Abstract
Objective
Controversy persists regarding appropriate radiographic surveillance strategies following lung cancer resection. We compared the impact of surveillance CT scan (CT) vs. chest radiograph (CXR) in patients who underwent resection for stage I lung cancer.
Methods
A retrospective analysis was performed of all patients undergoing resection for pathologic stage I lung cancer from January 2000–April 2013. After resection, follow-up included routine history and physical exam in conjunction with CXR or CT at the discretion of the treating physician. Identification of successive lung malignancy (i.e. recurrence at any new site or new primary) and survival were recorded.
Results
There were 554 evaluable patients with 232 undergoing routine postoperative CT and 322 receiving routine CXR. Postoperative five-year survival was 67.8% in the CT group vs. 74.8% in the CXR group (p = 0.603). Successive lung malignancy was found in 27% (63/232) of patients undergoing CT vs. 22% (72/322) receiving CXR (p = 0.19). The mean time from surgery to diagnosis of successive malignancy was 1.93 years for CT vs. 2.56 years for CXR (p = 0.046). For the CT group, 41% (26/63) of successive malignancies were treated with curative intent vs. 40% (29/72) in the CXR group (p = 0.639). Cox-proportional hazard analysis indicated imaging modality (CT vs. CXR) was not associated with survival (p = 0.958).
Conclusion
Surveillance CT may result in earlier diagnosis of successive malignancy vs. CXR in stage I lung cancer, although no difference in survival was demonstrated. A randomized trial would help determine the impact of postoperative surveillance strategies on survival.
Introduction
Currently there is no consensus on the optimal method of radiographic follow-up following resection of stage I NSCLC. Patients undergoing resection for pathologic stage I non-small cell lung cancer (NSCLC) are still at risk of developing recurrence but are also1– 4 at risk for developing a new primary lung cancer at an estimated rate of 1–6 cases per 100 person-years.5–9 The risk of identifying lung cancer in a high risk group of smokers in the National Lung Screening Trial (NLST) was approximately 0.6% per person-year.10 Because of the substantial combined risk of recurrence and development of new primary lung cancers, multiple guidelines have been recommended for routine postoperative radiographic surveillance after resection for NSCLC (Online Supplemental Table 1).9,11–13
Recently published results from the NLST demonstrated that routine low-dose CT scans of patients at high risk for developing a first primary lung cancer offered a lung cancer specific survival benefit compared to routine chest radiograph. However, it is unclear what impact radiographic surveillance will have on survivors of resected NSCLC, who are subject to competing risks of recurrence and new primary lung cancer. While CT has evolved as the standard for routine postoperative surveillance there remains a paucity of data demonstrating a distinct survival advantage for CT over less expensive imaging follow-up. A recent single arm prospective trial suggested that minimal dose CT (MnDCT) may be beneficial in the postoperative surveillance of patients with Stage I–III NSCLC.14 Historically, however, routine postoperative CT surveillance has resulted in inconsistent results regarding earlier detection of recurrence or new primary lung cancer with no conclusive evidence of improvement in survival (Online Supplemental Table 2).4–6,14–20
We performed a comparison of recurrence patterns and survival of resected stage I NSCLC patients who underwent routine CT versus chest x-ray for postoperative surveillance. Our objective was to determine whether routine CT imaging led to earlier diagnosis of recurrence or new primary lung cancer, and if so, whether early detection translated into a survival benefit. Our primary hypothesis was that CT surveillance would result in earlier detection and secondarily that earlier detection would increase survival.
Patients and Methods
All patients treated for pathologic stage I (T1–T2a N0 M0) NSCLC by pulmonary resection between January, 2000 and April, 2013 at Barnes-Jewish Hospital were identified from a prospective database and retrospectively analyzed in accordance with a protocol approved by the institutional review board at Washington University School of Medicine. Patients were staged using the 7th lung cancer TNM staging system. Initial exclusion criteria included: (1) malignancy within five years prior to resection (except squamous and basal cell skin carcinoma or carcinoma in situ) or a patient with contralateral nodules at the time of resection of the index lesion, (2) induction therapy, (3) incomplete resection, (4) missing surveillance records, (5) variability in surveillance imaging modality, (6) clinically detected recurrence before initiation of surveillance, (7) death before initiation of surveillance, and (8) age < 18 years. Online supplemental Figure 1 is a CONSORT diagram of patient selection.
