Abstract
We have examined the chemical and functional reversibility of oxidative modification in myosin. Redox regulation has emerged as a crucial modulator of protein function, with particular relevance to aging. We previously identified a single methionine residue in Dictyostelium discoideum (Dicty) myosin II (M394, near the myosin cardiomyopathy loop in the actin-binding interface) that is functionally sensitive to oxidation. We now show that oxidation of M394 is reversible by methionine sulfoxide reductase (Msr), restoring actin-activated ATPase activity. Sequence alignment reveals that M394 of Dicty myosin II is a cysteine residue in all human isoforms of skeletal and cardiac myosin. Using Dicty myosin II as a model for site-specific redox sensitivity of this Cys residue, the M394C mutant can be glutathionylated in vitro, resulting in reversible inhibition of actin-activated ATPase activity, with effects similar to those of methionine oxidation at this site. This work illustrates the potential for myosin to function as a redox sensor in both non-muscle and muscle cells, modulating motility/contractility in response to oxidative stress.
Keywords: Dictyostelium, myosin II, Reactive Oxygen Species (ROS), methionine, Methionine sulfoxide reductase (Msr), glutathionylation, redox
1. Introduction
To maintain functional homeostasis within cells, molecules must sensitively detect and respond to the cellular redox state. Reactive oxygen species (ROS) are a family of oxygen-derived molecules that cause covalent modification of proteins and DNA. Many physiological and pathological conditions are associated with the accumulation of ROS, which are countered by an elaborate system of antioxidant enzymes and small molecules [1]. An imbalance between the levels of ROS and antioxidants is generally termed oxidative stress. The most sensitive and selective targets of ROS and antioxidants within proteins, with particular relevance to aging, are the sulfur-containing amino acids, methionine (Met) and cysteine (Cys) [2].
The rapid rate and reversibility of sulfur oxidationgive Met and Cys important roles as functional redox switches in proteins. For example, methionine sulfoxide (MetO) can be reduced to Met by the thioredoxin-dependent enzyme methionine sulfoxide reductase (Msr). Cys oxidation can result in reversible intra- or intermolecular disulfide formation or other thiol modifications. One such modification is glutathionylation, which can covalently modify Cys by attachment of a glutathione (GSH) tripeptide. During episodes of oxidative stress, when the ratio of oxidized glutathione (glutathione disulfide, GSSG) to glutathione is high, susceptible Cys are more likely to be glutathionylated [3,4,5].
Sulfur-based redox switches are an integral component in cardiac and skeletal muscle function and regulation [6,7,8,9,10]. ROS-mediated mechanisms fundamental to muscle contractility include changes in muscle protein gene expression and post-translational modifications (PTMs)[11,12]. ROS-induced PTMs can indirectly affect muscle contractility, modifying proteins involved in calcium handling [13,14,15], enzymatic signaling [16,17], or proteolysis [18].
ROS-induced PTMs can also have direct effects on contractility by modifying sarcomeric proteins [18] such as myosin, the focus of the present study. Direct site-specific redox modifications to sarcomeric proteins are emerging as crucial regulators of muscle function. Met oxidation and glutathionylation in actin results in defects in polymerization [19] and activation of myosin ATPase activity [20,21,22,23]. Glutathionylation of specific Cys in troponin I and myosin binding protein C (MyBP-C) alter myofilament sensitivity to calcium [24,25]. Site-specific glutathionylation of titin can modulate muscle elasticity in muscle [26]. Myosin has been identified as a target of protein oxidation and glutathionylation [20,22,23,27,28], but little is known about the structural and functional significance of specific redox sites within myosin.
We have shown previously that site-specific methionine oxidation in the Dicty myosin II catalytic domain causes a functional decline in actomyosin interaction, correlated with changes in myosin internal dynamics and structure, specifically in the actin-binding cleft [29]. Oxidation of methionine at position 394 (M394), directly N-terminal to the cardiomyopathy (CM) loop of myosin, is responsible for the decline in function [29]. Sequence alignment of the Dicty myosin II catalytic domain with all human myosin II isoforms reveals that this residue is a conserved cysteine residue in all isoforms of human cardiac and skeletal myosin. This conserved Cys has been identified as a site of glutathionylation in β-cardiac myosin [27].
