Abstract
The Research Domain Criteria (RDoC) adopts a dimensional approach for examining pathophysiological processes underlying categorically defined psychiatric diagnoses. We used this framework to examine relationships among symptom dimensions, diagnostic categories, and resting state connectivity in behaviorally and emotionally dysregulated youth selected from the Longitudinal Assessment of Manic Symptoms study (n=42) and healthy control youth (n=18). Region of interest analyses examined relationships among resting state connectivity, symptom dimensions (behavioral and emotional dysregulation measured with the Parent General Behavior Inventory-10 Item Mania Scale [PGBI-10M]; dimensional severity measures of mania, depression, anxiety), and diagnostic categories (Bipolar Spectrum Disorders, Attention Deficit Hyperactivity Disorder, Anxiety Disorders, Disruptive Behavior Disorders). After adjusting for demographic variables, two dimensional measures showed significant inverse relationships with resting state connectivity, regardless of diagnosis: 1) PGBI-10M with amygdala-left posterior insula/bilateral putamen; and 2) depressive symptoms with amygdala-right posterior insula connectivity. Diagnostic categories showed no significant relationships with resting state connectivity. Resting state connectivity between amygdala and posterior insula decreased with increasing severity of behavioral and emotional dysregulation and depression; this suggests an intrinsic functional uncoupling of key neural regions supporting emotion processing and regulation. These findings support the RDoC dimensional approach for characterizing pathophysiologic processes that cut across different psychiatric disorders.
Keywords: fMRI, amygdala, insula, RDoC, adolescents
1. Introduction
Psychiatric disorders in youth characterized by behavioral and emotional dysregulation (difficulty regulating the experience and expression of behaviors and emotions) include major depressive disorder (MDD), bipolar spectrum disorders (BPSD), attention deficit hyperactivity disorder (ADHD), anxiety disorders, and disruptive behavior disorders (DBD). These disorders pose diagnostic and treatment challenges due to high comorbidity and the lack of clear biologic trait markers (Kowatch et al., 2005; Pavuluri et al., 2005; Arnold et al., 2011; Arnold et al., 2012). NIMH Research Domain Criteria (RDoC) offer an alternative approach to identifying transdiagnostic pathophysiologic processes (Cuthbert and Insel, 2013) and biological disease markers (Bebko et al., 2014) through dimensions of behavioral and emotional dysregulation.
Neuroimaging techniques examining brain-behavior relationships are ideal for applying RDoC to studying pediatric psychiatric disorders. One such technique, resting state functional connectivity (RSC), provides information about intrinsic connectivity in neural networks independent of specific cognitive contexts requiring sophisticated, and often challenging, cognitive tasks which may not represent daily activities. RSC may additionally be more ecologically valid, allowing observation of mind wandering, a commonly occurring activity in the daily lives of youth. Additionally, by focusing on neural regions shown to be important in fMRI task-related analyses [such as the amygdala (Altshuler et al., 2005; Foland et al., 2008), striatum (Deveney et al., 2013), prefrontal cortical (Pavuluri et al., 2008; Kalmar et al., 2009; Passarotti et al., 2010; Ladouceur et al., 2011) and anterior cingulate cortical (Gogtay et al., 2007; Kalmar et al., 2009) regions, and an insula-centered neural network supporting salience, interoception, and emotion perception (Rubia et al., 2009; Taylor et al., 2009; Kurth et al., 2010; Cauda et al., 2012; Cloutman et al., 2012)], RSC studies may increase our understanding of pathophysiologic processes in behaviorally and emotionally dysregulated youth.
The small number of RSC studies in youth with behavioral and emotional dysregulation across a variety of psychiatric disorders have used different methods and reported different patterns of abnormal RSC between the amygdala and key prefrontal cortical, anterior cingulate cortical, and insula regions supporting emotion regulation. Using Independent Component Analysis, increased RSC in a neural network comprising the amygdala, orbitofrontal cortex, anterior cingulate cortex, and insula (Wu et al., 2013) in unmedicated, manic, BPSD versus healthy control (HC) youth was reported. In contrast, when using an amygdala seed region, no RSC differences between BPSD and HC (Dickstein et al., 2010) were shown. For youth with MDD, decreases in both amygdala-prefrontal cortical, and amygdala-striatal, connectivity were reported relative to HC (Luking et al., 2011). In addition, differences were observed for MDD versus HC youth in RSC between right and left amygdala seed regions with prefrontal gyri (Pannekoek et al., 2014). Additional studies reported both increased (Davey et al., 2012) and decreased (Cao et al., 2006; Cullen et al., 2009; Sun et al., 2012; Pannekoek et al., 2014) RSC between prefrontal and anterior cingulate regions, as well as increased (Gabbay et al., 2013) and decreased (Bluhm et al., 2009; Davey et al., 2012) RSC between striatal and anterior cingulate cortical regions in youth with depression or ADHD versus HC. Together, these findings suggest inconsistent patterns of abnormal RSC in pediatric psychiatric disorders characterized by behavioral and emotional dysregulation.
While the variable nature of these findings may reflect differences in mood state, different analytic techniques, or both, it remains unclear whether there are more consistent patterns of abnormal amygdala-centered RSC associated with specific symptom dimensions across different diagnostic categories. A few studies examined relationships between RSC and symptom dimensions in youth with BPSD or MDD (Luking et al., 2011; Ford et al., 2013; Gabbay et al., 2013; Xiao et al., 2013) but may not represent the wider range of pediatric psychiatric disorders. Furthermore, these studies provided mixed findings of both positive and inverse relationships between mania and depression severity and RSC in networks linking amygdala, striatum, prefrontal, anterior cingulate, and other cortical regions (Luking et al., 2011; Gabbay et al., 2013; Xiao et al., 2013).
