Abstract
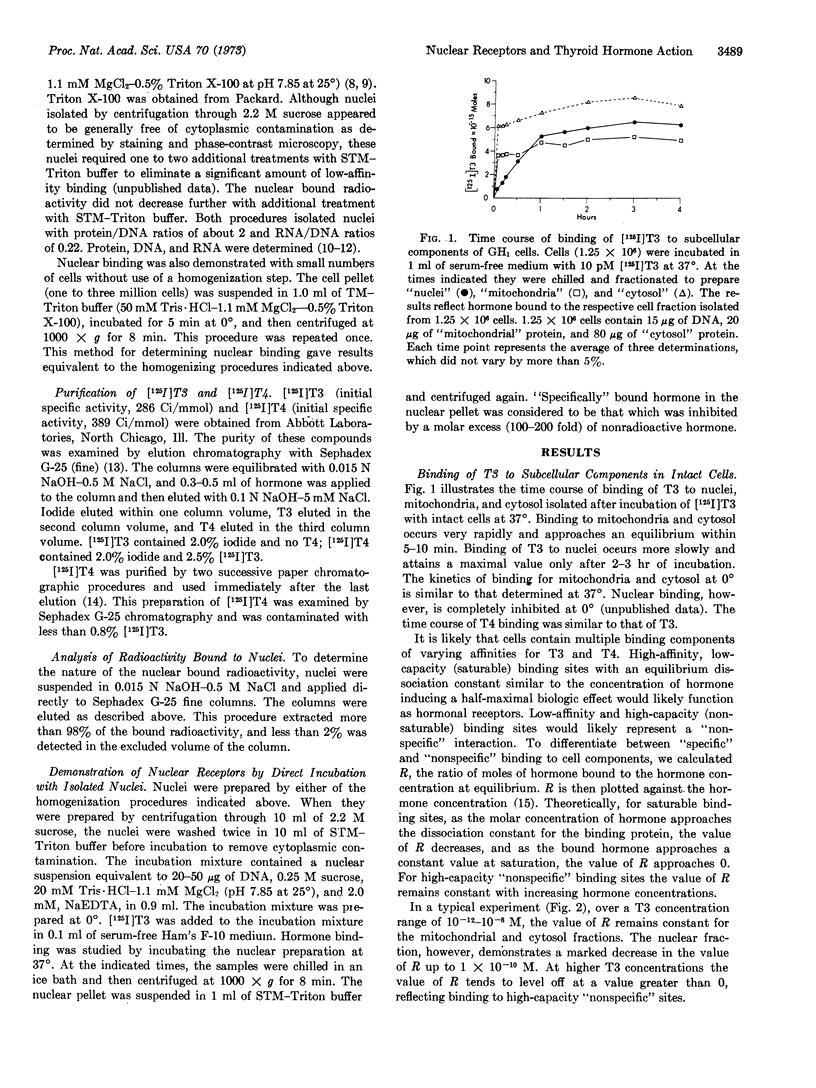
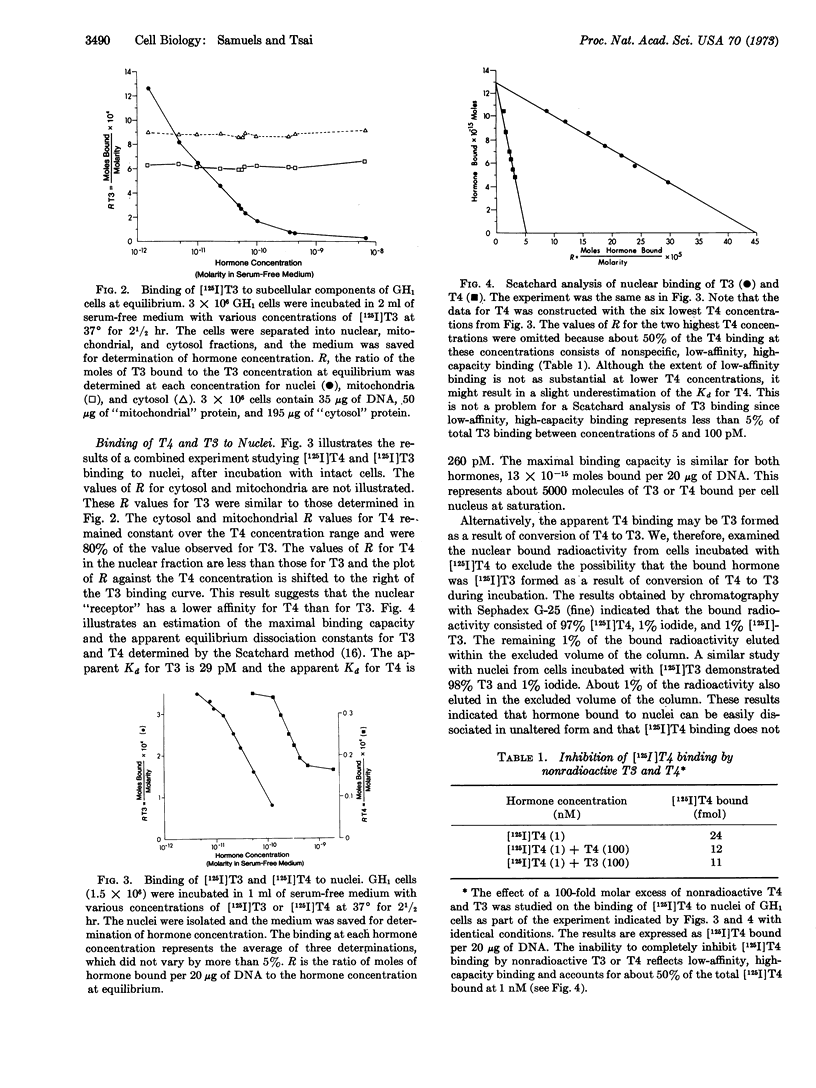
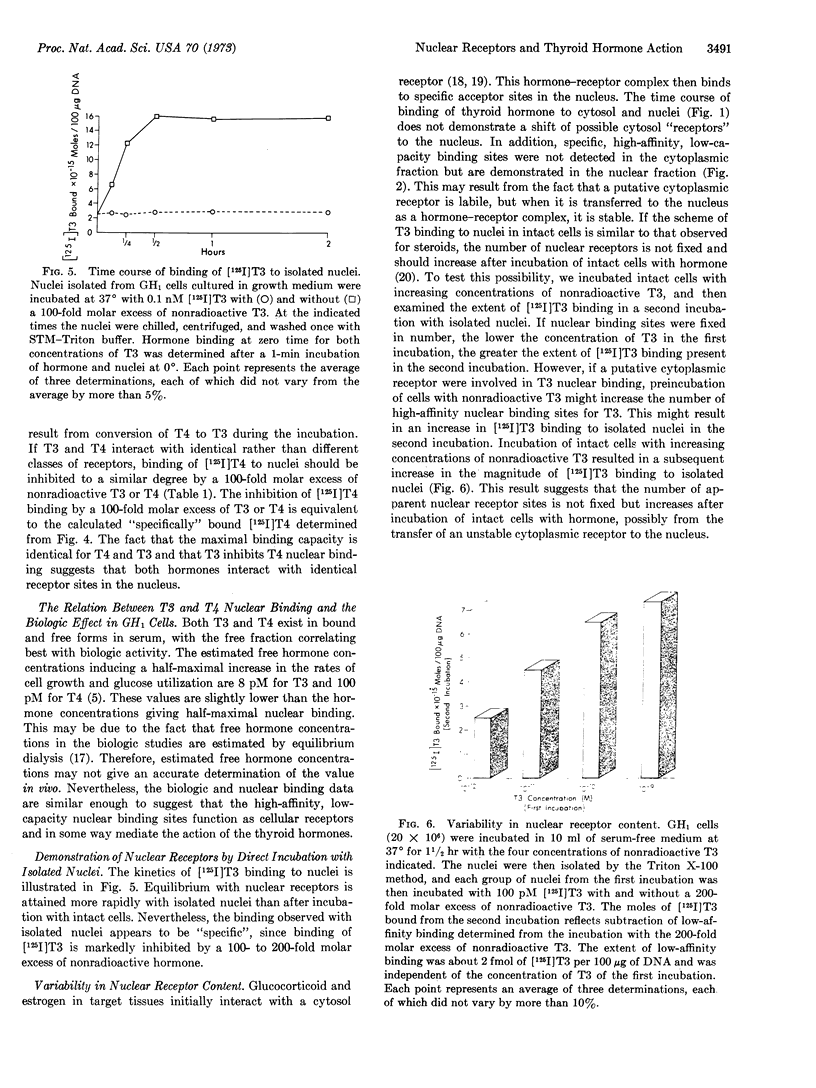
Triiodothyronine and thyroxine induce a 3-fold increase in the rate of growth of GH1 cells in culture. To study further the action of these hormones, we examined the binding of [125I]triiodothyronine and purified [125I]thyroxine to cellular fractions after incubation with intact cells in serum-free medium. High-affinity, low-capacity binding sites for the hormones were demonstrated in nuclear but not in mitochondrial or cytosol fractions. Chromatographic analysis of the bound nuclear radioactivity from cells incubated with [125I]thyroxine demonstrated 97% thyroxine, 1% iodide, and 1% triiodothyronine. Apparent equilibrium dissociation constants, determined by Scatchard analysis, were 29 pM for triidothyronine and 260 pM for thyroxine. The maximal binding capacity was identical for both hormones, with about 5000 sites per cell nucleus. [125I]Thyroxine binding was competitively inhibited by triiodothyronine. These data suggest that triiodothyronine and thyroxine interact with identical nuclear receptors, and that conversion of thyroxine to triiodothyronine may not be a prerequisite for biologic activity. Similar high-affinity, low-capacity nuclear binding sites were also demonstrated by incubation of [125I]triidothyronine directly with isolated nuclei. Incubation of cells with increasing concentrations of nonradioactive triidothyronine results in a subsequent increase in binding when [125I]triiodothyronine is then incubated directly with isolated nuclei. This result suggests that nuclear receptors are not fixed, but increase after exposure of intact cells to hormone. This increase in nuclear receptor content may result from the transfer of an unstable cytosol receptor to the nucleus.