In the present study, our goal is to examine directly the redox sensitivity of this residue. Using site-directed mutagenesis, chemical modification, and redox-specific enzymes, we have produced and reversed site-specific redox modifications, including Met oxidation and Cys glutathionylation, and determined quantitatively the associated chemical and functional changes in the myosin catalytic domain. Our results show that myosin is an oxidatively labile protein whose level of activity can be regulated by the redox status of selective sulfur-containing functional groups within its catalytic domain.
2. Methods
2.1. Protein preparations and assays
Site-directed mutagenesis of the Dicty myosin II gene, truncated at residue 762, containing only a single (non-reactive) cysteine at position 655 (“Cys-lite” S1dC construct [30]), was performed using the Quik Change II XL kit (Invitrogen). In addition, all constructs (except for the M394C mutant) contain a T688C mutation (needed for sufficient protein expression in Dicty) which was spin-labeled with IASL [4-(2-Iodoacetamido)-2,2,6,6-tetramethyl-1-piperidinyloxy] (Sigma-Aldrich, USA) to protect from oxidative modification. These proteins were expressed and purified from Dicty or f+ cells as previously described [31]. F-actin was prepared from rabbit skeletal muscle as previously described [32,33]. Human methionine sulfoxide reductase A protein was obtained from Abcam Biochemicals (Cambridge, England). Actin-activated ATPase activity was measured by detection of ADP using an NADH-coupled ATPase assay [34] using 0.2 μM S1dC, 2 mM ATP, and increasing concentrations of phalloidin-stabilized F-actin in 10 mM Tris, 2 mM MgCl2 (pH 7.5). The actin-dependent activity was analyzed to determine Vmax (specific activity at saturating actin) and KATPase (actin concentration needed for V = 0.5Vmax) [34].
2.2. Reversible Oxidation and Glutathionylation
Oxidative modification of Met in myosin was accomplished by treatment with 500 mM hydrogen peroxide (purchased as a stabilized 30% solution from Sigma-Aldrich) for 30 minutes on ice, followed by dialysis. Reversal of Met oxidation was achieved by incubating 20μM myosin for 30 min at 25°C with 4 μM MsrA (Abcam) and 1 mM DTT in 10 mM Tris (pH 7.5). Glutathionylation of the M394C mutant myosin was accomplished by treatment with varying concentrations of GSH in the presence of 10 mM diamide for 1 h on ice, followed by dialysis. Glutathionylation was reversed by addition of 1 mM DTT for 1 h on ice, followed by dialysis.
2.3 Mass spectrometry
Myosin samples for electrospray ionization mass spectrometry (ESI-MS) were prepared as previously described [29]. Myosin mass was determined using a QSTAR quadrupole-TOF mass spectrometer (ABI) with an electrospray ionization source. 20 μM myosin was introduced into the solvent stream using a 10 μL injection loop installed in the integrated loop injector with a total of five injections per sample. ESI spectra were acquired continuously over the range 500 – 2000 m/z, and were analyzed with BioAnalyst QS (ABI) software v 1.1.5.
3. Results
3.1 Site-directed redox modification of myosin residue 394
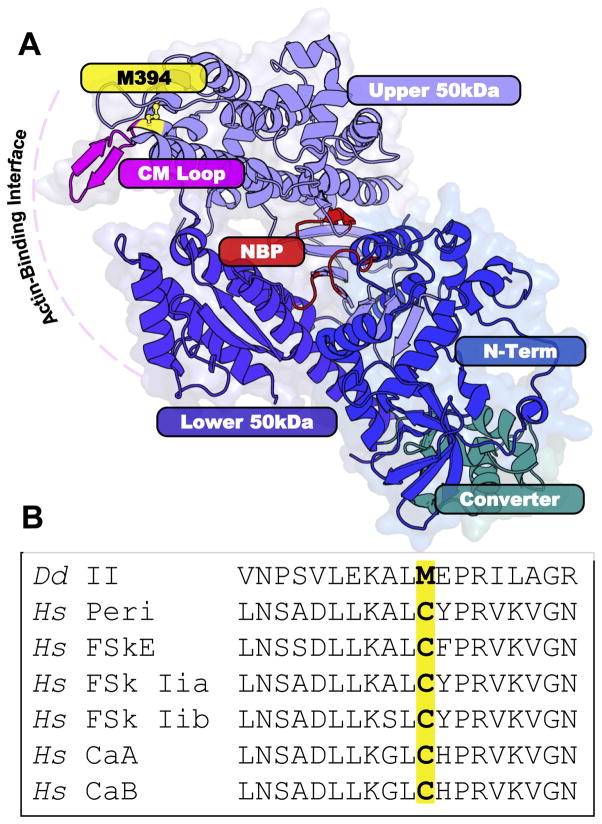
S1dC, a construct of the myosin II catalytic domain devoid of reactive cysteines, was used as the model system to examine the functional consequences of reversible oxidative modifications. Using Dicty myosin as such a model is justified by its high level of structural and functional homology with muscle myosin II [35,36]. S1dC contains nine Met residues, three of which are susceptible to oxidative modification by H2O2 [29]. Oxidation of Met 394, which is located at the C-terminus of an alpha-helix that transitions into the cardiomyopathy loop in the actin-binding interface (Fig. 1A), has been shown to be responsible for the observed functional and structural perturbations. M394 is a cysteine residue in all isoforms of human cardiac and skeletal myosin (Fig. 1).