In summary, prior studies provide inconsistent reports of RSC among amygdala (Dickstein et al., 2010; Luking et al., 2011), striatal (Bluhm et al., 2009; Davey et al., 2012; Gabbay et al., 2013; Xiao et al., 2013), prefrontal cortical (Cao et al., 2006; Cullen et al., 2009; Dickstein et al., 2010; Davey et al., 2012; Sun et al., 2012; Wu et al., 2013), anterior cingulate cortical (Bluhm et al., 2009; Cullen et al., 2009; Davey et al., 2012; Gaffrey et al., 2012; Gabbay et al., 2013; Wu et al., 2013; Xiao et al., 2013), and insula (Wu et al., 2013) regions in behaviorally and emotionally dysregulated youth. In the present study, we aimed to elucidate for the first time the nature and extent of relationships between pathological dimensions and RSC versus relationships between diagnostic categories and RSC in a clinical cohort of youth with behavioral and emotional dysregulation. Given the central role of the amygdala in emotion processing (Ochsner and Gross, 2005; Phillips et al., 2008) and the inconsistencies shown in the literature, we used an amygdala seed region. We recruited a subset of youth selected from the Longitudinal Assessment of Manic Symptoms (LAMS) study (Horwitz et al., 2010a), a longitudinal, multisite study of youth seeking treatment for behavioral and emotional dysregulation. The LAMS study was designed to assess relationships among the longitudinal course of symptoms, clinical, and functional outcomes in these youth (Methods). In addition to using DSM-IV classifications of pediatric psychiatric disorders and commonly-used dimensional symptom measures of emotional dysregulation in youth (rating scales of mania, depression, and anxiety), LAMS also uses the Parent General Behavior Inventory-10 Item Mania Scale (PGBI-10M), a parental report of manic-like behaviors associated with difficulty regulating mood and energy (Methods) (Youngstrom et al., 2005; Youngstrom et al., 2008).
Primary Hypothesis-Dimensional
As suggested by previous reports of altered RSC in behaviorally and emotionally dysregulated youth implementing dimensional approaches (Luking et al., 2011; Ford et al., 2013; Gabbay et al., 2013; Xiao et al., 2013), we hypothesized that, across all behaviorally and emotionally dysregulated LAMS youth, irrespective of diagnosis, RSC between amygdala, striatum, prefrontal cortices, anterior cingulate cortices, and insula would be significantly associated with dimensional measures of behavioral and emotional dysregulation (PGBI-10M score, mania, depression. and anxiety).
Secondary Hypothesis-Categorical
Based on previous findings of differential patterns of RSC among the above regions between youth with and without specific diagnoses (Dickstein et al., 2010; Luking et al., 2011; Wu et al., 2013), current diagnostic categories in LAMS youth would be differentiated by patterns of amygdala RSC.
Additionally, we recruited a comparison group of HC to examine the extent to which significant relationships between RSC and symptom dimensions, or diagnostic categories, represented abnormal RSC in LAMS youth versus HC.
2. Methods
2.1. Description of the Longitudinal Assessment of Manic Symptoms (LAMS) study
LAMS is a longitudinal NIMH-supported study of children and adolescents seeking treatment for behavioral and emotional dysregulation diagnoses such as BPSD, other mood disorders, ADHD, anxiety disorders, and disruptive disorders. Because behavioral and/or emotional dysregulation symptoms similar to manic-like behaviors are common to these disorders, the study name includes reference to “manic symptoms.”
The Parent General Behavior Inventory-10 Item Mania Scale (PGBI-10M; Supplemental) was used to screen potential LAMS study participants: 6–12 year old children recruited at their first visit to 9 mental health clinics associated with 4 universities (Findling et al., 2010). The PGBI-10M is parent-rated scale that assesses the child’s positive mood and energy dysregulation over the last six months and discriminates between BPSD and other comorbidities, such as ADHD (Youngstrom et al., 2005; Youngstrom et al., 2008). High PGBI-10M scores (>12) were common (present in 43% of these youth), regardless of diagnosis, and associated with worse overall functioning and higher rates of a variety of psychiatric disorders, including BPSD, ADHD, disruptive behavior disorders, other mood and anxiety disorders, in the initial screening of the LAMS cohort (Findling et al., 2010; Horwitz et al., 2010b). We invited children scoring >12 on the PGBI-10M, as well as a demographically matched sample of children who were also seeking mental health care but did not have severe behavioral or emotional dysregulation (scoring <11), to participate in the study. Independent of diagnosis, high PGBI-10M scores (>12) were associated with worse overall functioning (assessed by the Children’s Global Assessment Scale (Shaffer et al., 1983)), higher rates of psychiatric diagnoses (Findling et al., 2010; Horwitz et al., 2010a), and greater prefrontal cortical activity to reward (Bebko et al., 2014). Refer to Horwitz and colleagues (2010b) for more information on the background and study design of LAMS.
2.2. Participants
121 youth (9.89–17.71 years; Table 1) were selected from the LAMS1 cohort of 707 youth to participate in the neuroimaging component of the second phase of LAMS (LAMS2): Case Western Reserve University (CWRU; n=32); Cincinnati Children’s Hospital (CCH; n=46); and University of Pittsburgh Medical Center/Western Psychiatric Institute and Clinic (UPMC; n=43). For comparison, 32 age and sex ratio-matched HC were newly recruited via advertisement (8.03–16.92 years; CWRU n=13; CCH n=6; UPMC n=13; Table S1). See Supplemental for study inclusion/exclusion criteria. Written informed consent and assent were obtained from parents/guardians and youth prior to study participation.
Table 1.
Demographic information, clinical variables, and current medication usage for the total LAMS sample and comparisons of those included versus excluded from neuroimaging.
