Abstract
This study’s aim was to assess the histological and metabolic effects of N-3 polyunsaturated fatty acids (PUFA) versus placebo while adjusting for the impact of age and weight change in NASH patients. (ClinicalTrials.gov: NCT00681408).
Methods
Forty-one subjects with non-cirrhotic NASH were enrolled, and 34 completed the study. 17 received N-3 fish oil 3000 mg/day and 17 received placebo daily for 1 year with typical counseling on caloric intake and physical activity for all subjects.
Results
N-3- and placebo-treated groups showed no significant difference for the primary endpoint of NAS reduction ≥ 2 points without fibrosis progression after adjustment for known covariates (N-3, 4/17 (23.5%); placebo, 3/17, (17.6%), p=0.99). Among subjects with increased or stable weight, N-3 subjects showed a larger decrease in liver fat content by MRI than placebo-treated subjects (p=0.014 for 2nd quartile, p=0.003 for 3rd quartile of weight change). N-3 treatment showed significant fat reduction on paired analysis of image-assisted fat morphometry regardless of weight loss or gain. Exercise capacity remained markedly reduced in all subjects. No independent effects on markers of hepatocyte injury or insulin sensitivity indices were observed.
Conclusion
N-3 PUFA at 3000 mg/day for one year did not lead to improvement in the primary outcome of histological activity in NASH patients (≥ 2 point NAS reduction). N-3 led to reduced liver fat by multiple measures. Other metabolic effects were not seen, although no detrimental effects were apparent. Whether longer duration, higher dose, or different composition of N-3 therapy would lead to additional benefit is uncertain.
Keywords: N-3 fatty acid, fatty liver, steatohepatitis, exercise, cardiorespiratory fitness, obesity
INTRODUCTION
The recognition of non-alcoholic steatohepatitis (NASH) parallels an excess of N-6 and deficiency of N-3 polyunsaturated fatty acids (PUFA) in Western diets [1,2]. Reduction in cardiovascular risk by increased N-3 intake has been acknowledged by the American Heart Association and the US FDA [3]. A relationship between NASH and PUFA metabolism is supported by studies showing an increased dietary ratio of N-6:N-3 and lower intake of PUFA among NASH patients [4–6] and lipidomic studies that demonstrate lower PUFA and a higher N-6:N-3 ratio in NASH [7–8]. Additionally, insulin resistance is associated with lower PUFA content in skeletal muscle and a higher N-6:N-3 ratio [9]. Lower hepatic N-3 fatty acid has also been correlated to increased SREBP-1C (sterol receptor element-binding protein-1), increased fatty acid synthase, and diminished PPAR-α (peroxisome proliferator-activated receptor-α) mRNA, all of which favor lipogenesis over fatty acid oxidation [10].
Studies of supplemental N-3 in NAFLD patients have included only limited histological and metabolic outcomes [11]. Nine studies (four randomized, placebo-controlled) were analyzed in a meta-analysis [12] that showed a consistent reduction in liver fat by imaging, although confounding effects of weight loss and exercise, which independently affect steatosis [13], were not systematically accounted for.
The aim of this randomized, double-blind, placebo-controlled study was to assess the effects of N-3 PUFA compared to placebo on liver histology, anthropometric indices, exercise capacity, metabolic parameters, hepatic fat content, and serum markers of hepatocellular injury while adjusting for potential confounding effects of weight loss and activity.
METHODS
Eligible patients were recruited from the University of Virginia hepatology clinics from 2007 through 2010. All underwent evaluation to exclude viral hepatitis and autoimmune and metabolic liver diseases. Entry biopsies (see criteria below) demonstrated steatohepatitis, defined as steatosis with inflammation, hepatocellular ballooning and/or fibrosis [14,15]. Ethanol consumption was < 30 g/day for males or 20 g/day for females. Subjects diagnosed with cirrhosis or secondary forms of steatohepatitis or treated with thiazolidinediones were excluded.
Patients with a liver biopsy within six months of projected enrollment were eligible for consideration. After excluding patients with cirrhosis and those prescribed thiazolidinediones agents, approximately 90 patient biopsies were reviewed and scored for possible inclusion over the study period. 60 patients were eligible for possible enrollment, and 41 patients agreed to enroll. Reasons to decline participation included concerns regarding intensity of testing including the end of treatment biopsy, travel distance, and visit frequency.
Forty-one subjects were enrolled, and 20 and 21 subjects were randomized to N-3 or placebo using a stratified block 1:1 randomization scheme. An independent biostatistician generated the randomization list which was confidentially forwarded to the Investigational Pharmacy. All staff and subjects were blinded to therapy assignment throughout the study period. One subject was excluded because a remote prior biopsy showing NASH was inadvertently considered as the entry biopsy (the actual entry biopsy showed cirrhosis). Of the remaining 40 subjects, 6 (3 placebo and 3 N-3) dropped out due to relocation (1) and transportation difficulties (5). Thirty-four subjects completed the study – 17 received N-3 supplement (3000 mg/day) and 17 received placebo – and 33 underwent end-of-study biopsy (1 subject was declined due to immune thrombocytopenia).
Active Drug Therapy
Active drug and identical-appearing placebo were provided by Nordic Natural and administered under IND# 75335. The N-3 dose of 3000 mg/day was the FDA’s recommended safe dose and is the American Heart Association’s upper dose for triglyceride reduction [16]. Each 1000 mg capsule contained 70% total N-3 as triglyceride: 35% eicosapentaenoic acid (EPA), 25% docosahexanoic acid (DHA), 10% other N-3’s, and a scant amount of lemon oil. Placebo therapy consisted of identical appearing capsules containing predominantly soybean oil but also small amounts of fish and lemon oils (only 8% N-3) to protect blinding.
Visits, exercise and dietary counseling, and anthropometric measures
Each visit included physical examination, anthropometric measurements, laboratory evaluation, and counseling to maintain an aerobic exercise goal of 150 minutes/week and a hypocaloric diet with 500–1000 calories less than estimated for age- and weight-based basal metabolic rate and fat content less than 30% of the total calories. Cardiopulmonary fitness testing consisted of a graded ergometer exercise protocol with increasing power output to measure peak volume of oxygen consumption (VO2peak). Baseline assessment of physical activity included the Aerobics Center Longitudinal Study Physical Activity Questionnaire calculated as MET hours per week using the Compendium of Physical Activities [17]. A nutritionist performed dietary counseling and administered a food frequency questionnaire (Harvard Service Food Frequency Questionnaire) at the entry and study-end visits to quantify fructose intake, a factor connected to NASH severity [18].
