Structured abstract
Objective
To study causes and implications of intraoperative conversions to thoracotomy during VATS lobectomy.
Methods
We performed an institutional review of patients undergoing lobectomy for known or suspected lung cancer with root cause analysis of every conversion from VATS to open thoracotomy.
Results
Between 2004 and 2012, 1227 patients underwent lobectomy. Of these, 517 (42%) were completed VATS, 87 (7%) converted to open, and 623 (51%) were performed via planned thoracotomy. Patients undergoing thoracotomy were younger and had a higher incidence of prior lung cancers. Planned thoracotomy and conversion group patients had higher clinical T stage than the VATS group while the planned thoracotomy group had higher pathologic stage than the other groups. Postoperative complications were more frequent in the conversion group (46%) than VATS (23%, p<0.001), but similar to the open group (42%, p=0.56). Validating a previous classification of causes for conversion, 22/87 (25%) were due to vascular causes, 56 (64%) for anatomy (adhesions/tumor size), and 8 (9%) for lymph nodes. No specific imaging variables predicted conversion. Within the conversions, emergent (20/87, 23%) and planned (67/87, 77%) conversion groups were similar in patient- and tumor characteristics and incidence of perioperative morbidity. The conversion rate for VATS lobectomy dropped from 21/74 (28%), to 29/194 (15%), to 37/336 (11%) (p<0.001) over 3-year intervals. Over the same periods, the proportion of operations started VATS increased significantly.
Conclusions
With increasing experience, a higher proportion of lobectomy operations can be completed thoracoscopically. VATS should be strongly considered as the initial approach for the majority of patients undergoing lobectomy.
Introduction
Surgical resection via lobectomy and systematic mediastinal lymph node assessment is the gold standard for treatment of early-stage non-small cell lung cancer (NSCLC). The feasibility, safety, and oncologic efficacy of video-assisted thoracoscopic surgery (VATS) lobectomy have been established over the last two decades via large institutional series (1, 2) as well as multi-center trials.(3) Despite the favorable evidence, registry data as well as the Society of Thoracic Surgeons general thoracic database show that only 30–40% of anatomic lung resections are performed utilizing VATS.(4, 5)
Technical barriers, including a potential higher risk of intraoperative complications and perceptions about unplanned conversions to thoracotomy, are important issues preventing more widespread acceptance of VATS lobectomy. The incidence of intraoperative conversion to an open approach ranges from 5% to 23% with nearly half the conversions being performed emergently. (6–8) Retrospective series describing conversions show conflicting evidence with some centers reporting greater perioperative morbidity compared to successful VATS completion (6) with others showing equivalent outcomes.(9) Few reports compare unplanned conversions with planned thoracotomy for lobectomy.(6)
Preoperative patient-related variables have been studied for association with the likelihood of conversion and CT scan lymph node calcification score found to be a potential predictor.(6) The role of surgeon/institutional experience in conversion, though, remains inadequately understood. Additionally, the impact of conversion on immediate and delayed outcomes is debatable.
Our objective was to study causes of intraoperative conversions to thoracotomy during VATS lobectomy utilizing and validating an existing classification system.(8) We also assessed short- and long-term implications of conversion with respect to cases completed VATS and those undergoing planned thoracotomy.
Patients and methods
With approval from the institutional review board of Washington University School of Medicine, a single-center, retrospective review of our database was performed to identify patients who had undergone lobectomy for known or suspected lung cancer between December, 2004 and December, 2012. We chose a start date of 2004 for the study since we initially started offering VATS lobectomy in 2004. Additionally, electronic patient records have been reliably available for review since that time. Patients who underwent a complete anatomic lobectomy with individual division of hilar bronchovascular structures were included in the study cohort. Patients undergoing wedge resection, segmentectomy, bilobectomy, or pneumonectomy were excluded as were any patients who underwent lung transplantation.