Fig. 1.
A) Structure of the myosin catalytic domain (PDB:1FMV). Light blue: upper 50 kDa domain. Violet: lower 50 kDa domain. Dark blue: N-terminal domain. Teal: converter domain. Pink: actin-binding loops. Red: nucleotide-binding pocket. Inset: location of M394 (yellow) N-terminal to the cardiomyopathy (CM) loop (red) in the myosin actin-binding interface. B) Sequence alignment of Dicty (Dd) myosin II M394 residue with Homo sapien (Hs) skeletal and cardiac muscle myosin II isoforms (Peri = perinatal, FSkE = fast skeletal embryonic, FSk = fast skeletal, FSk ex = fast skeletal extraocular, and Ca=cardiac).
Site-directed mutagenesis and site-specific oxidation define the key redox-sensitive residues that are chemically and functionally vulnerable to oxidants. Methionine to Leucine (M-to-L) mutations control susceptibility of Met oxidation, since Leu conservatively substitutes for Met and is not modified under the conditions used for in vitro oxidation. Methionine to Glutamine (M-to-Q) mutations structurally and functionally mimic Met oxidation to Met sulfoxide, since the substitution of glutamine for methionine introduces an oxygen atom at the same position in the side chain as the sulfoxide oxygen. This site-directed substitution approach of M-to-L and M-to-Q has previously been validated for the functional analysis of site-specific Met oxidation in several protein systems [37,38,39]. Dicty mutants M394L and M394Q to were used here to examine the redox capabilities of this residue. M394 was also mutated to cysteine residue, as found in various human myosin II isoforms (Fig. 1B). As all other reactive Cys in S1dC have been mutated to other residues, this system is ideal for studying site-specific cysteine modification.
3.2 Reversible oxidation of Met 394
To quantify the use of methionine sulfoxide reductase (Msr) to repair individual methionine sulfoxides (MetO) in the myosin catalytic domain, we have used in vitro conditions to generate a homogeneous population of oxidatively modified myosin in which three of nine Mets are oxidized to their corresponding MetO, as revealed by mass spectrometry. Prior to oxidative modification, the Dicty myosin II catalytic domain exhibits one major peak with a molecular mass of 88930 Da (Fig. 2A), corresponding to native S1dC expressed in D. discoideum spin-labeled with IASL. The conditions used for in vitro oxidative modification of myosin result in a 49 Da increase in mass, corresponding to the addition of three oxygen atoms (Fig. 2A) as we have previously shown [29]. Upon treatment of this oxidized myosin with MsrA, the molecular mass of S1dC decreases by 16 Da, consistent with the reversal of one MetO back to Met (Fig. 2A). Treatment of native (unoxidized) S1dC with MsrA shows no shift in molecular mass (Fig. 2A). No additional MetO repair is observed following incubation of myosin with MsrA for longer periods of time.
Fig. 2.
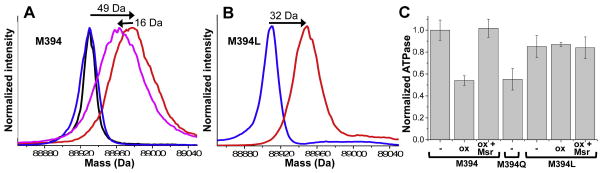
Chemical and functional reversal of oxidation, using MsrA. A: ESI-MS of wildtype residue M at position 394. Blue: no treatment. Red: after treatment with H2O2. Magenta: Red sample after treatment with MsrA. Black: Blue sample after treatment with MsrA. B: Mutant M394L, same color scheme as A. MsrA treatment had no effect on the mass of either sample in B (data not shown). C: Recovery of S1dC actin-activated ATPase activity (Vmax) upon repair of MetO with MsrA. Wildtype S1dC was oxidized with 500 mM H2O2 on ice for 30 minutes (ox), then treated with 0.5 μM MsrA for 30 minutes at 25°C (ox+Msr). The oxido-mimetic mutant M394Q (untreated) is shown for comparison. Error bars are SEM (n≥3).