Histologic assessment
Blinded histological assessment was performed separately by two observers: one hepatopathologist (CL) and one hepatologist (SC), both of whom have previously published on histological aspects of NASH [19, 20]. Biopsy cores were required to have length of ≥ 1.5 cm, width of ≥ 1.5 mm, and at least 11 portal tracts to be deemed adequate for diagnostic evaluation. Mean biopsy length was 1.9 cm (± 0.6 cm). The samples were independently and blindly scored to calculate the NAS (NASH Activity Score, 0–8) using H&E stained slides according to the NASH-CRN grading system and staged for fibrosis (0–4) using trichrome according to the Brunt system [21,22]. Recognizing that inter- and intra-observer variability in scoring histological activity in NASH is an inherent limitation [23–26], the authors used mean scores in the histological analyses to capture a range of activity and reflect common variation in histological interpretation especially relevant to ballooning [20]. To further resolve histological comparisons of steatosis, computer-assisted cell image analysis was used for fat quantification from liver biopsy specimens. Additional analysis of hepatocellular ballooning was performed with keratin-8,18 immunohistochemical staining when additional liver tissue was available for this secondary analysis, where ballooning was defined as loss of staining of the cytoskeleton (i.e. ‘K-8,18 empty cells’) [20].
Radiologic assessment
Abdominal MRI was performed at entry and study completion for quantitative assessment of hepatic fat using typical volumetric assessments of visceral and abdominal fat. Hepatic fat was specifically assessed using in-phase/out-of-phase and localized Dixon methodologies [27,28].
Biochemical studies and indices of insulin sensitivity
After an overnight 12-hour fast, subjects had serum drawn for AST, ALT, total cholesterol, LDL and HDL cholesterol, and triglycerides. Free fatty acids, insulin, and glucose levels were measured to calculate indices of insulin sensitivity including the QUICKI (quantitative insulin sensitivity check index), HOMA-IR (Homeostatic Model Assessment-Insulin Resistance), and QUICKI-FFA (free fatty acids) [29]. Serum was collected to assess markers of hepatocyte injury, including M30 and M65 keratin fragments and adiponectin. N-6:N-3 red blood cell membrane lipid composition was evaluated due to its link to hepatic lipid composition [30].
Additional technical details of Methods are included as an online data supplement.
Statistical Analysis
Power analysis and primary analysis
The primary outcome was decrease of at least 2 points in the NAS in the end-of-study as compared to the baseline liver biopsy (without fibrosis progression). The predicted difference in response rate for the primary outcome was estimated to differ by 42% (predicted response rate of 18% in placebo group and 60% in the N-3 group) between the two study populations; thus, using the chi-square statistic method [31] to evaluate the null hypothesis, a sample size of 18 patients per group would be sufficient to achieve 0.80 statistical power with a maximum two-side type I error rate of 0.05.
Data summarization
Categorical data were summarized as frequencies and percentages. Continuous data were summarized by mean, standard deviation, and range.
Secondary Analyses
The relative effects of recommended dietary changes and activity levels with N-3 PUFA versus placebo were examined via univariate and multivariate techniques. For univariate analyses, Student’s t-tests and Wilcoxon nonparametric tests were utilized to conduct within-group and between-group comparisons. For multivariate analyses, analysis of covariance (ANCOVA) was utilized to examine the impact of the intervention on the pre- to post-intervention difference in each outcome as influenced by: (a) age, (b) pre- to post-intervention differences in weight and (c) pre- to post-intervention differences in exercise capacity.
For hypothesis testing, a two-sided p≤0.05 decision rule was used as the null hypothesis rejection criterion for all comparisons. For multivariate analyses, the N-3 vs. placebo comparisons were adjusted for concomitant variables associated with outcome at p≤0.10. When interactions met this standard, comparisons between the two interventions were made at the 1st, 2nd, and 3rd quartiles of the concomitant variable measurement distribution. 95% confidence interval construction for the mean pre- to post-intervention difference in each outcome was based on the Student’s t-distribution.
SAS version 9.2 software (SAS Institute, Cary, NC) was used to conduct all analyses.
The study was approved by the University of Virginia Institutional Review Board for Health Sciences Research.
RESULTS
Cohort characteristics
The mean age of the cohort was 46.8 years ± 11.9 (range: 25–72 years), mean BMI: 32.5 kg/m2 ± 7.3, mean baseline ALT: 66.8 U/L ± 35.7, and mean HOMA-IR: 6.3 ± 4.3 (range: 1.1–22.3). Eleven patients were diabetic at baseline (5 in the N-3 group, 6 in the placebo group). There were few differences in demographic, laboratory, or histological parameters between the two groups (Table 1). The interventions were well-tolerated (the main adverse event was nausea which occurred infrequently and similarly between groups). The N-3 group had significantly higher hepatic fat by image morphometry (p=0.05) and higher self-reported daily fructose intake (p=0.01) at baseline. In addition, the N-3 group’s overall baseline liver fat by MRI was higher than the placebo group’s assessed volumetrically (p=0.08) and by the Dixon method (p=0.07). Histologically, the mean NAS score for the entire cohort was 5.2 ± 0.9, and the mean fibrosis stage was 1.8 ± 0.8, with no significant baseline differences between groups.
Table 1.