Each case was classified as having undergone a VATS lobectomy, open thoracotomy, or intraoperative unplanned conversion from VATS to open operation for lobectomy by an independent chart review by 2 observers (KM, VP). Cases with inconsistent classification by the 2 observers were resolved by joint review by authors KM, VP, and JB. VATS lobectomy was defined as per CALGB 39802 trial criteria.(3) Briefly, the technique mandated no rib spreading; a maximum length of 8 cm of the incision for removal of the lobectomy specimen; individual dissection of the vein, arteries, and airway; and standard lymph node sampling or dissection (identical to an open thoracotomy). Cases where a planned VATS operation was intraoperatively abandoned in favor of an open thoracotomy with rib spreading were classified as conversions. Each conversion was studied in detail by 2 observers (KM, VP) in a root cause analysis (10) and classified using the previously-described “VALT” system as either vascular, anatomy (related), lymph node (related), or technical (equipment failure).(8)
We utilized a prospectively maintained database to abstract information about patient demographics, diagnosis, preoperative workup, operation, perioperative course, and outcomes. Missing data were obtained by a review of patient charts. Perioperative events were defined per Society of Thoracic Surgeons (STS) data collection guidelines (Appendix 1) Follow-up data were obtained from clinic notes and supplemented by querying the social security death index to determine survival.
Data were managed using Microsoft Excel and analyzed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL). Descriptive statistics were expressed as mean ± standard deviation unless otherwise specified. Categorical data were expressed as counts and proportions. Comparisons were done with two-tailed t tests for means of normally distributed continuous variables. Differences among the categorical data were analyzed with either Fisher's exact test or chi-square comparison. We generated Kaplan Meier (product limit) survival plots, and survival comparisons between groups of patients were completed using the Mantel-Haenszel log rank test. All p values less that 0.05 were considered to be statistically significant.
After an initial exploratory comparison between the VATS, open, and conversion groups, logistic regression models were fitted to assess the influence of preoperative variables on the likelihood of conversion. The influence of increasing institutional experience with VATS lobectomy was studied separately by dividing the population into 3 year intervals. A secondary analysis of emergent and non-emergent conversions was also carried out. Emergent conversions were defined as those dictated by significant bleeding or airway injury while non-emergent conversions were related to lack of progress in the operation. For the purpose of this study, in keeping with STS definitions, conditions signifying major perioperative morbidity are summarized in Appendix 1.
Results
Between 2004 and 2012, 1227 patients underwent lobectomy for known or suspected lung cancer at our institution. Of these, 517 (42%) were completed via VATS, 87 (7%) converted to open, and 623 (51%) were performed via planned thoracotomy. Patients undergoing thoracotomy were younger and had a higher incidence of prior lung cancers. (Table 1) Patients undergoing successful VATS lobectomy were less likely to be male, current smokers or have undergone prior cardiothoracic surgery. Planned thoracotomy and conversion group patients had higher clinical T stage than the VATS group (Table 1) while the planned thoracotomy group had higher clinical N stage than the other 2 groups. There was no difference in the prevalence of intrathoracic granulomatous disease, including lymph node calcifications, between the groups. Patients undergoing successful VATS had a somewhat higher forced expiratory volume in one second (FEV1) percent predicted that those undergoing thoracotomy. The planned thoracotomy group had higher pathologic stage than the other groups. (Table 1)
Table 1.