To determine whether M394 was the MetO reversed to Met by MsrA, leucine was substituted for methionine at M394 to protect from oxidation. Oxidation of the M394L mutant with H2O2 caused a shift of 32 Da, corresponding to the formation of two MetOs (Fig. 2B), one less than observed in the presence of M394 (Fig. 2A). Treatment of oxidized M394L with MsrA had no effect on the mass (data not shown), showing that the other oxidized residues cannot be reduced by MsrA. These results, combined with the results of methionine sulfoxide reversal on globally oxidized Dicty myosin II, indicate that M394 is the only Met that, after oxidation to MetO, can be reduced back to Met by MsrA.
Oxidation of myosin with hydrogen peroxide induced a 2-fold decrease in maximum actin-activated ATPase rate (Vmax) in the presence of saturating actin, as observed previously [29], and this inhibition was completely rescued by subsequent treatment with MsrA (Fig. 2C, M394). Treatment with DTT alone had no effect (data not shown). Mutation M394Q produced a 2-fold decrease in actin-activated ATPase activity, matching the effect of oxidation, and mutation M394L protected myosin against the effects of oxidation (Fig. 2C).
3.3 Reversible glutathionylation of M394C
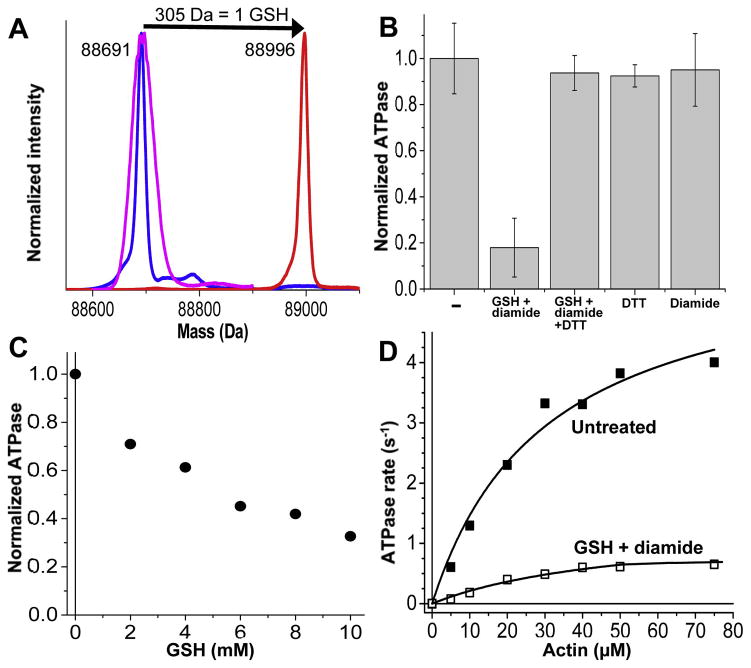
ESI-MS was used to quantify the modification of Cys 394 by glutathione (Fig. 3A). Prior to modification, the M394C mutant S1dC exhibits one major peak with a molecular mass of 88691 (Fig. 3A). Note the difference from the molecular mass in Fig. 2A&B due to the absence of spin label. Treatment with glutathione (GSH) and diamide produced a shift in molecular mass of ~305 Da, corresponding to the addition of 1 glutathione (Fig. 3A, blue to red). The reversibility of this modification was assessed by addition of DTT, which caused a shift back to 88691 (Fig. 3A, red to magenta), indicating that the modification with glutathione is indeed reversible.
Fig. 3.
A: ESI-MS illustrating glutathionylation and reversal in Dicty myosin II M394C. Blue: untreated. Red: treated with 10 mM GSH plus 10 mM diamide for 1 h on ice. Magenta: treated with DTT after glutathionylation. B: ATPase activity of M394C in the presence of 50 μM actin. Untreated (−), after treatment with 10 mM GSH plus 10 mM diamide, 10 mM GSH plus 10 mM diamide followed by 10 mM DTT, 10 mM DTT alone, or 10 mM diamide alone. Error bars are SEM (n≥3). C: ATPase activity of M394C S1dC, in the presence of 50 μM actin, after treatment with increasing concentrations of GSH (with equimolar diamide). D: Actin-dependence of ATPase activity of M394C, untreated (closed squares) and treated with 10 mM GSH plus 10 mM diamide (open squares).
