Abstract
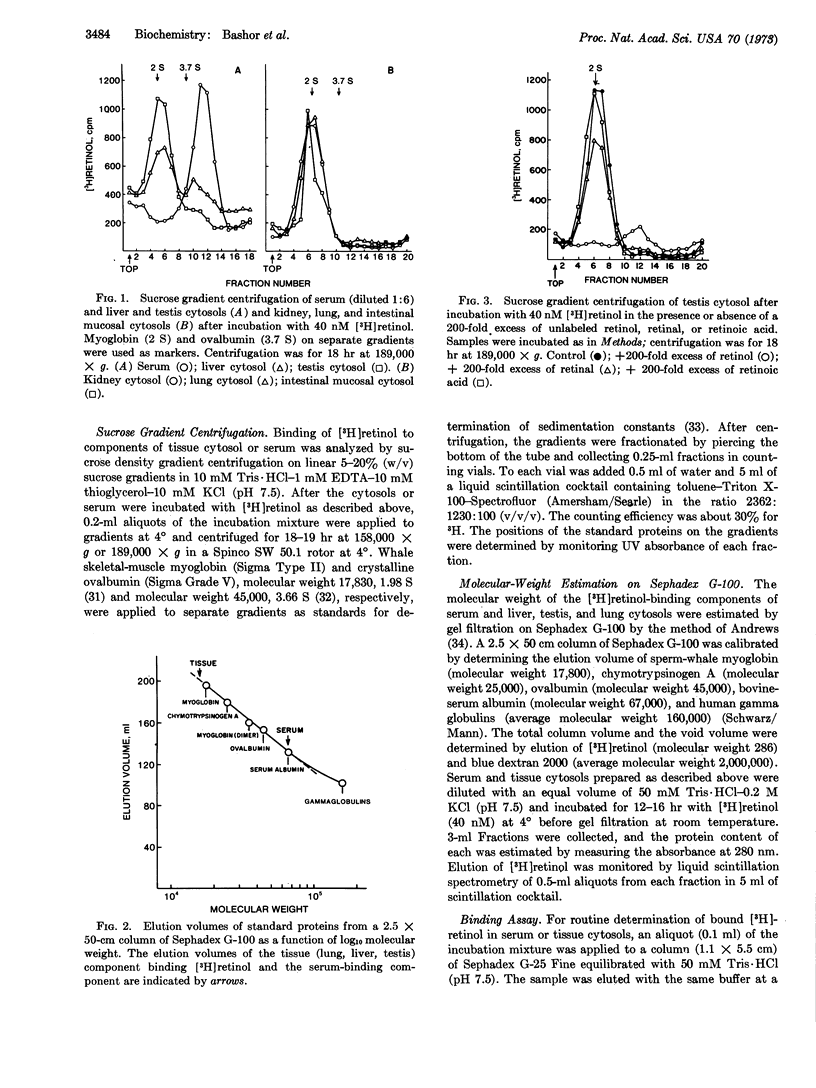
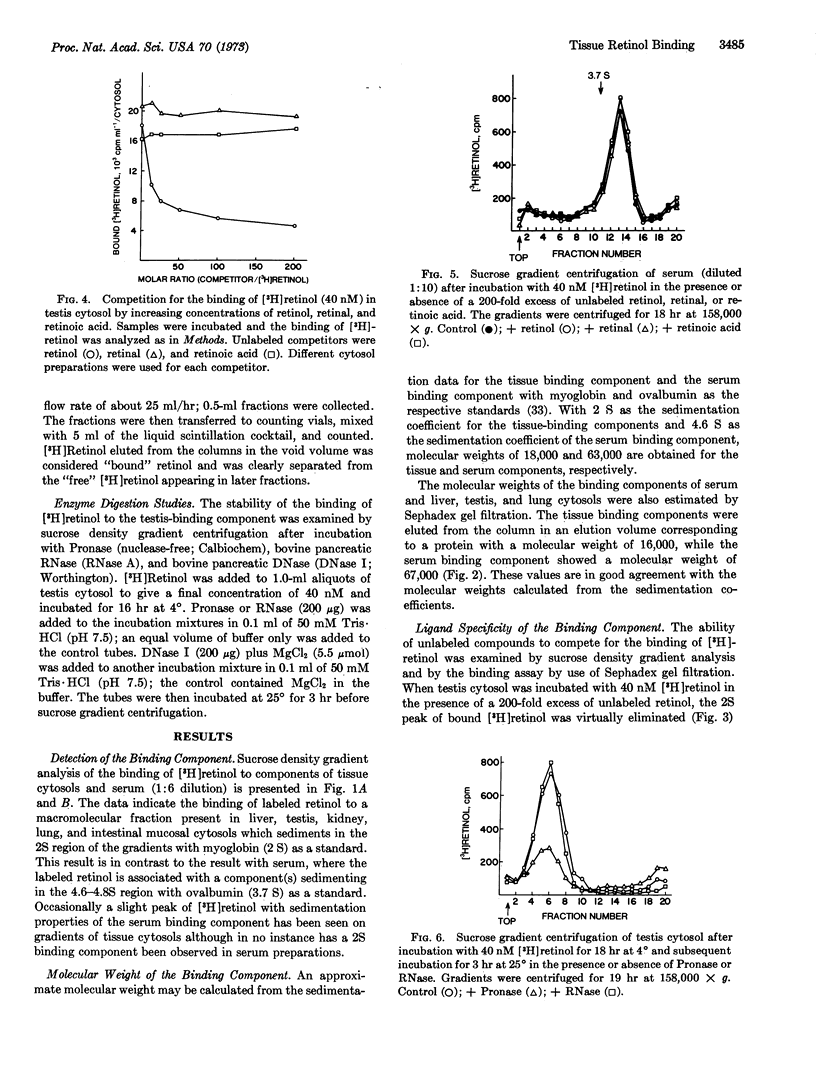
The high-speed supernatant fraction of rat liver, lung, kidney, testis, and intestinal mucosa contains a component capable of binding [3H]retinol in vitro when binding is analyzed by sucrose density gradient centrifugation or gel filtration. This binding component can be distinguished from one identified in rat serum. Whereas the tissue component sediments in the 2S region of sucrose gradients, the serum component sediments in the 4.6S region. Molecular weight estimations by gel filtration indicate molecular weights of 16,000 and 67,000 for the tissue and serum binding components, respectively. Unlabeled retinol, but not retinoic acid, competes for the binding of [3H]retinol in tissue cytosols. Competition for the binding of [3H]retinol by unlabeled retinal has also been observed in tissue cytosols, but may result from the in vitro reduction of retinal to retinol. Unlabeled retinol, retinal, and retinoic acid fail to compete for the binding of [3H]retinol in serum under the conditions used. The tissue binding component (testis) is sensitive to digestion with Pronase, but not with RNase or DNase, indicating a protein nature for this component.
Keywords: density gradient centrifugation, gel filtration, retinal, retinoic acid, serum
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRESLOW E. CHANGES IN SIDE CHAIN REACTIVITY ACCOMPANYING THE BINDING OF HEME TO SPERM WHALE APOMYOGLOBIN. J Biol Chem. 1964 Feb;239:486–496. [PubMed] [Google Scholar]
- Beato M., Brändle W., Biesewig D., Sekeris C. E. On the mechanism of hormone action. XVI. Transfer of (1,2-3H2)cortisol from the cytoplasm to the nucleus of rat-liver cells. Biochim Biophys Acta. 1970 Apr 14;208(1):125–136. [PubMed] [Google Scholar]
- Chamness G. C., McGuire W. L. Estrogen receptor in the rat uterus. Physiological forms and artifacts. Biochemistry. 1972 Jun 20;11(13):2466–2472. doi: 10.1021/bi00763a013. [DOI] [PubMed] [Google Scholar]
- De Luca L., Kleinman H. K., Little E. P., Wolf G. RNA metabolism in rat intestinal mucosa of normal and vitamin A-deficient rats. Arch Biochem Biophys. 1971 Jul;145(1):332–337. doi: 10.1016/0003-9861(71)90043-9. