increase. Therefore, future studies should place more emphasis on educating primary care professionals about the differential diagnosis of cervical cancer, better documentation of diagnosis and treatment of cervical cancer, improving patient access to treatment, and enhancing the quality of cancer registries in Zambia and other similar low-income countries.
Acknowledgements
Mulele Kalima was funded by the Fogarty International Training and Research Program (Grant D43 TW001429). His research utilized resources available through the Cancer Epidemiology Education in Special Populations Program (CEESP) of the University of Nebraska (Grant R25 CA112383).
We would like to thank staff at the CDH, UTH Pathology lab, National Cancer Registry, Livingstone General Hospital, Lewanika General Hospital, and Prospects Hill Clinic Laboratory in Zambia who helped in so many various ways with this work. Special gratitude also goes to those that helped with data acquisition, logistics support, and insightful comments to the research: Mr. Gilbert Phiri at the CDH; Dr. Mzaza Nthele, Dr. Robert Fubisha, Dr. Isaiah Hansingo and Dr. Jane Mutanga at Livingstone General Hospital; Dr. Andrew Silumesii and Dr. Kambinda Likambi at Lewanika General Hospital; Dr. Bellington Vwalika and Dr. Hamakwa Mantina at the UTH; and Dr. Evans Malyangu at Prospects Hill Clinic. We would like to thank Lynne Le from University of Nebraska Medical Center, College of Public Health, for her help in editing the manuscript and Melanie Wells coordinator of the CEESP Program for her extensive administrative work.
Disclosure: This work was supported in part by the Cancer Epidemiology Education in Special Populations (CEESP) Program of the University of Nebraska Medical Center through funding from the National Cancer Institute (R25CA112383). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflict of interests.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] 2012.
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] 2010.
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. Mar-Apr. [DOI] [PubMed] [Google Scholar]
- 4.Anorlu RI. Cervical cancer: the sub-Saharan African perspective. Reprod Health Matters. 2008 Nov;16(32):41–49. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- 5.Soliman AS, Schottenfeld D, Boffetta P. Cancer Epidemiology: Low- and Middle-Income Countries and Special Populations. Oxford University Press; New York, New York: 2013. [Google Scholar]
- 6.Bowa K, Wood C, Chao A, Chintu C, Mudenda V, Chikwenya M. The Epidemiology of Cancers at Lusaka University Teaching Hospital in Zambia. East and Central African Journal of Surgery. 2008;13(2):125–131. [Google Scholar]
- 7.Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, Pfaendler KS, Chibwesha C, Mkumba G, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Med. 2011 May;8(5):e1001032. doi: 10.1371/journal.pmed.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng'andwe C, Lowe JJ, Richards PJ, Hause L, Wood C, Angeletti PC. The distribution of sexually-transmitted Human Papillomaviruses in HIV positive and negative patients in Zambia, Africa. BMC Infect Dis. 2007 Jul 16;7:77. doi: 10.1186/1471-2334-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, Huh WK, Lyon MD, Stringer JS, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007 May 7;96(9):1480–1483. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntekim A. Chapter 4: Cervical Cancer in Sub Sahara Africa. In: Rajamanickam R, editor. Topics on Cervical Cancer With an Advocacy for Prevention. InTech; 2012. [Google Scholar]
- 11.Mwanahamuntu MH, Sahasrabuddhe VV, Blevins M, Kapambwe S, Shepherd BE, Chibwesha C, et al. Utilization of cervical cancer screening services and trends in screening positivity rates in a 'screen-and-treat' program integrated with HIV/AIDS care in Zambia. PLoS One. 2013 Sep 18;8(9):e74607. doi: 10.1371/journal.pone.0074607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministry of Health (MoH) Lusaka Provincial Health Office (LPHO). Annual Health Statistical Bulletin 2011. 2012 [Google Scholar]
- 13.Central Statistical Office (CSO). 2010 Census of Population and Housing: Population and Demographic Projections 2011-2035. 2013 [Google Scholar]
- 14.Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: A new WHO standard. 2001. GPE Discussion Paper Series: No. 31.
- 15.Central Statistical Office (CSO), Zambia Ministry of Health (MOH), Tropical Diseases Research Centre (TDRC), University of Zambia, Macro International Inc. Zambia: Demographic and Health Survey 2007. CSO and Macro International Inc; Calverton, Maryland, USA: 2009. [Google Scholar]
- 16.Central Statistical Office [Zambia], Central Board of Health [Zambia], ORC Macro. Zambia Demographic and Health Survey 2001-2002. 2003.
- 17.National Statistical Office (NSO) [Malawi], ORC Macro. Malawi demographic and health survey 2000. 2001.
- 18.National Statistical Office (NSO) [Malawi], ORC Macro. Malawi demographic and health survey 2004. 2005.
- 19.Republic of Zambia Central Statistical Office. Living conditions monitoring survey report 2006 & 2010. 2012.
- 20.Andrasik MP, Rose R, Pereira D, Antoni M. Barriers to cervical cancer screening among low-income HIV-positive African American women. J Health Care Poor Underserved. 2008 Aug;19(3):912–925. doi: 10.1353/hpu.0.0037. [DOI] [PubMed] [Google Scholar]
- 21.Moore KA. Breast cancer patients' out-of-pocket expenses. Cancer Nurs. 1999 Oct;22(5):389–396. doi: 10.1097/00002820-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Wani MA, Tabish SA, Warai ZA, Pandit KK, Yattoo GH, Rashid H. Analyzing cost of out-patient cancer chemotherapy in a resource-poor setup. Int J of Medicine and Allied Health Services. 2014;1(1):29–38. [Google Scholar]
- 23.Kim P. Cost of cancer care: The patient perspective. J Clin Oncol. 2007 Jan 10;25(2):228–232. doi: 10.1200/JCO.2006.07.9111. [DOI] [PubMed] [Google Scholar]
- 24.Ferrinho P, Siziya S, Goma F, Dussault The human resource for health situation in Zambia: deficit and maldistribution. Human Resources for Health. 9:30. doi: 10.1186/1478-4491-9-30. doi:10.1186/1478-4491-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United Nations World AIDS Day Report 2012. UNAIDS; Geneva, Switzerland: 2012. p. 13. [Google Scholar]