Baseline Demographics, Clinical, Laboratory Data
| Overall (n = 34) | Placebo (n = 17) | N-3 (n = 17) | P-value | |
|---|---|---|---|---|
| Age | 46.8 (11.9) | 47.2 (12) | 46.4 (12.1) | 0.84 |
| BMI | 32.5 (7.3) | 31.6 (6.7) | 33.3 (8.0) | 0.96 |
| Gender | ||||
| Female (%) | 61.8 | 58.8 | 64.7 | 0.98 |
| Ethnicity | ||||
| Caucasian (%) | 97.1 | 94.1 | 100 | 0.92 |
| Other (%) | 2.9 | 5.9 | 0 | |
| Diabetes (%) | 32.4 | 35.3 | 29.4 | 0.88 |
| Obese (%) | 58.8 | 52.9 | 58.8 | 0.91 |
| Metabolic Synd. Criteria | 3.3 (1.2) | 3.3 (1) | 3.2 (1.3) | 0.88 |
| ALT (U/L) | 66.8 (35.7) | 60.8 (40) | 72.8 (30.9) | 0.34 |
| AST (U/L) | 55.6 (39.7) | 45.7 (31.5) | 63.7 (44.7) | 0.21 |
| Fasting glucose (mg/dL) | 110.4 (18.5) | 114.5 (21.3) | 106.3 (14.7) | 0.20 |
| Fasting insulin (μIU/mL) | 22.8 (13.5) | 20.4 (13) | 25.2 (14) | 0.31 |
| HOMA-IR | 6.3 (4.3) | 5.9 (4.8) | 6.6 (3.8) | 0.66 |
| Total cholesterol (mg/dL) | 190.3 (35.0) | 189.2 (29.4) | 191.5 (41) | 0.86 |
| LDL cholesterol (mg/dL) | 120.5 (29.8) | 118.5 (27.7) | 122.6 (32.6) | 0.70 |
| Triglycerides (mg/dL) | 173.4 (75.0) | 190.7 (70.8) | 155 (77.1) | 0.17 |
| Free fatty acids (mmol/L) | 0.856 (0.367) | 0.893 (0.274) | 0.820 (0.448) | 0.57 |
| QUICKI-FFA | 0.316 (0.040) | 0.313 (0.030) | 0.318 (0.050) | 0.73 |
| N6/N3 ratio | 76.0 (24.2) | 82.0 (33.2) | 68.8 (34) | 0.22 |
| Adiponectin (pg/mL) | 5.9 (1.6) | 6.0 (1.7) | 5.9 (1.6) | 0.77 |
| M30 | 870.5 (314.5) | 856.2 (329.8) | 884.8 (307.8) | 0.80 |
| M65 | 1180.5 (1085) | 940.0 (436.3) | 1454.1 (352.7) | 0.20 |
| IGFBP-1 | 20.1 (20.9) | 18.7 (15.8) | 21.4 (25.4) | 0.71 |
| Visceral fat (cm3) | 218.3 (76.7) | 193.9 (82.8) | 242.6 (63.7) | 0.08 |
| MRI Dixon fat (%) | 14.8 (8.8) | 11.3 (6.5) | 18.4 (9.8) | 0.07 |
| Image fat (%) | 19.2 (8.5) | 16.3 (4.8) | 22.1 (10.4) | 0.05 |
| Waist circumference (cm) | 109.2 (11) | 106.8 (13.4) | 111.7 (16.6) | 0.36 |
| Steatosis | 2.0 (0.6) | 1.9 (0.6) | 2.2 (0.6) | 0.11 |
| Inflammation | 2.0 (0.5) | 2.0 (0.6) | 2.0 (0.5) | 0.87 |
| Ballooning | 1.2 (0.6) | 1.1 (0.5) | 1.3 (0.6) | 0.31 |
| NAS | 5.2 (0.9) | 4.9 (0.9) | 5.4 (0.9) | 0.09 |
| Fibrosis | 1.9 (0.9) | 1.6 (0.9) | 2.1 (0.8) | 0.16 |
| Daily fructose intake | 27.4 (16.6) | 19.8 (9.6) | 35.1 (18.8) | 0.01 |
| VO2max | 16.9 (5.8) | 16.7 (4.1) | 17.1 (7.3) | 0.84 |
Values are shown as mean (SD).
Primary analysis
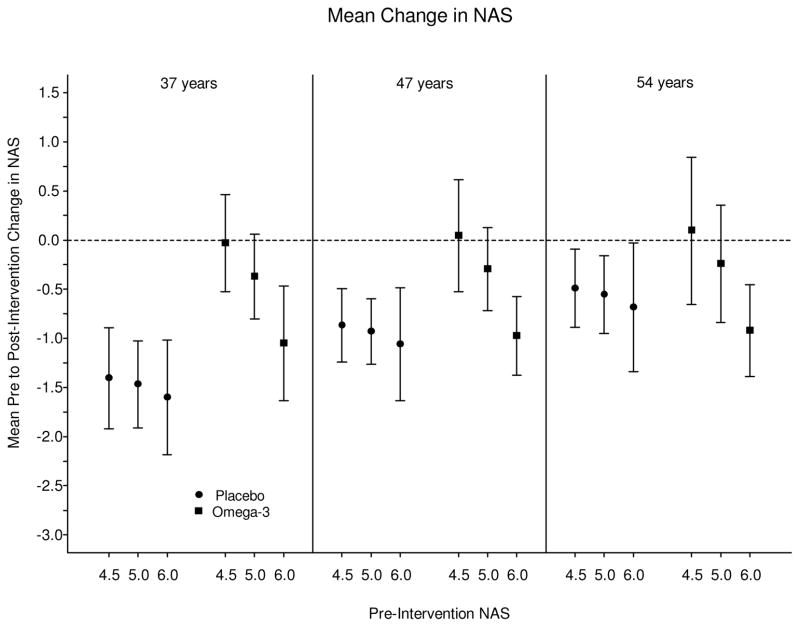
Four of 17 N-3- (24%) and 3 of 17 placebo-treated subjects (18%) had reduction in NAS of 2 points or more without progression of fibrosis (p=0.99); there was no significant difference between the two groups for the primary endpoint after adjustment for weight change, age, and baseline NAS (OR =1.53; 95% CI: [0.27, 9.72], p=0.64). (Figure 1).
Figure 1. Mean changes in NAS, stratified by age and baseline NAS score.
Overall, there was no difference in change in NAS across the two groups over the study period. There was also no difference in resolution of NASH (NAS < 4 at end-of-study). However, the magnitude of baseline score and age significantly influenced changes in these histological parameters.
Secondary Analyses
Univariate and multivariate analyses were conducted to compare the pre- and post-intervention differences within and between groups (Table 2). Covariates were identified by ANCOVA testing from independent variables for each dependent variable, and change in weight, age, and the magnitude of baseline value (more extreme baseline value inherently has more opportunity to improve) were all significant covariates. Exercise capacity was not a significant covariate in any analysis and was thus excluded. Weight change, on the other hand, was a significant covariate in nearly all analyses.