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Wald G. THE BIOLOGICAL FUNCTION OF VITAMIN A ACID. Proc Natl Acad Sci U S A. 1960 May;46(5):587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman I. S., Fimognari G. M. On the biochemical mechanism of action of aldosterone. Recent Prog Horm Res. 1968;24:1–44. doi: 10.1016/b978-1-4831-9827-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Fang S., Anderson K. M., Liao S. Receptor proteins for androgens. On the role of specific proteins in selective retention of 17-beta-hydroxy-5-alpha-androstan-3-one by rat ventral prostate in vivo and in vitro. J Biol Chem. 1969 Dec 25;244(24):6584–6595. [PubMed] [Google Scholar]
- Fidge N. H., Goodman D. S. The enzymatic reduction of retinal to retinol in rat intestine. J Biol Chem. 1968 Aug 25;243(16):4372–4379. [PubMed] [Google Scholar]
- Gardner R. S., Tomkins G. M. Steroid hormone binding to a macromolecule from hepatoma tissue culture cells. J Biol Chem. 1969 Sep 10;244(17):4761–4767. [PubMed] [Google Scholar]
- Gorski J., Toft D., Shyamala G., Smith D., Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res. 1968;24:45–80. doi: 10.1016/b978-1-4831-9827-9.50008-3. [DOI] [PubMed] [Google Scholar]
- Herman T. S., Fimognari G. M., Edelman I. S. Studies on renal aldosterone-binding proteins. J Biol Chem. 1968 Jul 25;243(14):3849–3856. [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. C., Kennedy M., Chiba N. Vitamin A and nuclear RNA synthesis. Am J Clin Nutr. 1969 Aug;22(8):1048–1058. doi: 10.1093/ajcn/22.8.1048. [DOI] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. G., Baker M. S., Smith J. M., Henderson W. R., Harris C. C., Sporn M. B., Saffiotti U. RNA metabolism in tracheal epithelium: alteration in hamsters deficient in vitamin A. Science. 1972 Sep 22;177(4054):1105–1108. doi: 10.1126/science.177.4054.1105. [DOI] [PubMed] [Google Scholar]
- Liao S., Fang S. Receptor-proteims for androgens and the mode of action of androgens on gene transcription in ventral prostate. Vitam Horm. 1969;27:17–90. doi: 10.1016/s0083-6729(08)61124-3. [DOI] [PubMed] [Google Scholar]
- Logan W. S. Vitamin A and keratinization. Arch Dermatol. 1972 May;105(5):748–753. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mainwaring W. I. A soluble androgen receptor in the cytoplasm of rat prostate. J Endocrinol. 1969 Dec;45(4):531–541. doi: 10.1677/joe.0.0450531. [DOI] [PubMed] [Google Scholar]
- Muto Y., Goodman D. S. Vitamin A transport in rat plasma. Isolation and characterization or retinol-binding protein. J Biol Chem. 1972 Apr 25;247(8):2533–2541. [PubMed] [Google Scholar]