Surveillance
Each patient had an initial postoperative visit with the treating surgeon, consisting of a physical examination and a chest x-ray, two to four weeks after resection. Subsequently, patients were followed by routine visits that consisted of a thorough history and physical examination and either chest x-ray or CT scan. The imaging modality was chosen at the discretion of the treating physician. Certain surgeons consistently utilized chest x-rays for surveillance while others initially utilized chest x-ray then transitioned to regimens that included a mixture of chest x-rays and CT or CT only. Some surgeons that utilized CT initially transitioned to a combination of chest x-ray and CT. Surgeon “intent” could not be reliably determined for this subset of patients thus they were not included in the final analysis. In a separate analysis, there was no association between the treating surgeon and overall survival (Online Supplemental Figure 2). Chest x-rays tended to be utilized more in the early period of this study. The mean dates of resection for patients undergoing routine CT surveillance was 5/15/2009 vs. 6/1/2005 for chest x-ray (p<0.001). CT scans included sections of the thorax and upper abdomen (including liver and adrenal glands), and were done with or without contrast, although the default was low-dose non-contrast CT. Generally, the standard follow-up regimen included a baseline CT scan approximately 3 months after resection, with follow-up imaging every 6 months for the first 2–3 years, then annually thereafter for at least 5 years. Standard CT technique included 5 mm contiguous sections until 2009, and 3 mm contiguous sections thereafter. Surveillance was defined as routine follow-up of asymptomatic patients after the initial postoperative visit for screening for recurrence or new primary lung cancer. For this study, patients were categorized in the CT group if they had routine CT scans, or in the chest x-ray group if they had routine chest x-ray for surveillance. To minimize heterogeneity of follow-up, patients were excluded from the study if (1) the modality of their surveillance imaging switched because of changes in attending physician or in surveillance protocol for reasons not related to suspicion of recurrence, (2) if they alternated between chest x-ray and CT or (3) if they had routine positron emission tomography (PET) scans. False positive surveillance scans resulting in invasive procedures, including bronchoscopy, needle biopsy, or surgical resection were noted.
Patient and Surveillance Characteristics
There were 1140 patients who underwent resection for pathologic stage I lung cancer. Of these, 586 patients were excluded for: malignancy within prior five years (n=217), induction therapy (n=21), incomplete resection (n=16), missing surveillance records, including those who followed-up with an outside physician without accessible records (n=163), inconsistency in imaging modality used for surveillance or routine PET imaging (n=149), recurrence before initiation of surveillance (n=7), expiration before initiation of surveillance (n=12) and age less than 18 years of age at time of treatment (n=1). Of the 149 patients excluded for inconsistency in imaging method, 123 switched method due to change in follow-up physician or protocol, 22 alternated between CT and CXR surveillance, 3 had routine CXR surveillance but had their successive malignancy detected by imaging obtained for unrelated issues, and 1 had routine PET imaging.
Among the 554 patients included, 232 underwent routine surveillance with CT and 322 with CXR (Table 1). Median duration of follow-up up was 2.5 years (range 0.3 – 9.9 years) for the CT group, and 3.5 years (range 0.1 – 13.1 years) for the CXR group. There were no significant differences between the groups in most baseline characteristics, with the exception of pathologic T stage of index tumor, type of surgical resection, and adjuvant therapy (Table 1).
Table 1.
Baseline characteristics and mean number of follow-up imaging performed for CT and CXR groups.