Table 2.
Comparison of Pre- and Post-Intervention Clinical and Laboratory Variables Within and Between Treatment Groups
| Placebo Baseline | Placebo Post | P Value Within Placebo Group (Paired) | N-3 Baseline | N-3 Post | P Value Within N-3 Group (Paired) | P Value For Difference Between Groups | |
|---|---|---|---|---|---|---|---|
| Weight (kg) | 89.0 (14.6) | 88.8 (16.2) | 0.85 | 95.2 (20.6) | 93.7 (22.9) | 0.21 | 0.38 |
| BMI (kg/m2) | 31.6 (6.8) | 31.7 (7.6) | 0.99 | 33.3 (8.0) | 32.8 (8.9) | 0.50 | 0.29 |
| ALT (U/L) | 60.8 (40.0) | 52.8 (31.0) | 0.30 | 72.8 (30.9) | 56.7 (28.3) | 0.13 | 0.41 |
| Fasting glucose (mg/dl) | 114.5 (21.3) | 110.7 (18.8) | 0.15 | 106.3 (14.7) | 111.6 (24.2) | 0.23 | 0.07 |
| Fasting insulin (mIU/l) | 20.4 (13.0) | 15.6 (10.2) | 0.11 | 25.2 (14.0) | 23.0 (22.6) | 0.69 | 0.67 |
| HOMA-IR | 6.0 (4.8) | 4.4 (3.2) | 0.13 | 6.6 (3.8) | 7.0 (8.6) | 0.85 | 0.39 |
| QUICKI | 0.304 (0.021) | 0.332 (0.056) | 0.04 | 0.301 (0.030) | 0.326 (0.062) | 0.07 | 0.87 |
| QUICKI-FFA | 0.313 (0.030) | 0.337 (0.056) | 0.07 | 0.318 (0.050) | 0.389 (0.213) | 0.20 | 0.37 |
| Tot Cholesterol (mg/dl) | 189.2 (29.4) | 198.6 (37.7) | 0.17 | 191.5 (41.0) | 193.9 (45.8) | 0.75 | 0.52 |
| LDL (md/dl) | 118.5 (27.7) | 128.2 (34.8) | 0.15 | 122.6 (32.6) | 124.1 (36.3) | 0.79 | 0.47 |
| Triglycerides (mg/dl) | 190.8 (70.8) | 187.7 (89.2) | 0.86 | 155.0 (77.1) | 132.2 (45.4) | 0.38 | 0.31 |
| Free fatty acids | 0.893 (0.274) | 1.007 (0.400) | 0.18 | 0.820 (0.448) | 0.830 (0.425) | 0.94 | 0.50 |
| M30 | 856.2 (329.8) | 762.9 (204.7) | 0.02 | 884.8 (307.8) | 723.0 (732.4) | 0.08 | 0.47 |
| M65 | 939.6 (436.3) | 811.9 (434.1) | 0.12 | 1421.4 (1454.1) | 839.0 (422.2) | 0.15 | 0.25 |
| Adiponectin (mmol/L) | 6.0 (1.7) | 6.4 (1.9) | 0.01 | 5.9 (1.6) | 6.2 (1.5) | 0.13 | 0.97 |
| IGFBP-1 | 18.7 (15.8) | 18.1 (21.0) | 0.88 | 21.4 (25.4) | 20.3 (34.0) | 0.84 | 0.93 |
| N6:N3 ratio | 82.0 (33.2) | 53.9 (31) | 0.47 | 68.8 (34) | 17.5 (19) | 0.001 | 0.16 |
| MRI Dixon Fat (%) | 11.3 (6.5) | 12.0 (5.6) | 0.82 | 18.4 (9.8) | 8.4 (5.2) | 0.015 | 0.02 |
| Image Fat (%) | 16.3 (4.7) | 14.3 (5.8) | 0.19 | 22.1 (10.4) | 16.4 (11.4) | 0.13 | 0.10 |
| Visceral Fat (cm2) | 193.9 (82.8) | 185.6 (87.4) | 0.78 | 242.6 (63.7) | 221.3 (76.8) | 0.10 | 0.48 |
| VO2max (ml/kg/min) | 16.7 (4.1) | 18.4 (4.1) | 0.33 | 17.1 (7.3) | 18.4 (6.9) | 0.43 | 0.78 |
| Fructose Intake | 19.8 (9.6) | 16.4 (6.8) | 0.23 | 35.1 (18.8) | 30.4 (14.1) | 0.64 | 0.99 |
| Activity Level (met-H/wk) | 133.6 (67.5) | 143.7 (84.5) | 0.55 | 129.5 (79.5) | 139.4 (74.7) | 0.63 | 0.99 |
Values are shown as mean (SD).
Histologic
Overall, there was no difference in change in NAS across the two groups over the study period. There was also no difference in resolution of NASH (NAS < 4 at end-of-study) (p=0.16). In the placebo group, 3 subjects had improved fibrosis stage and 5 worsened by one or more stages while 2 improved and 4 had worsened fibrosis in the N-3 group. No subjects developed overt signs of portal hypertension, although one subject had cirrhosis on the exit biopsy. At study end, 10 subjects showed advanced fibrosis (≥ stage 3), and 13 patients had stage 2 fibrosis, with no differences between treatment groups.
The magnitude of baseline score and age significantly influenced changes in these histological parameters. Placebo-treated subjects with lower baseline NAS scores had relative improvement in an age-related manner: subjects in the lowest 2 quartiles of age (≤37 years or 38–47 years) showed significant improvement when baseline NAS ≥ 5 (p=0.001) independent of weight loss, with the mean difference in NAS change in these age quartiles ranging from 0.64 to 1.37. However, this effect on NAS was not present in subjects with baseline NAS scores ≥ 6 or in the highest two age quartiles (≥ 54 years), and it was not observed in the N-3 treated group (Figure 1). In the overall cohort, N-3 did not show more histologic fat reduction by NAS scoring, but the fish oil group did show a greater fat reduction in the subgroup of subjects with baseline histologic steatosis score > 2 (p=0.009). This was mirrored in the MRI findings detailed below.