| Total LAMS Sample | Included Participants | Excluded Participants | Statistic Comparing Included vs Excluded Participants | ||
|---|---|---|---|---|---|
|
N=121 M(SD/Range) or Proportion |
N=42 M(SD/Range) or Proportion |
N=79 M(SD/Range) or Proportion |
|||
| Demographic Information | P value | ||||
| Age (years) | 13.65(2.05/9.89–17.17) | 14.32(2.32/9.89–17.71) | 13.30(1.81/9.99–17.07) | t=−2.45 | 0.017* |
| IQ | 101.28(16.44/70–140) | 97.31(16.96/70–137) | 103.39(15.86/71–140) | t=−1.96 | 0.052 |
| SES (maternal education) | χ2=1.96 | 0.580 | |||
| HS Diploma or less | 39/121 | 13/42 | 26/79 | ||
| Some post-HS | 27/121 | 9/42 | 18/79 | ||
| Associate’s Degree | 32/121 | 14/42 | 18/79 | ||
| Bachelor’s Degree or higher | 23/121 | 6/42 | 17/79 | ||
| Sex (males) | 75/121 | 24/42 | 51/79 | χ2=0.64 | 0.424 |
| Clinical Measures | |||||
| PGBI-10M | 5.97(6.12/0–24) | 4.18(4.33/0–18) | 6.91(6.72/0–24) | t=−2.72 | 0.008** |
| K-DRS | 3.68(4.51/0–24) | 3.86(5.29/0–24) | 3.58(4.07/0–20) | t=−0.32 | 0.751 |
| K-MRS | 4.22(6.44/0–28) | 3.07(6.10/0–24) | 4.83(6.57/0–28) | t=−1.44 | 0.152 |
| SCARED | 11.37(10.94/0–53) | 10.93(11.01/0–46) | 11.60(10.97/0–53) | t=−0.32 | 0.749 |
| Current Medication Use | |||||
| Antidepressant | 18/121 | 3/42 | 15/79 | χ2=3.04 | 0.081 |
| Antipsychotic | 25/121 | 7/42 | 18/79 | χ2=0.63 | 0.429 |
| Benzodiazepine | 2/121 | 1/42 | 1/79 | 1.00 | |
| Mood Stabilizer | 10/121 | 4/42 | 6/79 | 0.737 | |
| Non-stimulant1 | 9/121 | 2/42 | 7/79 | 0.494 | |
| Stimulant | 45/121 | 11/42 | 34/79 | χ2=3.33 | 0.068 |
Abbreviations: GED=general education development test; HS=high school; IQ=intelligence quotient Wechsler Intelligence test; K-DRS= Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Present Episode Depression Rating Scale; K-MRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale; M=mean; N=sample size; P=probability value; PGBI-10M=Parent General Behavior Inventory 10 Item Mania Scale; SCARED=Screen for Child Anxiety Related Emotional Disorders (child rating); SD=standard deviation; SES=socio-economic status; t=t-test statistical value; χ2=chi-squared test statistic value.
Non-stimulant ADHD medications included both prescription and non-prescription medications and supplements (Intuniv, Brightspark, etc.).
Difficulties with resting state acquisition resulted in unusable resting state data from CCH. Of the remaining 75 LAMS youth and 26 HC from CWRU and UPMC, 33 LAMS youth (Maximum displacement in translation or rotation: M=8.19, SD=11.63, Range=2.19–59.61) and 8 HC (Maximum displacement in translation or rotation: M=4.34, SD=2.23, Range=2.53–9.53) were excluded due to data loss and excessive motion (>2 mm maximum displacement in translation or rotation, as in previous resting state studies (Cao et al., 2006; Fair et al., 2010)), leaving 42 LAMS youth (Age: M=14.32, SD=2.32, Range=9.89–17.71; 24 males; n=28; Table 1) and 18 HC (Age: M=13.56, SD=2.37, Range=8.03–15.29; 9 males; Table S1).
Eighteen of the 42 LAMS youth were taking at least one psychotropic medication (Table 1). Of those, 10 were taking one class, 6 two classes, and 2 three classes, of psychotropic medications. Prescribed medication(s) could be taken before and on the day of the scan given ethical problems with restricting medication use for research participation.
Neural circuitry underlying emotion processing (Perlman et al., 2013), emotion regulation (Bertocci et al., 2014), and reward processing (Bebko et al., 2014) in LAMS participants is reported elsewhere.
2.3. Symptom assessment
LAMS youth and their parents/guardians completed several symptom assessment measures. To assess behavioral and emotional dysregulation, parents/guardians completed the PGBI-10M (Supplemental) at study entry, baseline, and 6-monthly intervals throughout LAMS1 and LAMS2. PGBI-10M scores showed stability over the three assessment points (one year) closest to scan day (Bebko et al., 2014). PGBI-10M score closest to scan day (Days between obtaining score and scan: M=18.48, SD=39.07, Range=87 days prior to scan and 91 days after scan) was used as the most recent PGBI-10M measure.
Both parents and children completed the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale (K-MRS) (Axelson et al., 2003) to assess hypo/manic symptoms and Depression Rating Scale (K-DRS) (Kaufman et al., 1997) to assess depressive symptom, both with good psychometric properties (Axelson et al., 2003; Kaufman et al., 1997) respectively, on scan day (Table 1). If parent and child responses differed on a question, interviewers used clinical judgment with all available information to make final decisions on the summary scores for that question. The Screen for Child Anxiety Related Emotional Disorders (SCARED) assessed anxiety symptoms over the last 6 months (Birmaher et al., 1997) from scan day (Table 1).
2.4. Diagnostic categories
Unmodified DSM-IV diagnoses were established by administering children and parents/guardians the K-SADS-PL-W (Kaufman et al., 1997). We defined BPSD as a diagnosis of bipolar I disorder, bipolar II disorder, or bipolar disorder not otherwise specified and DBD as a diagnosis of conduct disorder, disruptive behavior disorders not otherwise specified, or oppositional defiant disorder. BPSD was documented as a current diagnosis even if it was noted as “in partial/full remission.” As confirmed by a licensed child psychiatrist or psychologist, the 42 LAMS youth had a variety of current primary and secondary DSM-IV diagnoses: no primary or secondary diagnoses (n=8), ADHD (n=17), BPSD (n=12), depression (n=12), DBD (n=10), and anxiety disorders (n=2). See Supplemental for further breakdowns of the diagnoses. HC did not meet DSM-IV criteria for any current diagnoses.
2.5. Neuroimaging data analysis
We collected resting state data on a 1) 3T Philips Achieva X-series MRI scanner at CCH, 2) 3T Siemens Verio MRI scanner at CWRU, and 3) 3T Siemens Trio MRI scanner at UPMC. An axial 3D magnetization prepared rapid gradient echo (MP-RAGE) sequence (192 axial slices 1 mm thick; flip angle=9°; field of view=256 mm × 192 mm; TR=2300 msec; TE=3.93 msec; matrix=256×192) acquired T1-weighted volumetric anatomical images covering the whole brain. A reverse interleaved gradient echo planar imaging (EPI) sequence (38 axial slices 3.1 mm thick; flip angle=90°; field of view=205 mm; TR=2000 msec; TE=28 msec; matrix=64×64) acquired T2-weighted BOLD images covering the whole cerebrum and most of the cerebellum. Participants were instructed to remain still while viewing a fixation cross during a 6-minute resting state image acquisition.