- Muto Y., Smith J. E., Milch P. O., Goodman D. S. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J Biol Chem. 1972 Apr 25;247(8):2542–2550. [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Sherman M. R., Toft D. O. Progesterone "receptors" in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci U S A. 1970 Oct;67(2):501–508. doi: 10.1073/pnas.67.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley B. W., Toft D. O., Sherman M. R. Progesterone-binding components of chick oviduct. II. Nuclear components. J Biol Chem. 1971 Feb 25;246(4):1117–1122. [PubMed] [Google Scholar]
- Peterson P. A., Berggård I. Isolation and properties of a human retinol-transporting protein. J Biol Chem. 1971 Jan 10;246(1):25–33. [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Ostberg L., Andersson L., Kamwendo F., Pertoft H. Studies on the transport and cellular distribution of vitamin A in normal and vitamin A-deficient rats with special reference to the vitamin A-binding plasma protein. J Biol Chem. 1973 Jun 10;248(11):4009–4022. [PubMed] [Google Scholar]
- Sherman M. R., Corvol P. L., O'Malley B. W. Progesterone-binding components of chick oviduct. I. Preliminary characterization of cytoplasmic components. J Biol Chem. 1970 Nov 25;245(22):6085–6096. [PubMed] [Google Scholar]
- Smith J. E., Muto Y., Milch P. O., Goodman D. S. The effects of chylomicron vitamin A on the metabolism of retinol-binding protein in the rat. J Biol Chem. 1973 Mar 10;248(5):1544–1549. [PubMed] [Google Scholar]
- Spelsberg T. C., Steggles A. W., O'Malley B. W. Progesterone-binding components of chick oviduct. 3. Chromatin acceptor sites. J Biol Chem. 1971 Jul 10;246(13):4188–4197. [PubMed] [Google Scholar]
- Tryflates G. P., Krause R. F. Altered messenger RNA synthesis in vitamin A deficient rat liver. Life Sci II. 1971 Oct 8;10(19):1097–1103. doi: 10.1016/0024-3205(71)90261-x. [DOI] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968 Oct 11;162(3850):230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- Wong Y. C., Buck R. C. An electron microscopic study of metaplasia of the rat tracheal epithelium in vitamin A deficiency. Lab Invest. 1971 Jan;24(1):55–66. [PubMed] [Google Scholar]
- Zachman R. D. The stimulation of RNA synthesis in vivo and in vitro by retinol (vitamin A) in the intestine of vitamin A deficient rats. Life Sci. 1967 Oct 15;6(20):2207–2213. doi: 10.1016/0024-3205(67)90244-5. [DOI] [PubMed] [Google Scholar]
- Zile M., Deluca H. F. Vitamin A and ribonucleic acid synthesis in rat intestine. Arch Biochem Biophys. 1970 Sep;140(1):210–214. doi: 10.1016/0003-9861(70)90024-x. [DOI] [PubMed] [Google Scholar]