Hepatic Fat Content by Computer-Assisted Biopsy Image Analysis
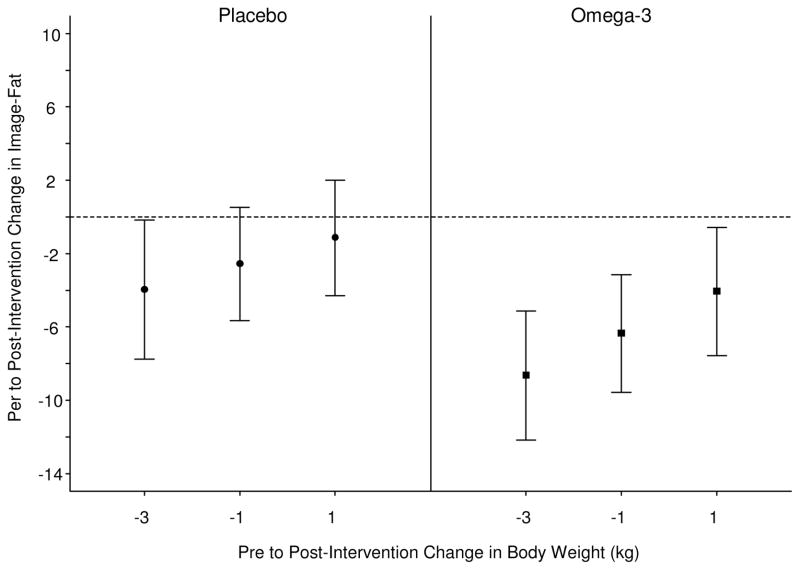
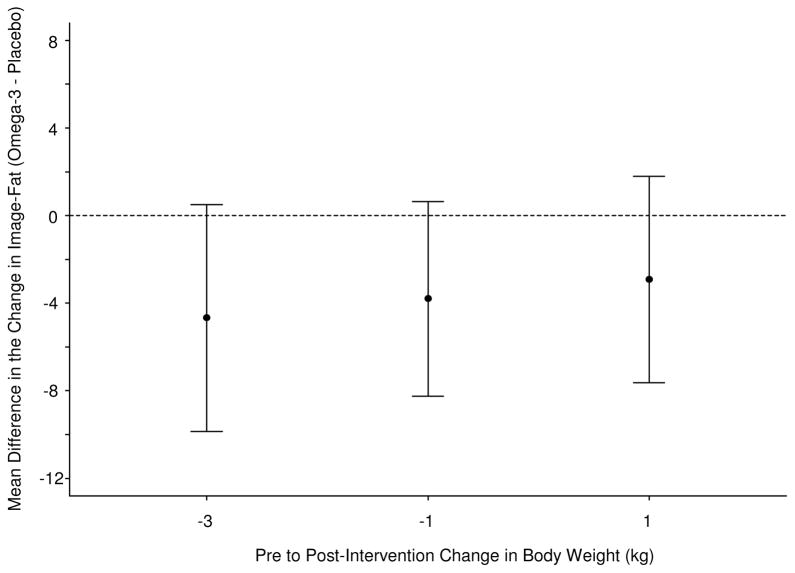
N-3 treatment showed strong trends towards greater fat reduction on fat morphometry, with the p-values for subjects in the lowest two quartiles of weight loss (> 3kg and 1–3 kg of weight loss) of 0.075 and 0.091 respectively (Figure 2a, 2b).
Figure 2. Mean change in liver fat assessed by computer-assisted image morphometry of liver biopsy tissue.
(A) 95% confidence intervals for the mean pre to post-intervention change in Image-Liver-Fat, evaluated at the 1st, 2nd, and 3rd quartiles of the pre to post-intervention change in body weight (kg) distribution. This figure demonstrates that N-3 intervention led to strong trends in improvement in liver fat by histological image analysis than placebo at the lowest 2 quartiles of weight change.
(B) Mean difference in pre to post-intervention change in Image-Liver-fat, between N-3 and placebo evaluated at the 1st, 2nd, and 3rd, quartiles of the pre to post-intervention change in body weight distribution. This figure shows that N-3 intervention led to similar change in Steatosis by image analysis regardless of weight change.
Radiologic
Hepatic Fat Content by MRI
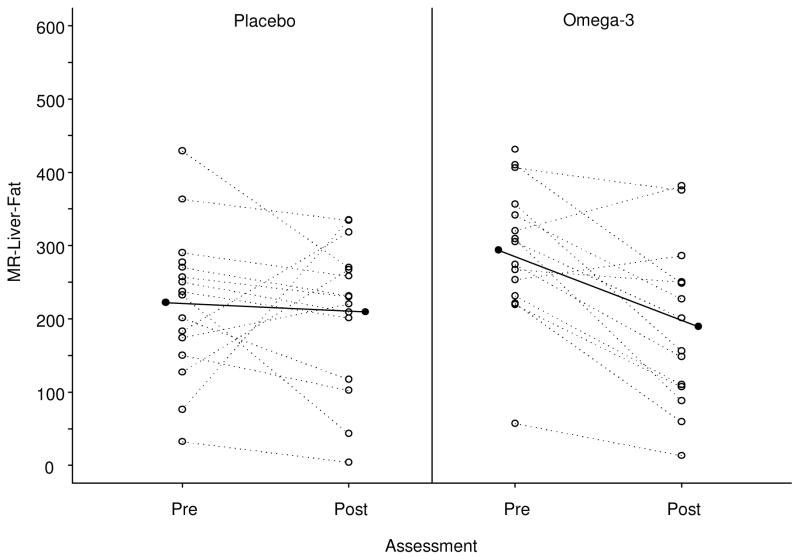
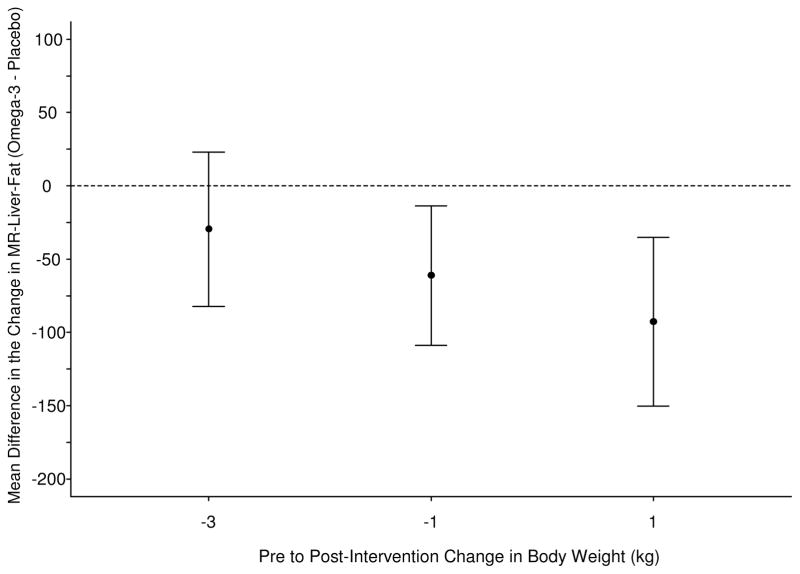
Consistent with findings by computer-assisted biopsy image analysis, among subjects who either gained or had stable weight, N-3 treated subjects showed a larger difference in liver fat content by MRI than placebo treated subjects (p=0.014 for 2nd quartile and p=0.003 for 3rd quartile for change in weight in the N-3 group) (Figure 3a, 3b). Visceral and abdominal fat loss was not significantly different between groups. Waist circumference decreased along with liver fat by biopsy image analysis and MRI in a similar fashion with a larger decrease in the fish oil group (3.2 cm mean decrease versus 0.1 cm mean increase in placebo group) which trended toward significance (p=0.084). The within-group difference in waist circumference was also significant in the fish oil group (p=0.018).