We conducted a seed-based resting state functional connectivity analysis using predominantly Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm) related tools. See Supplemental for our analytic approach, which was inspired by a rsfMRI study of amygdala connectivity (Kim et al., 2011) and previously employed (Chase et al., 2013).
2.6. Creation of a single anatomically-defined bilateral target region ROI mask
Based on previous studies (Altshuler et al., 2005; Ochsner and Gross, 2005; Gogtay et al., 2007; Foland et al., 2008; Pavuluri et al., 2008; Kalmar et al., 2009; Passarotti et al., 2010; Ladouceur et al., 2011; Deveney et al., 2013; Wu et al., 2013), we used the WFU PickAtlas (Maldjian et al., 2003) to create a single ROI mask including the striatum (caudate head and body, putamen, and ventral striatum [bilateral spheres centered on MNI coordinates −9, 9, −8 and 9, 9, −8; radius=8mm (Postuma and Dagher, 2006; Di Martino et al., 2008)]), prefrontal cortical regions: medial prefrontal cortex (mPFC; BA10), orbitofrontal cortex (OFC; BA11), ventrolateral prefrontal cortex (VLPFC; BA47), dorsal anterior cingulate cortex (dACC; BA24/32), and insula. Using one large ROI mask avoided problems associated with conducting multiple statistical tests over the entire brain.
2.7. Identifying resting state connectivity
We determined significant clusters of RSC between a) the average time course across all voxels within the bilateral amygdala and b) the time course of each voxel within the entire a priori, anatomically-defined bilateral (noncontiguous) ROI mask (P<0.005 voxelwise, P<0.05 corrected with AlphaSim, a Monte Carlo simulation cluster forming threshold to correct for multiple comparisons (Ward, 2002)) with scan site as a covariate of no interest. We saved these significant clusters of RSC for use in multiple regression analyses testing primary and secondary hypotheses.
2.8. Statistical approach
Similar to our previous statistical approach to examining dimensions versus diagnostic categories (Bebko et al., 2014), we performed a voxelwise multiple regression analysis in SPM8 to determine which dimensional (primary hypothesis) and categorical (secondary hypothesis) variables were significantly associated with RSC in the above clusters after accounting for other variables of no interest: demographic (age, IQ, sex), scan site, signal: noise ratio (SNR) closest to the scan date (Supplemental), and medication status (taking versus not taking psychotropic medication). This analysis involved two steps. First, to avoid model overfitting and to balance Type I and II errors, we examined the univariate relationship between each variable (i.e., variables of interest and other variables) and amygdala-target region RSC identified above (P<0.05 voxelwise, P<0.05 clusterwise significance threshold). Second, we created one final regression model that contained only those variables showing significant relationships with amygdala-target region RSC in step one.
When a significant relationship with a dimensional measure was identified, we conducted additional HC comparisons to examine the extent to which the relationships between the dimensional measure and RSC represented abnormal RSC. For this analysis we first identified the 18 highest and 18 lowest scoring LAMS youth on the relevant dimensional measure, and then compared amygdala-target region RSC in these two LAMS subgroups with the 18 HC (voxelwise threshold of P<0.013, corrected for the three pairwise between group tests; P<0.05, corrected threshold). All three groups were matched on sex ratio, age, and IQ. This analysis determined whether RSC patterns were specifically associated with dimensional constructs (i.e., if either LAMS subgroup differed significantly from HC in this RSC pattern), or were more generally associated with psychopathology (i.e., if both LAMS samples differed from HC in this RSC pattern).
To test our secondary hypothesis, we conducted similar analyses: identified diagnostic categories were followed-up with analyses comparing LAMS youth with and without the relevant diagnosis, relative to HC (matched on age, IQ, and sex ratio). Here, the n for the group comparisons was determined by the number of LAMS youth with the relevant diagnosis (i.e., 12 LAMS youth had a primary or comorbid BPSD diagnosis, so the n for the LAMS youth without the relevant diagnosis and for the HC was 12).
2.9. Accounting for the potential effect of site
We used two strategies to reduce inter-scan site variability. First, we used a Biomedical Informatics Research Network (BIRN) phantom at each site to monitor SNR monthly and ensure scanner signal stability over time as recommended (http://www.nbirn.net) (Table S2). SNR across all scan sites (CCH: M=162.43, SD=10.75; CWRU: M=170.74, SD=8.87; UPMC: M=166.45, SD=11.43) did not significantly differ overall, (F(2, 50)=2.51, P>.05) or between the two scan sites with usable data (t(38)=1.31, P>.05). Second, when statically appropriate, we used scan site and SNR as covariates in analyses (Section 2.7.–2.8.).
3. Results
In LAMS youth, right putamen (901 voxels), left putamen extending into posterior insula (1060 voxels), right BA47 extending into posterior insula (2 clusters: 19 voxels, 45 voxels), bilateral OFC/dACC (Left: BA 11/24, 130 voxels; Right: BA 11/32,34 voxels), and right dACC (BA24, 116 voxels) showed significant RSC with the amygdala (P<0.005, corrected P<0.05; Figure 1A; Table 2), controlling for scan site.