| Characteristic | CT (n = 232) | CXR (n = 322) | p value |
|---|---|---|---|
| Male gender | 101 (44%) | 121 (38%) | 0.158 |
| Caucasian race | 211 (91%) | 283 (88%) | 0.253 |
| Age (mean ± SD) | 65 ± 11 | 66 ± 11 | 0.346 |
| Charlson index (preoperative) (mean ± SD) | 0.91 ± 0.94 | 0.91 ± 1.08 | 0.927 |
| Tumor size (cm) (mean ± SD) | 2.3 ± 1.1 | 2.4 ± 1.1 | 0.332 |
| T2a tumor | 94 (41%) | 103 (32%) | 0.039 |
| Tumor histology | 0.366 | ||
| Adenocarcinoma | 128 (55%) | 159 (49%) | |
| Bronchoalveolar | 24 (10%) | 28 (9%) | |
| Squamous | 41 (18%) | 69 (21%) | |
| Adenosquamous | 5 (2%) | 8 (2%) | |
| Carcinoid | 20 (9%) | 43 (13%) | |
| Other or mixed | 14 (6%) | 15 (5%) | |
| Resection procedure | 0.002 | ||
| Wedge | 46 (20%) | 27 (8%) | |
| Segmentectomy | 17 (7%) | 19 (6%) | |
| Lobectomy | 152 (66%) | 260 (81%) | |
| Sleeve | 7 (3%) | 6 (2%) | |
| Bilobectomy | 4 (2%) | 4 (1%) | |
| Wedge and Lobectomy | 5 (2%) | 3 (1%) | |
| Pneumonectomy | 1 (0.4%) | 3 (1%) | |
| Adjuvant therapy | 48 (21%) | 11 (3%) | <0.001 |
| Chemotherapy | 39 (17%) | 7 (2%) | |
| Radiation | 8 (3%) | 3 (1%) | |
| Chemoradiation | 1 (0.4%) | 1 (0.3%) | |
| Number of follow-up CT or CXR per year (mean ± SD) | |||
| First year | 1.74 ± 0.81 | 2.12 ± 0.86 | <0.001 |
| Second year | 1.74 ± 0.83 | 1.66 ± 0.72 | 0.310 |
| After second year | 1.34 ± 0.55 | 1.25 ± 0.47 | 0.122 |
| Overall | 1.56 ± 0.51 | 1.58 ± 0.56 | 0.605 |
Successive Malignancy and Survival
Successive malignancy was defined as recurrence or new primary lung cancer. The Martini-Melamed criteria3 was used to identify new primary lung cancers, as lesions that were (1) of different histology than the index cancer, (2) found in a different lobe, with no evidence of extra-pulmonary or lymphatic metastasis, or (3) found at least two years after the index cancer. Successive malignancies were categorized according to whether they were detected during a scheduled or unscheduled visit, presence of relevant symptoms at time of detection, and imaging modality responsible for detection. Relevant symptoms included dyspnea or cough, pleuritic chest pain, hemoptysis, pneumonia, pneumothorax, hoarseness, weight loss, musculoskeletal pain, subcutaneous mass or swelling, or neurological changes. Also included were all new-onset symptoms noted in the records by the physician as suspicious for recurrence or warranting further imaging, and symptoms that prompted an unscheduled visit where recurrence or new primary was subsequently diagnosed. For treatment of successive malignancies, therapy was defined as “curative-intent” when it included surgical resection or stereotactic radiation therapy for a localized pulmonary lesion or solitary brain or adrenal metastasis, or definitive chemoradiation for locoregional disease such as an isolated recurrence in mediastinal lymph nodes. “Palliative therapy” was defined as chemotherapy alone, radiation therapy for symptom management, or pain management.
Overall survival was defined as starting from date of resection for the index lung cancer, and ending on date of expiration or censored at date of last observation of follow-up. For cancer-specific survival all deaths were reviewed and cause of death determined by the authors (TC/SC). Time to diagnosis of successive malignancy was defined as starting from date of resection for the index lung cancer, and ending on date of diagnosis of successive malignancy.
Statistical Analysis
Means for parametric continuous data were compared using t-tests. Counts and proportions for categorical data were compared using χ2 tests. Overall and cancer-specific survival curves were constructed using the Kaplan-Meier method, and compared using log rank tests. Multivariate analysis of prognostic effect of covariates on overall and cancer-specific survival was performed using Cox proportional hazard analysis. To account for potentially confounding covariates as well as imbalance between the CT and CXR groups with respect to resection procedure and tumor T-stage, propensity score matching using the nearest-neighbor caliper method was carried out to match the CT and CXR groups based on covariates of age, peri-operative Charlson comorbidity index, sub-lobar resection, T-stage of index tumor, and adjuvant therapy with a caliper radius of 0.03 of the logit of the standard deviation of the propensity score. All statistical tests were 2-sided. Analyses were conducted using SPSS software, version 21.0 (IBM, Armonk, NY). Propensity score matching was performed using the Propensity Score Matching for SPSS plug-in, version 3.0 (Felix Thoemmes, Cornell University, Ithaca, NY).