This glutathionylation treatment produced a substantial decrease in actin-activated ATPase activity that was reversed by DTT, with no effect caused by DDT or diamide alone (Fig. 3B,C). In untreated myosin, Vmax (activity at saturating actin) and KATPase (actin concentration needed for half-maximal activation) are 7.0 ± 1.1 s−1 and 41.0 ± 11.8 μM; glutathionylation decreased Vmax by a factor of 5 (to 1.3 ± 0.3 s−1), with no significant effect on KATPase (52.7 ± 17.5 μM) (Fig. 3D). This result suggests that the effect of glutathionylation on myosin function is not simply due to decreased actin affinity, but is due to the reduced activity of actin-bound myosin.
4. Discussion
We previously identified at least three Dicty myosin II methionines that are susceptible to oxidation by peroxide: M394, M486 and M642, and showed that M394 is responsible for the oxidation-induced functional decline in myosin actin-activated ATPase activity and actomyosin interaction [29]. The present study shows that the oxidation of M394 is reversible by MsrA and that the reduction of this MetO completely rescues the decline in ATPase activity (Fig. 2). Mutation of this Met to Cys, mimicking the residue present in all human isoforms of skeletal and cardiac myosin, shows that this Cys can also be oxidatively modified by glutathione, decreasing actomyosin functional interaction (Fig. 3).
Methionine sulfoxide reductase most readily reverses oxidized methionines that are solvent-exposed and located in protein regions composed predominantly of random-coil [8,40,41]. It is therefore not surprising that only oxidation of M394, but not M486 and M642, is reversible. Based on the crystal structure, M394 is solvent-exposed when myosin is not bound to actin, and exists at the extreme end of an alpha-helix, transitioning into a random coil of the CM-loop (Fig. 1A). Although both M486 and M642 are also predicted to be solvent-exposed, they exist in highly structured regions of S1dC, in the force-generating domain. Using site-directed spin labeling (SDSL) and electron paramagnetic resonance (EPR), we previously showed that oxidation of M394 changes the structural dynamics of the actin-binding cleft, [29]. This change in structural dynamics involves a perturbation of the actin-binding cleft’s open-to-closed transition, which is necessary for proper force production and movement [29,42].
Glutathionylation of Cys is a reversible oxidative modification that plays a key role in redox regulation of proteins and signal transduction. In β-cardiac myosin, three Cys within the myosin heavy chain are susceptible to glutathionylation [27], which inhibits myosin ATPase activity at high levels of GSSG but potentiates ATPase activity at low levels of GSSG [27]. Glutathionylation of these three Cys produced changes in the structure of myosin detected by changes in fluorescence and increased susceptibility to proteolytic cleavage [27]. Two of these Cys residues, Cys 400 and Cys 695, are located within the myosin catalytic domain. Cys400 is equivalent to M394 in Dicty myosin, located in the actin-binding cleft adjacent to the CM-loop, while Cys695 is the reactive cysteine SH2 in the force-generating domain of myosin. Our mutagenesis strategy allows for site-specific investigation of Cys glutathionylation in the myosin catalytic domain. The M394C S1dC mutant can be modified specifically by glutathione in vitro (Fig. 3), resulting in a decrease in actin-activated ATPase activity (Fig. 3). In addition to this direct effect of modifying myosin function, it is likely that glutathionylation can also play a protective role [43], protecting myosin against irreversible protein thiol oxidation at Cys residues in crucial subdomains of myosin such as the actin-binding cleft.
Oxidation of methionine to methionine sulfoxide, as well as glutathionylation of cysteine, has been shown to affect protein secondary and tertiary structure. The presence of MetO can cause αhelical destabilization [44,45,46,47,48]. Glutathionylation has a similar effect, having been shown to decrease α-helical content of proteins [49,50]. As both Met oxidation and glutathionylation at residue 394 in Dicty myosin II cause a decrease in actin-activated ATPase activity of myosin (Fig. 2 & Fig. 3), we hypothesize that oxidative modification at this site, whether Met oxidation or Cys glutathionylation, destabilizes the adjacent helix, changing the structural dynamics of the entire CM-loop and hence interaction with actin. This change in structural dynamics at the actin-binding cleft probably perturbs the weak-to-strong transition of myosin, necessary for proper force production. This hypothesis will be the subject of future testing by spectroscopic methods in both muscle and nonmuscle myosin.