Preoperative variables for entire cohort of patients undergoing lobectomy (N=1227)
| Variable | Thoracotomy only (n=623) |
VATS only (n=517) |
VATS conversion to Thoracotomy (n=87) |
P |
|---|---|---|---|---|
| Mean age (years) | 62.4 ± 12.3 | 64.8 ± 10.9 | 63.9 ± 10.4 | 0.002* |
| Male Gender | 321 (52%) | 223 (43%) | 47 (54%) | 0.010 |
| Caucasian | 553 (88%) | 444 (86%) | 73 (84%) | 0.079 |
| Current smoker | 202 (32%) | 131 (25%) | 26 (30%) | 0.001 |
| Prior lung cancer | 54 (9%) | 28 (5%) | 10 (12%) | 0.039 |
| Mean Body Mass Index | 26.8 ± 6.2 | 27.6 ± 6.9 | 27.7 ± 5.8 | 0.082 |
| Hypertension | 306 (49%) | 300 (58%) | 45 (52%) | 0.011 |
| Granulomatous disease on CT | 154 (25%) | 138 (27%) | 23 (26%) | 0.739 |
| Prior cardiothoracic surgery | 87 (14%) | 42 (8%) | 13 (15%) | 0.005 |
| Mean FEV1 percent predicted † | 0.79 ± 0.20 | 0.83 ± 0.18 | 0.80 ± 0.19 | 0.002** |
| Mean DLCO percent predicted ‡ | 0.73 ± 0.23 | 0.74 ± 0.23 | 0.69 ± 0.20 | 0.274 |
| Clinical stage I II III IV X |
319 (57%) 91 (16.1%) 103 (18.3%) 47 (8.3%) 2 (0.3%) |
330 (75.2%) 42 (9.6%) 27 (6.2%) 35 (7.9%) 5 (1.1%) |
52 (69.3%) 6 (8%) 12 (16%) 5 (6.7%) 0 |
<0.0001 |
| Clinical tumor (T) stage T1 T2 T3 T4 TX |
225 (40%) 236 (42%) 55 (9.9%) 44 (7.8%) 2 (0.3%) |
306 (69.7%) 102 (23.3%) 21 (4.8%) 5 (1.1%) 5 (1.1%) |
45 (60%) 21 (28%) 3 (4%) 6 (8%) 0 |
<0.0001 |
| Clinical nodal (N) stage N0 N1 N2 N3 |
400 (71.2%) 72 (12.8%) 87 (15.5%) 3 (0.5%) |
381 (86.8%) 28 (6.4%) 26 (5.9%) 4 (0.9%) |
64 (85.4%) 4 (5.3%) 6 (8%) 1 (1.3%) |
<0.0001 |
| Pathologic stage 0 I II III IV X |
15 (2.7%) 300 (53.4%) 126 (22.4%) 100 (17.8%) 19 (3.4%) 2 (0.3%) |
1 (0.2%) 315 (71.8%) 69 (15.7%) 45 (10.3%) 9 (2%) 0 |
0 49 (66.2%) 16 (21.6%) 8 (10.8%) 1 (1.4%) 0 |
<0.0001 |
Post-hoc testing noted statistical difference in age between Thoracotomy only and VATS only, p=0.001
FEV1= Forced expiratory volume in 1 second, data available in 1160 patients
Post-hoc testing noted statistical difference in FEV1% between Thoracotomy only and VATS only, p=0.001
DLCO=Diffusing capacity of the lung for carbon monoxide, data available in 1025 patients
Postoperative STS database-defined complications were more frequent in the conversion group, 40/87 (46%) than VATS, 119/517 (23%, p<0.001), but similar to the open group, 264/623 (42%, p=0.56). The higher incidence of perioperative morbidity in the two open groups was largely related to greater incidence of atelectasis, pneumonia, arrhythmias, and respiratory failure. (Table 2) The length of stay was also longer if patients underwent a planned or unplanned thoracotomy. (Table 2) Surgical mortality was similarly low across the groups (Thoracotomy 5/623, 0.8%, VATS 0/517, Conversion 1/87, 1%, p=0.1).
Table 2.