Results
Successive Malignancies
Recurrence or new primary lung cancer was found in 24% (135/554) of patients (Table 2). Overall, successive malignancy was found in 27% (63/232) in the CT group and 22% (72/322) in the CXR group (p = 0.195). The rate of successive malignancy was 10.7% per person-year for CT vs. 6.3% per person-year for CXR (p=0.002). The majority of successive malignancies had thoracic manifestation. The rate of new primary lung cancers overall was 2.1% per person-year. In the CT group, 9/232 (4%) had false positive surveillance imaging that resulted in an invasive diagnostic procedure (bronchoscopy, biopsy, or surgery) vs. 1/322 (0.3%) in CXR (p=0.002). Among patients developing successive malignancy, 49% (31/63) in the CT group were asymptomatic at presentation, versus 19% (14/72) in the CXR group (p<0.001).
Table 2.
Characteristics of successive malignancies diagnosed during follow-up.
| Characteristic | CT (n = 63) | CXR (n = 72) | p value |
|---|---|---|---|
| Type | 0.275 | ||
| New primary | 14 (22%) | 22 (31%) | |
| Recurrence | 49 (78%) | 50 (69%) | |
| Region | 0.187 | ||
| Extrathoracic | 16 (25%) | 10 (14%) | |
| Thoracic | 41 (65%) | 51 (71%) | |
| Both | 6 (10%) | 11 (15%) | |
| Location | 0.295 | ||
| Local | 3 (5%) | 4 (6%) | |
| Regional | 11 (17%) | 14 (19%) | |
| Distant | 34 (54%) | 36 (50%) | |
| Local and regional | 5 (8%) | 1 (1%) | |
| Local and distant | 1 (2%) | 0 | |
| Regional and distant | 9 (14%) | 17 (24%) | |
| Symptomatic at time of detection | 32 (51%) | 58 (81%) | <0.001 |
| Detected during scheduled surveillance | 46 (73%) | 33 (46%) | 0.001 |
| Treatment | 0.670 | ||
| Curative | 26 (41%) | 29 (40%) | |
| Palliative | 29 (46%) | 37 (51%) | |
| Refused | 0 | 1 (1%) | |
| None | 1 (1%) | 1 (1%) | |
| Unknown | 7 (11%) | 4 (6%) |
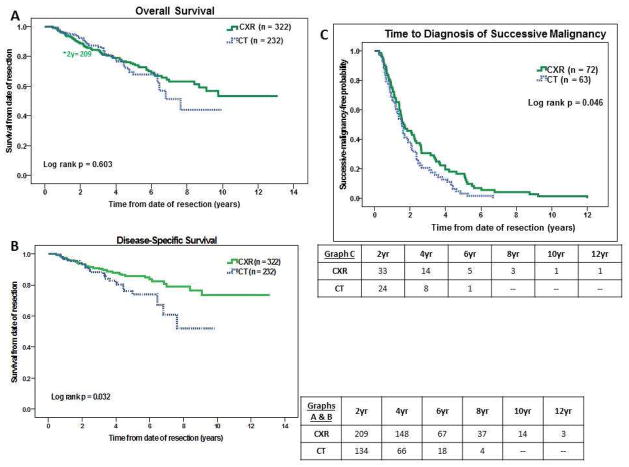
Among patients with successive malignancy, time to diagnosis was shorter for the CT group (p=0.046, Figure 1), with mean times of 1.93 years versus 2.56 years for CXR and median times of 1.56 versus 1.63 years (95% CI 1.29–1.83 years vs. 1.03–2.23 years).
Figure 1.
Figure 1A–C. Kaplan-Meier curves for overall survival, cancer-specific survival, and time to diagnosis of relapse.
Survival
In unmatched analysis, overall survival was similar between the groups, with five-year survival rates of 67.8% for CT versus 74.8% for CXR (mean survival 7.00 vs. 9.19 years, median survival 7.63 years vs. not reached p=0.603, Figure 1)(95% CI 5.78–9.48 years). Five-year cancer-specific survival was 74.1% for CT vs. 85.5% for CXR (p=0.032, Figure 1). Among patients with successive malignancy, there was no difference in overall survival, with five-year survival rates of 33.5% in CT and 40.2% in CXR (mean 4.24 vs. 5.34 years, median 3.96 vs. 3.47 years, p=0.843)(95% CI 2.71–5.21 years vs. 2.72–4.22 years). Among patients with successive malignancy, cancer-specific survival was also similar, with five-year survival rates of 39.1% in CT and 50.7% in CXR (mean 4.47 vs. 6.51 years, median 4.44 vs. 5.70 years, p=0.353)(95% CI 3.33–5.54 years vs. 3.47–7.93 years). These results were unchanged if survival was measured from date of diagnosis of successive malignancy.