5. Conclusion
Redox regulation has emerged as a crucial modulator of protein function. The production of ROS in muscle can be due to many pathophysiological and physiological conditions, including aging, heart failure, inflammation, and strenuous exercise. ROS-induced oxidative modifications occur frequently in sarcomeric proteins, including myosin. This work illustrates the potential for myosin to function as a redox sensor in both nonmuscle and muscle cells, potentially modulating motility/contractility in response to oxidative stress.
Acknowledgments
We thank Margaret Titus for expert consultation regarding Dicty myosin. We thank Ivan Rayment for the S1dC plasmid. This work was supported by NIH grants to DDT (R37 AG026160) and RJM (F31 AG037303) and from AHA to DEO (12UFEL12040063). We received excellent assistance with mass spectrometry from Bruce Witthuhn at the Center for Mass Spectrometry and Proteomics at the University of Minnesota. The Biophysical Spectroscopy Center and Biomedical Genomics Center at the University of Minnesota were essential for this work.
ABBREVIATIONS
- ESI-MS
Electrospray ionization mass spectrometry
- Dicty
Dictyostelium discoideum
- S1dC
Cys-lite catalytic domain of Dicty myosin II
- ROS
reactive oxygen species
- Met
methionine
- MetO
methionine sulfoxide
- Msr
Methionine sulfoxide reductase
- Cys
cysteine
- GSH
glutathione
- GSSG
oxidized glutathione
References
- 1.Jackson MJ. Redox regulation of skeletal muscle. IUBMB Life. 2008;60:497–501. doi: 10.1002/iub.72. [DOI] [PubMed] [Google Scholar]
- 2.Stadtman ER. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 3.Pastore A, Piemonte F. Protein glutathionylation in cardiovascular diseases. Int J Mol Sci. 2013;14:20845–20876. doi: 10.3390/ijms141020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen HH, Hamilton EJ, Liu CC, Figtree GA. Reversible oxidative modification: implications for cardiovascular physiology and pathophysiology. Trends Cardiovasc Med. 2010;20:85–90. doi: 10.1016/j.tcm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Thomas JA, Mallis RJ. Aging and oxidation of reactive protein sulfhydryls. Exp Gerontol. 2001;36:1519–1526. doi: 10.1016/s0531-5565(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 6.Bigelow DJ, Squier TC. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim Biophys Acta. 2005;1703:121–134. doi: 10.1016/j.bbapap.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Emes MJ. Oxidation of methionine residues: the missing link between stress and signalling responses in plants. Biochem J. 2009;422:e1–2. doi: 10.1042/BJ20091063. [DOI] [PubMed] [Google Scholar]
- 8.Ghesquiere B, Jonckheere V, Colaert N, Van Durme J, Timmerman E, Goethals M, Schymkowitz J, Rousseau F, Vandekerckhove J, Gevaert K. Redox proteomics of protein-bound methionine oxidation. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshi T, Heinemann S. Regulation of cell function by methionine oxidation and reduction. J Physiol. 2001;531:1–11. doi: 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadtman ER, Van Remmen H, Richardson A, Wehr NB, Levine RL. Methionine oxidation and aging. Biochim Biophys Acta. 2005;1703:135–140. doi: 10.1016/j.bbapap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 12.Sumandea MP, Steinberg SF. Redox signaling and cardiac sarcomeres. J Biol Chem. 2011;286:9921–9927. doi: 10.1074/jbc.R110.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zima AV, Bovo E, Mazurek SR, Rochira JA, Li W, Terentyev D. Ca handling during excitation-contraction coupling in heart failure. Pflugers Archiv : European journal of physiology. 2014;466:1129–1137. doi: 10.1007/s00424-014-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donoso P, Sanchez G, Bull R, Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Frontiers in bioscience. 2011;16:553–567. doi: 10.2741/3705. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo C, Donoso P. Cell signaling. Getting to the heart of mechanotransduction. Science. 2011;333:1388–1390. doi: 10.1126/science.1212183. [DOI] [PubMed] [Google Scholar]
- 16.Humphries KM, Juliano C, Taylor SS. Regulation of cAMP-dependent protein kinase activity by glutathionylation. J Biol Chem. 2002;277:43505–43511. doi: 10.1074/jbc.M207088200. [DOI] [PubMed] [Google Scholar]