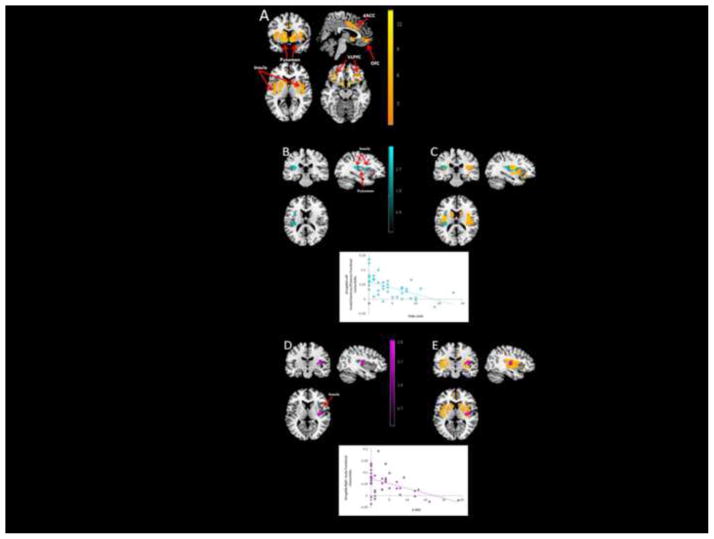
Figure 1. Entire bilateral ROI mask resting state analysis in LAMS youth (n=42) displayed in neurological convention.
(A) Amygdala resting state functional connectivity (orange)
(B) Amygdala-left insula/putamen connectivity associated with PGBI-10M (teal) (r=−0.44)
(C) Overlap between amygdala resting state connectivity displayed in (A) and (B)
(D) Amygdala-right insula connectivity associated with K-DRS (purple) (r=−0.52)
(E) Overlap between amygdala resting state connectivity displayed in (A) and (D)
Table 2.
Amygdala resting state connectivity in 42 LAMS youth.
| MNI Coordinates | Statistic | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Region | BA | k | x | y | z | Test Statistic(df) | Puncorrected |
| Right Putamen | 901 | 27 | −7 | −8 | t(40)=14.03 | <0.001*** | |
| Left Putamen/Insula | 1060 | −24 | −7 | −8 | t(40)=13.95 | <0.001*** | |
| Right VLPFC/Insula | 47 | 19 | 27 | 11 | −20 | t(40)=10.96 | <0.001*** |
| 47 | 45 | 30 | 32 | −14 | t(40)=5.84 | <0.001*** | |
| Right OFC/dACC | 11, 32 | 34 | 3 | 50 | −14 | t(40)=6.06 | <0.001*** |
| Left OFC/dACC | 11, 24 | 130 | −6 | 53 | −11 | t(40)=5.34 | <0.001*** |
| Right dACC | 24 | 116 | 3 | 14 | 31 | t(40)=5.30 | <0.001*** |
Region of interest analyses using voxelwise P<0.005 and P<0.05, AlphaSim corrected. Table rows represent the peak voxel within the specified region.
Abbreviations: BA=Brodmann area; dACC=dorsal anterior cingulate cortex; df=degrees of freedom; k=cluster size in voxels; MNI=Montreal Neurological Institute coordinates; OFC=orbitofrontal cortex; Puncorrected=uncorrected voxelwise probability value; t=t-test statistical value; VLPFC=ventrolateral prefrontal cortex
3.1. Primary hypothesis (dimensional)
Initial univariate analyses revealed that two dimensional variables showed significant inverse relationships (P<0.05, corrected) with RSC: PGBI-10M with amygdala-left posterior insula and putamen (210 voxels); and K-DRS with amygdala-right posterior insula (150 voxels). Univariate analyses also revealed a significant inverse relationship (P<0.05, corrected) between sex and amygdala-right posterior insula RSC (72 voxels).
We created one final regression model containing the two significant dimensional measures (K-DRS, PGBI-10M) and sex. The two inverse relationships between dimensional measures and resting connectivity observed in univariate analyses remained significant in the final regression model (both P<0.05, voxelwise, P<0.05, corrected within the RSC mask): PGBI-10M with amygdala-left posterior insula putamen (163 voxels; Pearson r=−0.52; P<0.001; Spearman r=−0.57; P<0.001 on extracted amygdala-left posterior insula putamen beta values; Figure 1B–C; Table 3); and K-DRS with amygdala-right posterior insula (74 voxels; Pearson r=−0.44, P=0.004; Spearman r=−0.39, P=0.010; Figure 1D–E; Table 3). Sex was not associated with RSC within this regression model. See Supplementary Material for regression fit statistics.
Table 3.
Symptom measures associated with amygdala resting state connectivity in 42 LAMS youth.
| MNI Coordinates | Statistic | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Region | BA | k | x | y | z | Test Statistic(df) | Puncorrected |
| K-DRS | |||||||
| Right Insula | 13 | 74 | 36 | −13 | 10 | r(38)= −0.44 | 0.003** |
| PGBI-10M | |||||||
| Left Insula | 13 | 163 | −30 | −28 | 10 | r(38)= −0.52 | 0.001** |
| Left Claustrum | −30 | 5 | 13 | ||||
| Left Putamen | −27 | −7 | 10 | ||||
Regression analyses using a voxelwise P<0.05 and P<0.05, AlphaSim corrected. Table rows represent the peak voxel within the specified region.
Abbreviations: BA=Brodmann area; df=degrees of freedom; k=cluster size in voxels; K-DRS= Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Present Episode Depression Rating Scale; MNI=Montreal Neurological Institute coordinates; Puncorrected=uncorrected voxelwise probability value; PGBI-10M=Parent General Behavior Inventory 10 Item Mania scale, r=Pearson’s correlation coefficient.