Multivariate analysis and propensity matching
In a Cox proportional hazard model, modality of surveillance imaging was not associated with survival (p=0.958), and only age, Charlson comorbidity index, and type of resection (sublobar vs. non-sublobar resection) were associated with survival. (Table 3) Identical results were seen in a model for cancer-specific survival. (Online Supplemental Table 3).
Table 3.
Regression analysis for overall survival.
| Covariate | Hazard Ratio | 95% CI | p value |
|---|---|---|---|
| Imaging (CXR vs. CT) | 0.988 | 0.629 – 1.551 | 0.958 |
| Age | 1.051 | 1.029 – 1.073 | <0.001 |
| Charlson index | 1.359 | 1.151 – 1.605 | <0.001 |
| Resection (sublobar vs. non-sublobar) | 2.577 | 1.614 – 4.115 | <0.001 |
| Tumor T-stage (1 vs. 2a) | 0.862 | 0.510 – 1.456 | 0.578 |
| Tumor size | 1.108 | 0.876 – 1.401 | 0.393 |
| Histology (non-BAC vs. BAC) | 1.581 | 0.812 – 3.077 | 0.178 |
| Adjuvant therapy (absent vs. present) | 0.691 | 0.389 – 1.226 | 0.206 |
| Gender (male vs. female) | 1.265 | 0.864 – 1.852 | 0.227 |
| Race (non-Caucasian vs. Caucasian) | 1.085 | 0.566 – 2.080 | 0.806 |
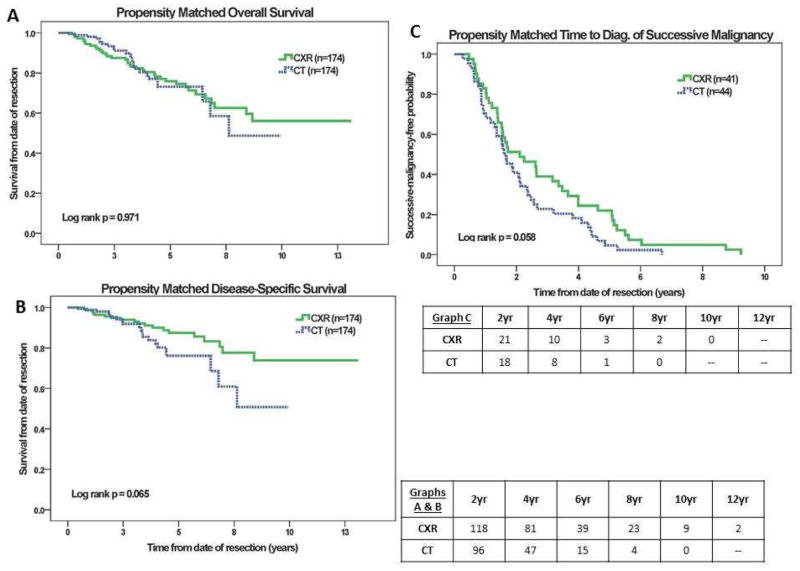
Using a caliper-based method, 174 propensity-matched pairs were identified (Online Supplemental Table 4). There was no significant difference in overall or cancer-specific survival between the groups. Time to diagnosis of successive malignancy trended towards being shorter for CT (median 1.60 years vs. 2.10 years for CXR (95% CI 1.23–1.96 years vs. 0.87–3.34 years, p=0.058)(Figure 2).
Figure 2.
Figure 2A–C. Kaplan-Meier curves for propensity matched overall survival, cancer-specific survival, and time to diagnosis of relapse.
There was no difference between groups in the proportion of successive malignancies that were treated with curative-intent, accounting for 41% (26/63) in the CT group versus 40% (29/72) in the CXR group (p=0.907)(Online Supplemental Figure 3). Overall and cancer-specific survival was similar between groups when successive malignancy was treated with curative intent (p=0.369) or when patients received palliative therapy (p=0.655) (Online Supplemental Figure 3). Overall and cancer-specific survival was also similar between groups among patients developing new primary lung cancers.