Figure 3. Changes in liver fat assessed by MRI.
(A) Scatterplot and trend-line of adjusted MRI liver fat measurements at baseline and end-of-study. N-3 led to significant improvement in liver fat compared to placebo.
(B) Mean difference in pre to post-intervention change in MR-Liver-fat, between N-3 and placebo evaluated at the 1st, 2nd, and 3rd, quartiles of the pre to post-intervention change in body weight distribution. N-3 intervention appears to have greater effects in patients with lesser weight loss or weight gain.
Biochemical
The magnitudes of change for ALT, total cholesterol, and LDL cholesterol effects were not significantly different between treatment groups although serum triglycerides trended toward a larger decrease in the N-3 group (p=0.084). Analyses of ALT were stratified by baseline ALT level and weight. Patients with baseline ALT ≥ 90 IU/L had significant improvement regardless of weight change or treatment assignment. Weight loss was the common significant variable for improvement in ALT in the lower quartiles of baseline ALT (1st quartile: ALT 45 IU/L, 2nd quartile: ALT 65 IU/L), with even modest weight loss of at least 1 kg resulting in statistically significant improvement in ALT. In subjects with weight loss of ≥ 5 kg, fasting serum glucose showed a significant mean difference in change between groups of 11.5 g/dl (p=0.013), with a decrease in the placebo group versus a small increase in the N-3 group.
Metabolic
Despite the difference in change in fasting blood glucose between groups, both showed similar improvements in insulin resistance indexes. There was no difference in change in HOMA-IR or QUICKI between groups, although changes within each group was closely associated with magnitude of weight loss, but the N-3 group did show a marginal but significant within-group difference in QUICKI-FFA over the study period (p=0.05). Adiponectin increased similarly in both groups and was not different.
Serum markers of inflammation and cell injury
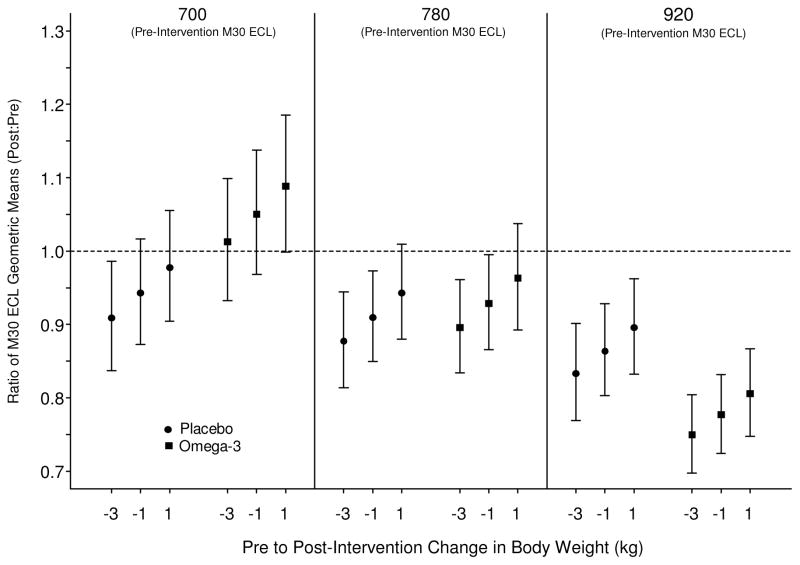
N-3 did not have an independent effect on either the M30 or M65 compared to placebo but weight loss was associated with improvement in both groups (Figure 4a, 4b). Among subjects with higher baseline M30 levels treated with N-3 supplements, those with concomitant weight loss had significant reduction in M30 (p=0.04), suggesting a possible synergistic effect.
Figure 4. Mean changes in M30 serum biomarker.
95% confidence intervals for the ratio of M30 ECL geometric means (post-intervention:pre-intervention), evaluated at the 1st, 2nd, and 3rd quartiles of the pre-intervention M30-ECL and Δ body weight distributions. This figure demonstrates the consistent effects of weight loss on M30 and that N-3 intervention resulted in greater positive impact (decrease in Post:Pre M30 ratio on y-axis) at higher baseline M30 levels, which has been linked to increased baseline necroapoptosis.