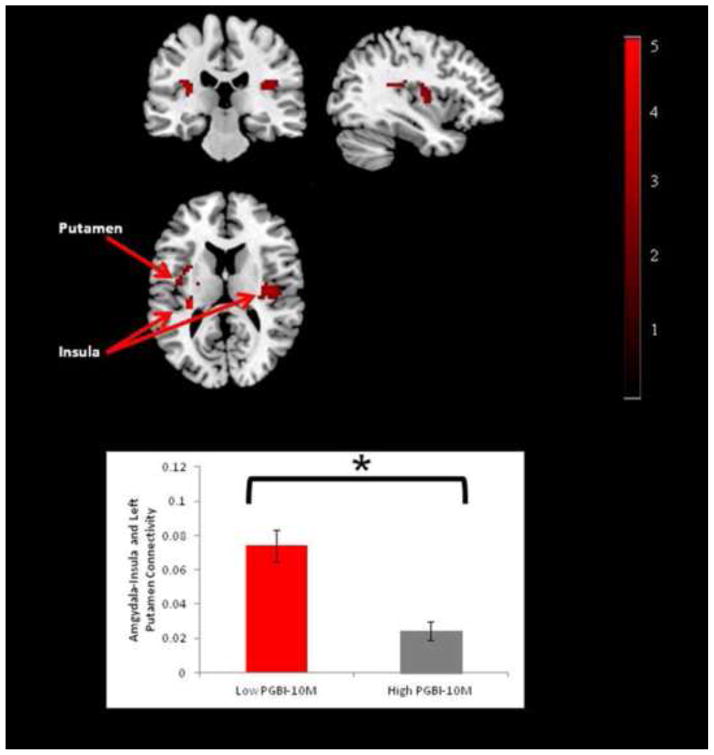
LAMS youth with the lowest PGBI-10M scores showed significantly greater amygdala-bilateral posterior insula (Left 46 voxels; Right 98 voxels)/left putamen (50 voxels) RSC relative to LAMS youth with highest PGBI-10M scores (voxelwise P<0.01; P<0.05, corrected; Figure 2; Table S3). The 18 highest and lowest KDRS scoring LAMS youth and the 18 HC did not differ significantly from each other on RSC (Table S4).
Figure 2.
Comparison of amygdala resting state connectivity in 18 LAMS youth with low PGBI-10M scores and 18 LAMS with high PGBI-10M scores displayed in neurological convention
3.2. Secondary hypothesis (categorical)
Univariate analyses revealed no significant differences in amygdala-target region RSC between diagnostic categories. Thus, no multivariate regression analyses were conducted.
4. Discussion
This study aimed to identify relationships between intrinsic (resting state) connectivity in neural regions supporting emotion processing/regulation and measures of behavioral and emotional dysregulation in LAMS youth presenting with a variety of psychiatric diagnoses. Dimensional and categorical relationships were tested. Supporting our dimension-focused primary hypothesis, we found significant inverse relationships between 1) amygdala-left posterior insula and putamen RSC and PGBI-10M score, and 2) amygdala-right posterior insula RSC and depression symptom severity. Importantly, these findings remained significant even when co-varying for demographic variables that showed significant relationships with RSC. In contrast, we did not find support for our secondary diagnostic category-focused hypothesis.
Overall, LAMS youth with behavioral and emotional dysregulation showed RSC between bilateral amygdala and several a priori regions supporting emotion regulation (Phillips et al., 2008): bilateral putamen, right VLPFC, bilateral OFC/dACC, and bilateral posterior insula. Altered connectivity among these regions has previously been reported in BPSD and depressed youth during resting state (Wu et al., 2013) and in BPSD youth during implicit emotion processing (Wang et al., 2012), suggesting that altered connectivity may underlie behavioral and emotional dysregulation both at rest and during task performance. Our key findings of significant inverse relationships resulted from decreased positive RSC with increasing severity of two dimensional measures. These findings suggest intrinsic functional uncoupling between amygdala and posterior insula in more behaviorally and emotionally dysregulated, as well as in more depressed, youth. Roles of the amygdala and putamen in emotion processing (Altshuler et al., 2005; Ochsner and Gross, 2005; Phillips et al., 2008), and that of the insula in the experience of, and response to, emotion are well-established (Dupont et al., 2003; Cauda et al., 2011). Studies also indicate divergent patterns of connectivity and related functions of anterior and posterior insula subregions (Dupont et al., 2003; Cauda et al., 2012). While the anterior insula has strong connectivity with orbitofrontal and ventrolateral prefrontal cortices (Dupont et al., 2003; Cauda et al., 2011), and is implicated in salience and emotion perception (Dolan, 2002; Dupont et al., 2003) and control of emotional and goal directed behavior (Cloutman et al., 2012), the posterior insula has connectivity with sensorimotor, temporal, and posterior cingulate cortices, and is implicated in interoceptive and emotional processing (Dupont et al., 2003; Cauda et al., 2011; Cauda et al., 2012), and visceral experiences (Dupont et al., 2003). The above patterns of intrinsic functional uncoupling among amygdala, putamen, and posterior insula that are common across different diagnoses in LAMS youth may therefore represent pathophysiological processes predisposing youth to impaired integration of external emotional and interoceptive information (Dupont et al., 2003; Cauda et al., 2011; Cauda et al., 2012), that in turn may result in behavioral and emotional dysregulation.
Our findings of decreased amygdala-posterior RSC in LAMS youth and subsequent relationships with clinical variables have some support from the literature. Negative relationships between amygdala-posterior insula, dACC, and prefrontal RSC in BPSD youth and measures of behavioral and emotional dysregulation using the BRIEF have been reported (Wu et al., 2013). Contrary to our findings are reports of 1) increased amygdala-posterior insula, dACC, and prefrontal RSC in BPSD youth relative to HC (Wu et al., 2013), 2) a lack of a relationship between amygdala-posterior insula, dACC, and prefrontal RSC and clinical measure of depression and mania, and 3) a positive relationship between a clinician-reported bipolar index and RSC in a network comprising putamen, insula and claustrum in young adults with either BPSD or MDD (Ford et al., 2013). The reason for these discrepancies is unclear, although may result from different clinical approaches: diagnostic categorical framework (Dickstein et al., 2010; Xiao et al., 2013), broadly defined bipolar index (Ford et al., 2013), dimensional approach focusing on more narrowly-defined symptom measures (e.g., depression severity), and a transdiagnostic measure of more stable, trait-like behavioral and emotional dysregulation (PGBI-10M score) (current study). Furthermore, previous studies focused on relationships between RSC and different measures of bipolarity in general, whereas our approach examined relationships between RSC and a transdiagnostic dimensional measure of behavioral and emotional dysregulation.