In both groups, those given palliative treatment had shorter survival compared to patients given curative-intent treatment, with a median difference of 52 months (median survival 2.64 vs. 6.98 years, p<0.001). Patients whose successive malignancy was detected during a scheduled follow-up visit had similar overall and cancer-specific survival vs. those who presented outside a scheduled follow-up. The difference between overall survival of patients with asymptomatic vs. symptomatic presentation of successive malignancy trended towards statistical significance (mean 5.41 vs. 4.78 years, median 4.98 vs. 3.23 years, p=0.070)(95% CI 3.30–6.66 years vs. 2.45–4.00 years). However, asymptomatic patients were more likely to be offered curative-intent treatment compared to those presenting symptomatically, [56% (25/45) vs. 33% (30/90) (p=0.013)] and have longer cancer-specific survival (mean 6.32 vs. 5.45 years, median 6.15 vs. 3.47 years, p=0.019)(95% CI 4.88–7.42 years vs. 2.67–4.27 years).
Discussion
Given significant variability in the risk of recurrence and treatment strategies for early stage versus locally advanced non-small cell lung cancers, we chose to examine the stage I population alone. These data demonstrate that for pathologic stage I NSCLC, surveillance with CT imaging was associated with earlier detection of successive malignancy compared to CXR imaging in the unmatched comparison with a similar trend in the propensity matched comparison. Patients with successive malignancy who underwent CT were more likely to be diagnosed at an asymptomatic stage or at a scheduled follow-up visit, than those who underwent CXR. Patients with asymptomatic successive malignancies were significantly more likely to receive curative-intent treatment compared to those presenting symptomatically. However, there was no demonstrable improvement in overall survival with CT surveillance vs. CXR. Furthermore, despite the association between CT and the detection of asymptomatic successive malignancies, CT did not result in a greater likelihood of patients receiving curative-intent therapy. Thus, earlier identification of lesions by CT and identification of asymptomatic lesions did not result in a survival benefit in our cohort.
The appropriate modality and regimen for postoperative radiographic surveillance of patients following surgical resection for NSCLC remains unresolved. Although findings from the NLST trial indicate improved mortality with CT screening for a first primary lung cancer among high risk smokers,10 it is unproven whether CT surveillance after resection for lung cancer improves mortality from a second lung cancer. (Online Supplemental Table 2) A primary goal of postoperative surveillance after lung resection is the early detection of new primary lung cancers, which have been shown to occur at a rate of 1–6% per person-year (with most studies reporting 1–2% per person-year).5–9 Intuitively, the risk of developing a new primary lung cancer would seem higher among patients that have undergone resection for lung cancer compared to high risk smokers without prior history of lung cancer as represented in the NLST. The risk of new primary lung cancer is additive to the risk of recurrence in previously resected patients, and our data reinforces the lack of clarity in whether intensive surveillance improves survival of patients with recurrence, even with earlier detection.
Various studies have similarly examined the effectiveness of surveillance CT imaging of patients after resection for lung cancer. A widely cited rationale for the use of CT imaging in surveillance of early stage patients is to detect new primary lung cancers early.5,6,11 In a study of stage I and II patients undergoing CT surveillance after resection,5 scheduled CT scans detected a majority of successive malignancies, consistent with our observation. Most series have demonstrated that the rate of new primary lung cancer development after cancer resection is 1–2% per person-year6–9. Similarly, we observed a rate of new primary lung cancers of 2.1% per person-year for our pathologic stage I NSCLC patients. Others have observed that the majority of second primary lung cancers were detected at stage I and treated by surgical resection.5 We demonstrated that new primary lung cancers were associated with significantly longer survival than recurrences. Furthermore, among patients diagnosed with new primaries, CT was not associated with significantly longer survival. Thus, it remains unclear whether surveillance CT imaging enhances the survival of patients who develop new primary lung cancers.