- 17.Ward NE, Stewart JR, Ioannides CG, O’Brian CA. Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry. 2000;39:10319–10329. doi: 10.1021/bi000781g. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg SF. Oxidative stress and sarcomeric proteins. Circulation research. 2013;112:393–405. doi: 10.1161/CIRCRESAHA.111.300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung RJ, Pak CW, Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science. 2011;334:1710–1713. doi: 10.1126/science.1211956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passarelli C, Di Venere A, Piroddi N, Pastore A, Scellini B, Tesi C, Petrini S, Sale P, Bertini E, Poggesi C, Piemonte F. Susceptibility of isolated myofibrils to in vitro glutathionylation: Potential relevance to muscle functions. Cytoskeleton (Hoboken) 2010;67:81–89. doi: 10.1002/cm.20425. [DOI] [PubMed] [Google Scholar]
- 21.Pizarro GO, Ogut O. Impact of actin glutathionylation on the actomyosin-S1 ATPase. Biochemistry. 2009;48:7533–7538. doi: 10.1021/bi900669m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prochniewicz E, Spakowicz D, Thomas DD. Changes in actin structural transitions associated with oxidative inhibition of muscle contraction. Biochemistry. 2008;47:11811–11817. doi: 10.1021/bi801080x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prochniewicz E, Thomas DD, Thompson LV. Age-related decline in actomyosin function. J Gerontol A Biol Sci Med Sci. 2005;60:425–431. doi: 10.1093/gerona/60.4.425. [DOI] [PubMed] [Google Scholar]
- 24.Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol. 2012;590:1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel BG, Wilder T, Solaro RJ. Novel control of cardiac myofilament response to calcium by S-glutathionylation at specific sites of myosin binding protein C. Front Physiol. 2013;4:336. doi: 10.3389/fphys.2013.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alegre-Cebollada J, Kosuri P, Giganti D, Eckels E, Rivas-Pardo JA, Hamdani N, Warren CM, Solaro RJ, Linke WA, Fernandez JM. S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding. Cell. 2014;156:1235–1246. doi: 10.1016/j.cell.2014.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passarelli C, Petrini S, Pastore A, Bonetto V, Sale P, Gaeta LM, Tozzi G, Bertini E, Canepari M, Rossi R, Piemonte F. Myosin as a potential redox-sensor: an in vitro study. J Muscle Res Cell Motil. 2008;29:119–126. doi: 10.1007/s10974-008-9145-x. [DOI] [PubMed] [Google Scholar]
- 28.Prochniewicz E, Lowe DA, Spakowicz DJ, Higgins L, O’Conor K, Thompson LV, Ferrington DA, Thomas DD. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–626. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein JC, Moen RJ, Smith EA, Titus MA, Thomas DD. Structural and functional impact of site-directed methionine oxidation in myosin. Biochemistry. 2011;50:10318–10327. doi: 10.1021/bi201279u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein JC, Burr AR, Svensson B, Kennedy DJ, Allingham J, Titus MA, Rayment I, Thomas DD. Actin-binding cleft closure in myosin II probed by site-directed spin labeling and pulsed EPR. Proc Natl Acad Sci U S A. 2008;105:12867–12872. doi: 10.1073/pnas.0802286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moen RJ, Thomas DD, Klein JC. Conformationally trapping the actin-binding cleft of myosin with a bifunctional spin label. J Biol Chem. 2013;288:3016–3024. doi: 10.1074/jbc.M112.428565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochniewicz E, Chin HF, Henn A, Hannemann DE, Olivares AO, Thomas DD, De La Cruz EM. Myosin isoform determines the conformational dynamics and cooperativity of actin filaments in the strongly bound actomyosin complex. J Mol Biol. 2010;396:501–509. doi: 10.1016/j.jmb.2009.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prochniewicz E, Walseth TF, Thomas DD. Structural dynamics of actin during active interaction with myosin: different effects of weakly and strongly bound myosin heads. Biochemistry. 2004;43:10642–10652. doi: 10.1021/bi049914e. [DOI] [PubMed] [Google Scholar]