Interestingly, amygdala-posterior insula RSC was associated with behaviors measured by the PGBI-10M, not the K-MRS. The PGBI-10M, a parent-rated measure, captures trait-like information about specific manic symptoms (positive mood/energy dysregulation over the last six months), while the K-MRS is a traditional clinician-rated measure that captures state-like information about a broader range of current manic symptoms. We previously reported a significant relationship between PGBI-10M score, but not K-MRS, and neural activity in LAMS youth (Bebko et al., 2014). Our findings suggest the PGBI-10M may better reflect underlying pathophysiologic processes across different diagnoses than the conventionally defined measures of psychiatric comorbidity commonly used in previous studies. A possible explanation for the stronger relationship between the parent assessment and amygdala-posterior RSC than was found with the clinical assessment in this case is that the parent assessment is based on the parents’ historical knowledge of participant behavior whereas the clinical assessment reveals a state that is more circumscribed in time.
Contrary to our secondary hypothesis, none of the diagnostic categories examined (ADHD, BPSD, DBD) showed disorder-specific RSC abnormalities. This may be due to the greater statistical power of a dimensional, rather than a diagnostic/categorical, approach for examining pathophysiology of behavioral and emotional dysregulation. Given the absence of disorder-specific findings in this study, as well as the paucity and inconsistent findings of RSC studies in youth with disorders characterized by behavioral and emotional dysregulation, combining both dimensional and diagnostic approaches in future RSC studies may be a promising way forward to elucidate patterns of abnormal intrinsic neural connectivity in these youth,.
LAMS youth with the lowest PGBI-10M scores showed significantly greater amygdala-bilateral posterior insula RSC than the subset of LAMS youth with the highest PGBI-10M scores. Neither LAMS subset differed significantly from HC in this connectivity. Interpretation of these findings is speculative given that PGBI-10M scores were not collected from HC. One possibility is that greater RSC among these regions may be a compensatory process occurring in the presence of lower levels of behavioral and emotional dysregulation. Alternatively, the greater RSC among these regions may reflect a recruitment failure in the presence of higher levels of behavioral and emotion dysregulation. In a similar analysis, the K-DRS did not show the same phenomenon. Because of the non-independence of our regression and t-test analyses, we prudently suggest that these findings may further reflect greater sensitivity of a dimensional versus a group-based analytic approach to detect relationships between RSC and symptom severity in youth (MacCallum et al., 2002).
The significance of left and right laterality RSC abnormalities associated with PGBI-10M and K-DRS, respectively, is unclear. Previous research highlights putative roles of the left forebrain in approach-related and affiliative emotions and the right forebrain in arousal and withdrawal-related emotion processing (Craig, 2005; Harmon-Jones et al., 2010). Greater functional decoupling between the amygdala and left posterior insula associated with greater PGBI-10M scores thus may represent a neural mechanism underlying the approach nature of some abnormal behaviors, such as difficulty regulating positive mood and energy, measured by this scale. In contrast, greater functional decoupling between amygdala and right posterior insula associated with greater K-DRS score may underlie the withdrawal nature of depressive symptoms measured by the K-DRS.
Limitations include data loss from data acquisition difficulties and from excessive motion (>2mm), especially in younger children with more severe pathology. We chose this conservative movement threshold because seed-based resting state approaches are sensitive to small head motion (Van Dijk et al., 2012). Many participants were taking psychotropic medication (n=18); of those, eight were taking more than one class of medication. Power was insufficient to examine the influence of multiple psychotropic medications on RSC, thus, the impact of specific or multiple medication use on the brain, brain development, emotion regulation, and cognitive function is unknown. Univariate analyses, however, showed no significant effect of medication status (taking versus not taking psychotropics) on RSC. LAMS youth were scanned at multiple sites; however, advantages of multi-site neuroimaging, including increased statistical power and a large, diverse population, likely outweighed potential limitations. Furthermore, we implemented several measures to minimize inter-scanner differences. The mean PGBI-10M score was low (4.16), although the range (0–18) captured both low and high levels of behavioral and emotional dysregulation in LAMS youth. Further studies should replicate our findings and examine youth with higher PGBI-10M scores. PGBI-10M scores were not collected for HC, so we could not compare this dimensional measure between LAMS youth and HC. Finally, measures of pubertal status were not collected, precluding examination of the relationship between pubertal change and RSC.
The LAMS cohort provided a unique opportunity to examine the extent to which intrinsic neural connectivity either reflected dimensions of psychopathology or was associated with specific diagnostic categories in a large cohort of behaviorally and emotionally dysregulated youth. We found significant relationships between RSC and dimensional measures of behavioral and emotional dysregulation. Findings support using a dimensional approach, such as RDoC, to study neural mechanisms underlying psychopathology and utilizing RSC as a measure of intrinsic connectivity among neural regions. Ultimately, by combining dimensional and diagnostic categorical approaches, future research may identify objective biomarkers to help identify and treat youth with, or at risk for, psychopathology characterized by behavioral and emotional dysregulation.
Supplementary Material
Highlights.