Hanna et al.14 studied a predominantly early-stage NSCLC population (79% stage I) that underwent surveillance with simultaneous CXR and minimal-dose CT (MnDT) scans, and found that MnDCT resulted in improved sensitivity and negative predictive value vs. CXR in the detection of successive malignancies. They demonstrated that patients whose successive malignancies were detected asymptomatically had a markedly greater rate of curative-intent treatment compared to symptomatic patients, and that those treated with curative intent had improved survival compared to those treated palliatively. We did not observe a significant difference in the rate of curative-intent treatment for the CT and CXR groups, even though CT surveillance was more likely to detect successive malignancies asymptomatically. The minimal-dose CT evaluated by Hanna et al results in less radiation exposure (0.2 mSv) but may be limited in the ability to delineate early subsolid adenocarcinomas or mediastinal recurrence. Our observations suggest that the efficacy of CT imaging in detecting successive malignancies asymptomatically may not be a sufficiently reliable surrogate marker for assessing impact on treatment or survival.
The results of our study are consistent with those from earlier studies that have directly compared CT versus radiograph follow-up regimens. Nakamura et al.17 reported similar survival for stage I patients utilizing surveillance with CT and CXR vs. CXR alone. Benamore et al.15 found that, for stage IIB–III patients, follow-up with routine CT offered earlier detection of successive malignancy but did not result in improved survival, versus CXR. Virgo et al.,18 comparing ‘non-intensive’ vs. ‘intensive’ follow-up that included more frequent CT imaging, found that intensive follow-up did not improve survival. Similarly, Younes et al20 observed that routine follow-up with CT and CXR did not improve survival compared to symptom-prompted follow-up.
Any survival advantage with CT surveillance must be considered in the context of cost-effectiveness and potential hazards of false-positive CT imaging that may result in unnecessary cost and patient morbidity. Postoperative CT imaging has been associated with a nontrivial false-positive rate of 50% for patients with Stage I–IV disease.21 Hanna et al.14 found that the positive predictive value of minimal-dose CT was significantly lower than that of CXR for surveillance of stage I–IV patients (25.1% vs. 91.7%). Furthermore, reports have demonstrated that 4–5% of patients undergoing CT surveillance underwent invasive diagnostic procedures for false-positive imaging.5,21 The rate of false-positives necessitating invasive diagnostic procedures of 4% in our CT group was similar, and was higher than the CXR group. Therefore, the use of intensive CT screening carries a small but meaningful risk of unnecessary invasive procedures.
We certainly recognize the significant limitations of our study in its retrospective approach and the potential lack of power based on our cohort size to detect an actual survival difference. Since we sought to identify and compare patients who consistently received CXR or CT surveillance imaging, we had to exclude patients whose surveillance protocol was inconsistent in the imaging modality used for routine surveillance. The lack of difference in the rate of curative intent therapy may be related to a nonsignificant increase in the number of new primary cancers diagnosed in the CXR group vs. the CT group given that the length of follow-up was longer for the CXR group allowing for more time to diagnose new primary lesions. Our simple power calculation indicated that with a power of 80% and an alpha level of 5%, our sample size was sufficient to detect a 29% difference in median survival; differences beneath 29% could not be detected. Given the limitations of our sample size we’ve included sample size calculations for theoretical scenarios of surveillance trials in Online Supplemental Table 5. It was also not possible to assess whether the quality of life of patients was enhanced or diminished by earlier diagnosis of successive malignancies in the CT group. The ongoing IFCT-0302 trial,22 comparing follow-up using CXR versus CT and bronchoscopy, will help determine the survival benefits of intensive follow-up after resection.
Consistent with our primary hypothesis, our study suggests that for stage I lung cancer patients treated by surgical resection, routine surveillance CT was associated with earlier diagnosis of recurrence or new primary lung cancer compared to routine CXR. However, this earlier diagnosis did not result in a demonstrable difference in treatment approach or overall survival. Recognizing the limitations of our study but also acknowledging the consistency of our findings with the findings of others, we conclude that the appropriate imaging modality and regimen for surveillance remains unclear and unsubstantiated and recommend that a randomized controlled, multi-institutional prospective clinical trial be performed to help define the appropriate radiographic modality for surveillance after resection for lung cancer.