- 34.De La Cruz EM, Ostap EM. Kinetic and equilibrium analysis of the myosin ATPase. Methods Enzymol. 2009;455:157–192. doi: 10.1016/S0076-6879(08)04206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroder RR, Manstein DJ, Jahn W, Holden H, Rayment I, Holmes KC, Spudich JA. Three-dimensional atomic model of F-actin decorated with Dictyostelium myosin S1. Nature. 1993;364:171–174. doi: 10.1038/364171a0. [DOI] [PubMed] [Google Scholar]
- 36.Bauer CB, Holden HM, Thoden JB, Smith R, Rayment I. X-ray structures of the apo and MgATP-bound states of Dictyostelium discoideum myosin motor domain. J Biol Chem. 2000;275:38494–38499. doi: 10.1074/jbc.M005585200. [DOI] [PubMed] [Google Scholar]
- 37.Balog EM, Norton LE, Thomas DD, Fruen BR. Role of calmodulin methionine residues in mediating productive association with cardiac ryanodine receptors. Am J Physiol Heart Circ Physiol. 2006;290:H794–799. doi: 10.1152/ajpheart.00706.2005. [DOI] [PubMed] [Google Scholar]
- 38.Binger KJ, Griffin MD, Howlett GJ. Methionine oxidation inhibits assembly and promotes disassembly of apolipoprotein C-II amyloid fibrils. Biochemistry. 2008;47:10208–10217. doi: 10.1021/bi8009339. [DOI] [PubMed] [Google Scholar]
- 39.Chin D, Means AR. Methionine to glutamine substitutions in the C-terminal domain of calmodulin impair the activation of three protein kinases. J Biol Chem. 1996;271:30465–30471. doi: 10.1074/jbc.271.48.30465. [DOI] [PubMed] [Google Scholar]
- 40.Sun H, Gao J, Ferrington DA, Biesiada H, Williams TD, Squier TC. Repair of oxidized calmodulin by methionine sulfoxide reductase restores ability to activate the plasma membrane Ca-ATPase. Biochemistry. 1999;38:105–112. doi: 10.1021/bi981295k. [DOI] [PubMed] [Google Scholar]
- 41.Tarrago L, Kaya A, Weerapana E, Marino SM, Gladyshev VN. Methionine sulfoxide reductases preferentially reduce unfolded oxidized proteins and protect cells from oxidative protein unfolding. J Biol Chem. 2012;287:24448–24459. doi: 10.1074/jbc.M112.374520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moen RJ, Klein JC, Thomas DD. Electron Paramagnetic Resonance Resolves Effects of Oxidative Stress on Muscle Proteins. Exercise and Sport Sciences Reviews. 2013 doi: 10.1249/JES.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mieyal JJ, Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation. Antioxid Redox Signal. 2012;16:471–475. doi: 10.1089/ars.2011.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balog EM, Lockamy EL, Thomas DD, Ferrington DA. Site-Specific Methionine Oxidation Initiates Calmodulin Degradation by the 20S Proteasome (dagger) Biochemistry. 2009;48:3005–3016. doi: 10.1021/bi802117k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colombo G, Meli M, Morra G, Gabizon R, Gasset M. Methionine sulfoxides on prion protein Helix-3 switch on the alpha-fold destabilization required for conversion. PLoS One. 2009;4:e4296. doi: 10.1371/journal.pone.0004296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao J, Yao Y, Squier TC. Oxidatively modified calmodulin binds to the plasma membrane Ca-ATPase in a nonproductive and conformationally disordered complex. Biophys J. 2001;80:1791–1801. doi: 10.1016/S0006-3495(01)76149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao B, Heinecke JW. Impact of HDL oxidation by the myeloperoxidase system on sterol efflux by the ABCA1 pathway. J Proteomics. 2011;74:2289–2299. doi: 10.1016/j.jprot.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Younan ND, Nadal RC, Davies P, Brown DR, Viles JH. Methionine oxidation perturbs the structural core of the prion protein and suggests a generic misfolding pathway. J Biol Chem. 2012;287:28263–28275. doi: 10.1074/jbc.M112.354779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butturini E, Darra E, Chiavegato G, Cellini B, Cozzolino F, Monti M, Pucci P, Dell’Orco D, Mariotto S. S-glutathionylation at Cys328 and Cys542 impairs STAT3 phosphorylation. ACS Chem Biol. 2014 doi: 10.1021/cb500407d. [DOI] [PubMed] [Google Scholar]
- 50.Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR, Jr, Hutchens S, Tew KD. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res. 2009;69:7626–7634. doi: 10.1158/0008-5472.CAN-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]