Keratin-8,18 Immunohistochemistry
Keratin-8,18 staining was available in 24 subjects due to inaccessible tissue blocks and scarcity of biopsy tissue for post-hoc processing and analysis. Defining ballooning as enlarged hepatocytes with absent keratin-8,18 (keratin ‘empty’ cells), 17 of these 24 subjects scored ≥ 1 for ballooning by this criterion at baseline. The fibrosis stage was not significantly different in specimens without versus with empty cells (1.5 ± 0.7 versus 1.9 ± 0.9, p=0.16). However, taking M30 and M65 as the most established surrogate markers for hepatocellular injury, there were baseline differences in these parameters between subjects without versus with empty cells: M30, 726.3 ± 66 vs. 807.3 ± 136 (p=0.08) and M65, 711 ± 186 vs. 994 ± 424 (p=0.05). In 11 placebo-treated subjects with positive baseline ‘empty’ cells, the empty cell score declined from 1.3 ± 0.5 to 0.55 ± 0.8 (p=0.02). Among six N-3 treated subjects, this change was less apparent with a decline from 1.5 ± 0.6 to 0.83 ± 0.7 (p=0.1). However, serum M30 declined significantly only in the N-3 treated subjects with keratin-8,18-confirmed ballooning from 1081 ± 413 to 682 ± 114 (p=0.05).
Red Blood Cell (RBC) Lipid Composition
Over the study period, RBC lipid composition (N-6:N-3 ratio) decreased significantly only in N-3 treated patients from 68.8 ± 34 (cohort baseline mean) to 17.5 ± 19 in N-3 subjects compared to 53.9 ± 31 in placebo subjects (p=0.001) in unadjusted analysis.
Cardiovascular Conditioning
All subjects began and remained in the 10–20th percentile for VO2peak at the end of the study compared to age- and gender-matched normal subjects despite reported increases in mean weekly physical activity in both groups. No subjects crossed percentiles during the study despite corresponding changes in weight.
DISCUSSION
Our results demonstrate that N-3 PUFA at 3000 mg/day for one year did not lead to significant changes in overall histological activity in NASH patients (primary endpoint: ≥ 2 point reduction in NAS), despite adjusting for weight loss and other covariates. N-3 therapy was associated with reduced liver fat independent of weight loss as measured histologically by steatosis score and image morphometry and radiologically by MRI assessment. However, improvement in cell injury biomarkers (M30 and M65) did not occur uniformly with N-3 PUFA and only occurred in the setting of concomitant weight loss. N-3 administered in the setting of weight loss (even modest declines) was synergistic in decreasing liver fat compared to placebo. Fish oil had no independent effects on glucose or insulin levels, which indicates that N-3 PUFA probably affect liver fat independent of changes in insulin resistance. All subjects were severely physically deconditioned (VO2peak of 10th–20th percentile for age and gender) at baseline and at the close of study regardless of starting weight or change in weight and despite reported increased activity in the majority of subjects. This refractoriness to exercise suggests skeletal muscle dysfunction may be a key feature of disease pathogenesis.
A number of potential health benefits have been attributed to N-3 PUFA including reduction in cardiovascular risks, improved lipoprotein and insulin metabolism, and reduction in hepatic fat content. In this randomized, placebo controlled trial which focused on their effects in patients with NASH, we did not detect consistent beneficial effects on blood lipid composition (aside from a trend in triglyceride reduction) or insulin sensitivity at the selected dose and duration of therapy although one year of N-3 was associated with a substantial change in RBC membrane lipid composition. Because of the relatively short life-span of RBCs compared to other tissues, one can speculate that a longer duration of therapy may have produced more evident systemic effects.
Our study has several limitations. We encountered similar problems to other studies in inter-observer variation in evaluating NAS score, especially hepatocyte ballooning [23–26, 32–33]. There was reasonable agreement in overall activity score within ranges of low, intermediate or high NAS, but there were systematic scoring differences between observers in evaluating individual parameters, with cellular ballooning being especially problematic. To address this issue further, we reanalyzed our data using blinded reading of K-8,18 stained specimens to address ballooning [20]. This showed that baseline M30 and M65 levels were significantly higher in subjects with ballooning by this more stringent criterion, and N-3 therapy appeared to be associated with larger improvements in M30 and M65 in this subgroup with more strictly defined ballooning.
In addition, our study was mildly underpowered, given the desired sample size of 18 subjects per group. We had a significant number of screen failures in the proscribed enrollment period, and patient drop-out was greater than expected, leaving a lower-than-desired number of evaluable patients estimated to be necessary to detect a between-group difference in the primary outcome, which may have led to type II error. Travel expenses in our rural setting and budgetary constraints for travel support contributed to this limitation.
Despite performing analysis with baseline covariate adjustment and accounting for the confounders of weight loss and physical activity, we may have not controlled for all confounding factors. Several of our conclusions are based on subgroup analyses for which the study was not specifically powered; clearly, these post-hoc findings should be interpreted with caution. Additionally, it is unclear whether our selected dose of N-3 fish oil (3 g daily) or duration of therapy was optimal. Finally, we had a large number of subjects with advanced fibrosis (56% of subjects had stage 2 or greater and 29% had stage 3 at baseline), and despite accurate allocation and randomization, more patients with high fibrosis were enrolled in the N-3 vs. placebo groups (12 vs. 7 subjects with stage 2 or more, 6 vs. 4 subjects with stage 3), and this may have altered the treatment response.
We did observe consistent effects on liver fat content. N-3 has been postulated to affect steatosis by several different pathways. With reduction in plasma triglycerides, delivery of fatty acids to the liver may be reduced. PUFA also favorably alters hepatic fat synthetic pathway transcription factors such as PPAR-alpha and SREBP-1 [34,35]. Dietary PUFA also alters inflammatory cell membranes and prostaglandin metabolism: increased intake of N-3 is associated with decreased arachidonic acid production related to cell membrane phospholipid composition [36]. It seems likely that these potential mechanisms are affected by interactions between hepatic, muscle and peripheral fat stores, membrane lipid composition and insulin sensitivity [9,37]. Our results support that N-3 fats reduce liver fat by a mechanism other than modulating insulin sensitivity, as liver fat content decreased in the N-3 group despite no significant change in insulin sensitivity while the placebo group had tangible improvements in insulin resistance without appreciable changes in liver or visceral fat on MRI. A recent placebo-controlled trial of N-3 PUFA agent containing solely eicosapentaenoic acid (EPA) did not demonstrate a significant difference in EPA- versus placebo-treated patients with regards to overall NAS score, steatosis, inflammation, ballooning, or fibrosis, or to several serum parameters aside from mildly lower serum triglyceride levels [38]. One reason our study may have shown different results is that the docosahexanoic acid (DHA) component contained in our preparation is important, either alone or synergistically with EPA, in the efficacy of N-3 in lowering liver fat; however, further studies are needed to test this hypothesis. Another possibility for the differences is that we measured liver fat by several different means – MRI imaging and histologically with conventional methods and with computer-assisted image morphometry – and this may have improved our sensitivity to detect changes in liver fat. The disconnect between liver fat reduction and lack of histological improvement in steatohepatitis raises the question of whether liver fat is a sufficient target in NASH trials.