Keywords: receptor, thyroxine, triiodothyronine
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Clark J. H., Peck E. J., Jr Oestrogen and nuclear binding sites. Determination of specific sites by ( 3 H)oestradiol exchange. Biochem J. 1972 Feb;126(3):561–567. doi: 10.1042/bj1260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. The relationship between glucocorticoid binding and tyrosine aminotransferase induction in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1970 Mar;65(3):709–715. doi: 10.1073/pnas.65.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W. L. Separation of iodo compounds in serum by chromatography on Sephadex columns. J Chromatogr. 1972 Oct 5;72(1):83–91. doi: 10.1016/0021-9673(72)80010-4. [DOI] [PubMed] [Google Scholar]
- Griswold M. D., Fischer M. S., Cohen P. P. Temperature-dependent intracellular distribution of thyroxine in amphibian liver. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1486–1489. doi: 10.1073/pnas.69.6.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYMER W. C., KUFF E. L. ISOLATION OF NUCLEI FROM MAMMALIAN TISSUES THROUGH THE USE OF TRITON X-100. J Histochem Cytochem. 1964 May;12:359–363. doi: 10.1177/12.5.359. [DOI] [PubMed] [Google Scholar]
- Hollander C. S., Mitsuma T., Nihei N., Shenkman L., Burday S. Z., Blum M. Clinical and laboratory observations in cases of triiodothyronine toxicosis confirmed by radioimmunoassay. Lancet. 1972 Mar 18;1(7751):609–611. doi: 10.1016/s0140-6736(72)90408-4. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Numata M., Brecher P. I., Desombre E. R. Hormone-receptor interaction as a guide to biochemical mechanism. Biochem Soc Symp. 1971;32:133–159. [PubMed] [Google Scholar]
- Mitsuma T., Nihei N., Gershengorn M. C., Hollander C. S. Serum triiodothyronine: measurements in human serum by radioimmunoassay with corroboration by gas-liquid chromatography. J Clin Invest. 1971 Dec;50(12):2679–2688. doi: 10.1172/JCI106769. [DOI] [PMC free article] [PubMed] [Google Scholar]
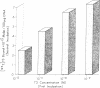
- Oppenheimer J. H., Koerner D., Schwartz H. L., Surks M. I. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972 Aug;35(2):330–333. doi: 10.1210/jcem-35-2-330. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Cintron R. Thyroid hormone action: a cell-culture system responsive to physiological concentrations of thyroid hormones. Science. 1973 Sep 28;181(4106):1253–1256. doi: 10.1126/science.181.4106.1253. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Surks M. I., Oppenheimer J. H. Quantitation of extrathyroidal conversion of L-thyroxine to 3,5,3'-triiodo-L-thyronine in the rat. J Clin Invest. 1971 May;50(5):1124–1130. doi: 10.1172/JCI106584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding S. W., Gregerman R. I. Free thyroxine in serum by equilibrium dialysis: effects of dilution, specific ions and inhibitors of binding. J Clin Endocrinol Metab. 1972 Jun;34(6):974–982. doi: 10.1210/jcem-34-6-974. [DOI] [PubMed] [Google Scholar]