Examined resting state in behaviorally/emotionally dysregulated youth
Recruited participants from Longitudinal Assessment of Manic Symptoms (LAMS) study
Decreased resting state connectivity associated with greater depressive symptoms
Resting state connectivity negatively related to behavioral/emotional dysregulation
Supports Research Domain Criteria (RDoC) dimensional approach
Acknowledgments
Supported by the National Institute of Mental Health grants 2R01 MH73953-09A1 (Birmaher and Phillips, University of Pittsburgh), 2R01 MH73816-09A1 (Holland, Children’s Hospital Medical Center), 2R01 MH73967-09A1 (Findling, Case Western Reserve University), and 2R01 MH73801-09A1 (Fristad, Ohio State University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: A functional magnetic resonance imaging study. American Journal of Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Demeter C, Mount K, Frazier TW, Youngstrom EA, Fristad M, Birmaher B, Findling RL, Horwitz SM, Kowatch R. Pediatric bipolar spectrum disorder and ADHD: comparison and comorbidity in the LAMS clinical sample. Bipolar Disorders. 2011;13:509–521. doi: 10.1111/j.1399-5618.2011.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LE, Mount K, Frazier T, Demeter C, Youngstrom EA, Fristad MA, Birmaher B, Horwitz S, Findling RL, Kowatch R. Pediatric bipolar disorder and ADHD: Family history comparison in the LAMS clinical sample. Journal of Affective Disorders. 2012;141:382–389. doi: 10.1016/j.jad.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Bebko G, Bertocci MA, Fournier JC, Hinze AK, Bonar L, Almeida JR, Perlman SB, Versace A, Schirda C, Travis M. Parsing Dimensional vs Diagnostic Category–Related Patterns of Reward Circuitry Function in Behaviorally and Emotionally Dysregulated Youth in the Longitudinal Assessment of Manic Symptoms Study. JAMA psychiatry. 2014;71:71–80. doi: 10.1001/jamapsychiatry.2013.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci MA, Bebko G, Olino T, Fournier J, Hinze AK, Bonar L, Almeida JR, Perlman SB, Versace A, Travis M, Gill MK, Demeter C, Diwadkar VA, White R, Schirda C, Sunshine JL, Arnold LE, Holland SK, Kowatch RA, Birmaher B, Axelson D, Youngstrom EA, Findling RL, Horwitz SM, Fristad MA, Phillips ML. Behavioral and emotional dysregulation trajectories marked by prefrontal-amygdala function in symptomatic youth. Psychol Med. 2014;27:1–13. doi: 10.1017/S0033291714000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, NEER SM. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, Neufeld R, Osuch E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. NeuroReport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D’Agata F, Duca S, Geminiani G, Fox PT, Vercelli A. Meta-analytic clustering of the insular cortex: characterizing the meta analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Chase HW, Moses-Kolko EL, Zevallos C, Wisner KL, Phillips ML. Disrupted posterior cingulate amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, Lambon Ralph MA. The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. Neuroimage. 2012;59:3514–3521. doi: 10.1016/j.neuroimage.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42:2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- Deveney CM, Connolly ME, Haring CT, Bones BL, Reynolds RC, Kim P, Pine DS, Leibenluft E. Neural Mechanisms of Frustration in Chronically Irritable Children. American Journal of Psychiatry. 2013;4:12070917. doi: 10.1176/appi.ajp.2013.12070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, Biswal B, Walters J, Castellanos F, Milham M. Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, Kelly C, Gee DG, Zuo XN, Castellanos FX, Milham MP. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol Psychiatry. 2010;68:839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, Cognition, and Behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surg Radiol Anat. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TGC, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter C. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. The Journal of clinical psychiatry. 2010;71:1664. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Research - Neuroimaging. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Theberge J, Neufeld RJ, Williamson PC, Osuch EA. Correlation of brain default mode network activation with bipolarity index in youth with mood disorders. J Affect Disord. 2013;24:00467–00469. doi: 10.1016/j.jad.2013.05.088. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52:628–641. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53:964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Ordonez A, Herman DH, Hayashi KM, Greenstein D, Vaituzis C, Lenane M, Clasen L, Sharp W, Giedd JN, Jung D, Nugent TF, 3rd, Toga AW, Leibenluft E, Thompson PM, Rapoport JL. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Horwitz SM, Demeter CA, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA, Frazier TW, Findling RL. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. The Journal of Clinical Psychiatry. 2010a;71:1511–1517. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz SMC, Demeter C, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA. Longitudinal Assessment of Manic Symptoms (LAMS) Study: background, design and initial screening results. The Journal of clinical psychiatry. 2010b;71:1511. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Spencer L, Edmiston E, Lacadie CM, Martin A, Constable RT, Duncan JS, Staib LH, Papademetris X, Blumberg HP. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc. 2009;15:476–481. doi: 10.1017/S1355617709090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disorders. 2005;7:483–496. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox P, Laird A, Eickhoff S. A link between the systems: functional differentiation and integration within the human insula revealed by meta analysis. Brain Structure and Function. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, Birmaher B, Phillips M. Differential Patterns of Abnormal Activity and Connectivity in the Amygdala–Prefrontal Circuitry in Bipolar I and Bipolar NOS Youth. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:1275–1289. e1272. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM. Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry. 2011;50:1027–1041. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological methods. 2002;7:19. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, van der Werff SJA, Meens PHF, van den Bulk BG, Jolles DD, Veer IM, van Lang NDJ, Rombouts SARB, van der Wee NJA, Vermeiren RRJM. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naïve clinically depressed adolescents. Journal of Child Psychology and Psychiatry. 2014:n/a–n/a. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion Processing Influences Working Memory Circuits in Pediatric Bipolar Disorder and Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Research: Neuroimaging. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Fournier JC, Bebko G, Bertocci MA, Hinze AK, Bonar L, Almeida JR, Versace A, Schirda C, Travis M. Emotional Face Processing in Pediatric Bipolar Disorder: Evidence for Functional Impairments in the Fusiform Gyrus. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:1314–1325. e1313. doi: 10.1016/j.jaac.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A children’s global assessment scale (CGAS) Archives of General Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, Zuo X, An L, Song Y, Zang Y, Wang Y. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naïve boys with attention deficit hyperactivity disorder. Psychiatry Research: Neuroimaging. 2012;201:120–127. doi: 10.1016/j.pscychresns.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Bobrow L, Liu J, Spencer L, Blumberg HP. Corticolimbic functional connectivity in adolescents with bipolar disorder. PLoS One. 2012;7:e50177. doi: 10.1371/journal.pone.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. AlphaSim. National Institute of Mental Health; Bethesda, MD: 2002. [Google Scholar]
- Wu M, Lu LH, Passarotti AM, Wegbreit E, Fitzgerald J, Pavuluri MN. Altered affective, executive and sensorimotor resting state networks in patients with pediatric mania. J Psychiatry Neurosci. 2013;38:120073. doi: 10.1503/jpn.120073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xiao Q, Zhong Y, Lu D, Gao W, Jiao Q, Lu G, Su L. Altered regional homogeneity in pediatric bipolar disorder during manic state: a resting state fMRI study. PLoS One. 2013;8:6. doi: 10.1371/journal.pone.0057978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom E, Meyers O, Demeter C, Youngstrom J, Morello L, Piiparinen R, Feeny N, Calabrese JR, Findling RL. Comparing diagnostic checklists for pediatric bipolar disorder in academic and community mental health settings. Bipolar Disorders. 2005;7:507–517. doi: 10.1111/j.1399-5618.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a Ten Item Mania Scale from the Parent General Behavior Inventory for Children and Adolescents. The Journal of clinical psychiatry. 2008;69:831. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.