In summary, our results show that N-3 PUFA does not have substantial effects on NASH histological activity with 1 year of therapy, although our findings do show that N-3 PUFA causes a decrease in hepatic fat content and changes in RBC lipid composition, which were independent of dietary weight loss, insulin sensitivity, and changes in physical conditioning. Whether or not longer term treatment or higher dose of N-3 would show different results is speculative and uncertain; however, therapeutic trials of NASH evaluating other future agents should consider the possible confounding effect of N-3 intake given our findings.
Supplementary Material
Acknowledgments
Support: This study was supported by NIH NCCAM Grant 5R21AT2901–2 and 5 M01 RR00847. Study medication and identical appearing placebo was provided at no charge by Nordic Natural. RBC phospholipid profile was performed by Metametrix (www.metametrix.com). M30, M65, adiponectin, and IGFBP-1 electrochemiluminescence assays were performed by Wellstat Diagnostics (www.wellstatdiagnostics.com).
The authors wish to express their gratitude to Dr. Keith Lindor, Dr. Anthony McCall, Dr. Jamal Ibdah and Dr. Mark Conaway for guidance and to Marty Phillips for database construction.
Footnotes
Conflict of Interest Statement: The authors do not have any commercial associations that might pose a conflict of interest in connection with the submitted manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos AP. Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. World Rev Nutr Diet. 2011;102:10–21. doi: 10.1159/000327785. [DOI] [PubMed] [Google Scholar]
- 3.RE: Health Claim Petition: Omega-3 Fatty Acids and Reduced Risk of Coronary Heart Disease (Docket No. 2003Q-0401) Sep 8, 2004. [Accessed on April 19, 2013]. Letter responding to a request to reconsider the qualified claim for a dietary supplement health claim for omega-3 fatty acids and coronary heart disease. [Google Scholar]
- 4.Cortez-Pinto H, Jesus L, Barros H, et al. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr. 2006;25:816–23. doi: 10.1016/j.clnu.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Musso G, Gambino R, De Michieli F, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–16. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 6.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol. 2007;47:711–7. doi: 10.1016/j.jhep.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci. 2004;106:635–43. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 8.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 9.Borkman M, Storlien LH, Pan DA, et al. The relationship between insulin sensitivity and the fatty acid composition of skeletal muscle. N Engl J Med. 1993;328:238–44. doi: 10.1056/NEJM199301283280404. [DOI] [PubMed] [Google Scholar]
- 10.Pettinelli P, del Pozo T, Araya J, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta–Mol Basis Dis. 2009;1792:1080–6. doi: 10.1016/j.bbadis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Masteron GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids – a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:679–92. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 12.Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–51. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–12. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 14.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 15.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 16.Kris-Etherton PM, Harris WS, Appel LJ. AHA scientific statement: fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32 (Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 18.Abdelmalek MF, Suzuki A, Guy C, et al. NASH Clinical Research Network (CRN) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–71. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caldwell S, Ikura Y, Dias D, et al. Hepatocellular Ballooning in NASH. J Hepatol. 2010;53:719–23. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lackner C, Gogg-Kamerer M, Zatloukal K, et al. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48:821–8. doi: 10.1016/j.jhep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, et al. NASH Clinical Research Network (CRN) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 23.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–82. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, Yah MM, Guy CD, et al. Creation of a continuous visual scale of ballooned hepatocytes in non-alcoholic fatty liver disease [abstract] Hepatology. 2008;48(4 Suppl):815A. [Google Scholar]
- 25.Juluri R, Vuppalanchi R, Olson J, et al. Generalizability of the NASH-CRN histologic scoring system for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2011;45:55–8. doi: 10.1097/MCG.0b013e3181dd1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gawrieh S, Knoedler DM, Saeian K, et al. Effects of interventions on intra- and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann Diagnost Path. 2011;15:19–24. doi: 10.1016/j.anndiagpath.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Raptis DA, Fischer MA, Graf R, et al. MRI: the new reference standard in quantifying hepatic steatosis? Gut. 2012;61:117–27. doi: 10.1136/gutjnl-2011-300155. [DOI] [PubMed] [Google Scholar]
- 28.Noworolski SM, Lam MM, Merriman RB, et al. Liver steatosis: concordance of MR imaging and MR spectroscopic data with histologic grade. Radiology. 2012;264:88–96. doi: 10.1148/radiol.12110673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perseghin G, Caumo A, Caloni M, et al. Incorporation of the fasting plasma FFA concentration into QUICKI improves its association with insulin sensitivity in nonobese individuals. J Clin Endocrinol Metab. 2001;86:4776–81. doi: 10.1210/jcem.86.10.7902. [DOI] [PubMed] [Google Scholar]
- 30.Elizondo A, Araya J, Rodrigo R, et al. Effects of weight loss on liver and erythrocyte polyunsaturated fatty acid pattern and oxidative stress status in obese patients with non-alcoholic fatty liver disease. Biol Res. 2008;41:59–68. [PubMed] [Google Scholar]
- 31.Dupont WD, Plummer WD. Power and sample size calculationsL a review and computer program. Control Clin Trials. 1990;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 32.Brunt EM, Kleiner DE, Wilson LA, et al. NASH Clinical Research Network. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–20. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal AJ, Chalasani N, Kowdley KV, et al. NASH Clinical Research Network. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekiya M, Yahagi N, Matsuzaka T, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–39. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Emilia IP, Farrell GC, Roberston G, et al. Central role of PPARα-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38:123–132. doi: 10.1053/jhep.2003.50307. [DOI] [PubMed] [Google Scholar]
- 36.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 37.Green RM. NASH–hepatic metabolism and not simply the metabolic syndrome. Hepatology. 2003;38:14–17. doi: 10.1053/jhep.2003.50325. [DOI] [PubMed] [Google Scholar]
- 38.Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377–84. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.