Supplementary Material
Footnotes
Presented at the 94th Annual Meeting of the American Association for Thoracic Surgery, Toronto, Ontario, Canada. April 29, 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feld R, Rubinstein LV, Weisenberger TH the Lung Cancer Study Group. Sites of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J Clin Oncol. 1984;2:1352–8. doi: 10.1200/JCO.1984.2.12.1352. [DOI] [PubMed] [Google Scholar]
- 2.Goodgame B, Viswanathan A, Zoole J, Gao F, Miller CR, Subramanian J, et al. Risk of recurrence of resected stage I non-small cell lung cancer in elderly patients as compared with younger patients. J Thorac Oncol. 2009;4:1370–4. doi: 10.1097/JTO.0b013e3181b6bc1b. [DOI] [PubMed] [Google Scholar]
- 3.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–12. [PubMed] [Google Scholar]
- 4.Walsh GL, O’Connor M, Willis KM, et al. Is follow-up of lung cancer patients after resection medically indicated and cost-effective? Ann Thorac Surg. 1995;60:1563–72. doi: 10.1016/0003-4975(95)00893-4. [DOI] [PubMed] [Google Scholar]
- 5.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–82. doi: 10.1016/j.jtcvs.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Lamont JP, Kakuda JT, Smith D, Wagman LD, Grannis FW. Systematic postoperative radiologic follow-up in patients with non-small cell lung cancer for detecting second primary lung cancer in stage IA. Arch Surg. 2002;137:935–9. doi: 10.1001/archsurg.137.8.935. [DOI] [PubMed] [Google Scholar]
- 7.Senthi S, Lagerwaard FJ, Haasbeek CJA, Slotman BJ, Senan S. Pattern of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–9. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Nat Ca Inst. 1998;90:1335–45. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 9.Colt HG, Murgu SD, Korst RJ, Slatore CG, Unger M, Quadrelli S. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy. Chest. 2013;143:e437S–54S. doi: 10.1378/chest.12-2365. [DOI] [PubMed] [Google Scholar]
- 10.The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaklitsch MT, Jacobson FL, Austin JHM, Field JK, Jett JR, Keshavjee S, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J thorac Cardiovasc Surg. 2012;144:33–8. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 12.Crinò L, Weder W, van Meerbeeck J, Felip E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v103–v15. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 13.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–71. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 14.Hanna WC, Paul NS, Darling GE, Moshonov H, Allison F, Waddell TK, et al. Minimal-dose computed tomography is superior to chest x-ray for the follow-up and treatment of patients with resected lung cancer. J Thorac Cardiovasc Surg. 2014;147:30–5. doi: 10.1016/j.jtcvs.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Benamore R, Shepherd FA, Leighl N, et al. Does intensive follow-up alter outcome in patients with advanced lung cancer? J Thorac Oncol. 2007;2:273–81. doi: 10.1097/01.JTO.0000263708.08332.76. [DOI] [PubMed] [Google Scholar]
- 16.Chiu C-H, Chern M-S, Wu M-H, et al. Usefulness of low-dose spiral CT of the chest in regular follow-up of postoperative non–small cell lung cancer patients: preliminary report. J Thorac Cardiovasc Surg. 2003;125:1300–5. doi: 10.1016/s0022-5223(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura R, Kurishima K, Kobayashi N, et al. Postoperative follow-up for patients with non-small cell lung cancer. Onkologie. 2010;33:14–8. doi: 10.1159/000264623. [DOI] [PubMed] [Google Scholar]
- 18.Virgo KS, McKirgan LW, Caputo MC, et al. Post-treatment management options for patients with lung cancer. Ann Surg. 1995;222:700–10. doi: 10.1097/00000658-199512000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westeel V, Choma D, Clément F, et al. Relevance of an intensive postoperative follow-up after surgery for non-small cell lung cancer. Ann Thorac Surg. 2000;70:1185–90. doi: 10.1016/s0003-4975(00)01731-8. [DOI] [PubMed] [Google Scholar]
- 20.Younes RN, Gross JL, Deheinzelin D. Follow-up in lung cancer: how often and for what purpose? Chest. 1999;115:1494–9. doi: 10.1378/chest.115.6.1494. [DOI] [PubMed] [Google Scholar]
- 21.Korst RJ, Kansler AL, Port JL, Lee PC, Altorki NK. Accuracy of surveillance computed tomography in detecting recurrent or new primary lung cancer in patients with completely resected lung cancer. Ann Thorac Surg. 2006;82:1009–15. doi: 10.1016/j.athoracsur.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 22.Westeel V, Lebitasy MP, Mercier M, Girard P, Barlesi F, Blanchon F, et al. IFCT-0302 trial: randomised study comparing two follow-up schedules in completely resected non-small cell lung cancer [in French] Rev Mal Respir. 2007;24:645–52. doi: 10.1016/s0761-8425(07)91135-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.