Perioperative outcomes in entire cohort of patients undergoing lobectomy (N=1227)
| Variable | Thoracotomy only (n=623) |
VATS only (n=517) |
VATS conversion to Thoracotomy (n=87) |
P |
|---|---|---|---|---|
| Mean length of stay (LOS), days | 7.5 ± 7.2 | 4.6 ± 4.7 | 7.6 ± 7.4 | <0.0001* |
| Any post-operative complication | 264 (42%) | 119 (23%) | 40 (46%) | <0.0001 |
| Pneumonia | 48 (8%) | 20 (4%) | 11 (13%) | 0.002 |
| Empyema | 2 (0.3%) | 3 (0.6%) | 0 | 0.653 |
| Arrhythmia | 97 (16%) | 52 (10%) | 16 (18%) | 0.009 |
| Atelectasis | 27 (4%) | 5 (1%) | 7 (8%) | <0.0001 |
| Air leak > 5 days | 39 (6%) | 21 (4%) | 2 (2%) | 0.115 |
| Pulmonary embolus | 3 (0.5%) | 1 (0.2%) | 2 (2%) | 0.034 |
| Respiratory failure | 29 (5%) | 1 (0.2%) | 7 (8%) | <0.0001 |
| Re-operation for bleeding | 7 (1%) | 3 (0.6%) | 1 (1%) | 0.605 |
| Myocardial infarction | 2 (0.3%) | 1 (0.2%) | 0 | 0.811 |
| Cerebrovascular accident | 3 (0.5%) | 2 (0.4%) | 2 (2%) | 0.083 |
| Wound infection | 6 (1%) | 0 | 0 | 0.054 |
| Operative mortality | 5 (0.8%) | 0 | 1 (1%) | 0.10 |
| Pathologic upstaging † | 105 (19%) | 72 (16%) | 12 (16%) | 0.610 |
Post-hoc testing noted statistical difference in LOS between Thoracotomy only and VATS only, and between VATS only and VATS conversion to thoracotomy
Pathologic upstaging data in 1075 patients
Validating a previous classification of causes for conversion, 22/87 (25%) were due to vascular causes, 56 (64%) for anatomy (adhesions/tumor size), 8 (9%) for lymph nodes, and 1 (1%) was related to a technical failure of equipment. Of the 22 conversion due to vascular causes, 15 were related to pulmonary artery injury or subadventitial hematoma, 6 due to pulmonary venous laceration, and 1 due to azygous vein injury. Of the 56 operations converted due to anatomy, only 4 were due to tumor size alone (mean size 6 cm), while the remaining were related to intrathoracic adhesions, either malignant or inflammatory in nature. In a logistic regression model, only male gender was associated with an elevated risk of unplanned conversion (OR 1.78, 95% CI 1.02–3.11). No other specific preoperative patient-related or imaging variables predicted the likelihood of conversion (Table 3). Within the conversions, emergent (20/87, 23%) and non-emergent (67/87, 77%) conversion groups were similar in patient and tumor characteristics and incidence of perioperative morbidity. (Online supplemental table 1)
Table 3.
Logistic regression analysis for predictors of conversion in patients started VATS (n=604)
| Variable | Odds ratio | 95% Confidence Interval |
P value |
|---|---|---|---|
| Age | 0.985 | 0.957 – 1.013 | 0.289 |
| Body Mass Index | 1.001 | 0.962 – 1.043 | 0.947 |
| Male Gender | 1.78 | 1.015 – 3.110 | 0.043 |
| Granulomatous disease on CT | 0.940 | 0.484 – 1.824 | 0.855 |
| Clinical nodal stage, N1 | 1.038 | 0.453 – 2.376 | 0.930 |
| Prior lung cancer | 2.347 | 0.942 – 5.848 | 0.084 |
| Prior cardiothoracic surgery | 1.567 | 0.610 – 4.030 | 0.365 |
| FEV1 percent predicted* | 1.305 | 0.240 – 7.107 | 0.758 |
| DLCO percent predicted† | 0.435 | 0.117 – 4.614 | 0.203 |
FEV1= Forced expiratory volume in 1 second
DLCO=Diffusing capacity of the lung for carbon monoxide
Intraoperative blood transfusion data were reliably available for patient operated between 2008 and 2012 (n=754). The rate of transfusion was lower for those undergoing successful VATS (6/464, 1.3%) than patients undergoing conversion (11/66, 16.7%) or planned thoracotomy (23/224, 10/3%) (p <0.001). Patients undergoing emergent conversions were more likely to be transfused intraoperatively (9/19, 47.4%) than those undergoing non-emergent conversion (2/47, 4.3%, p<0.001).
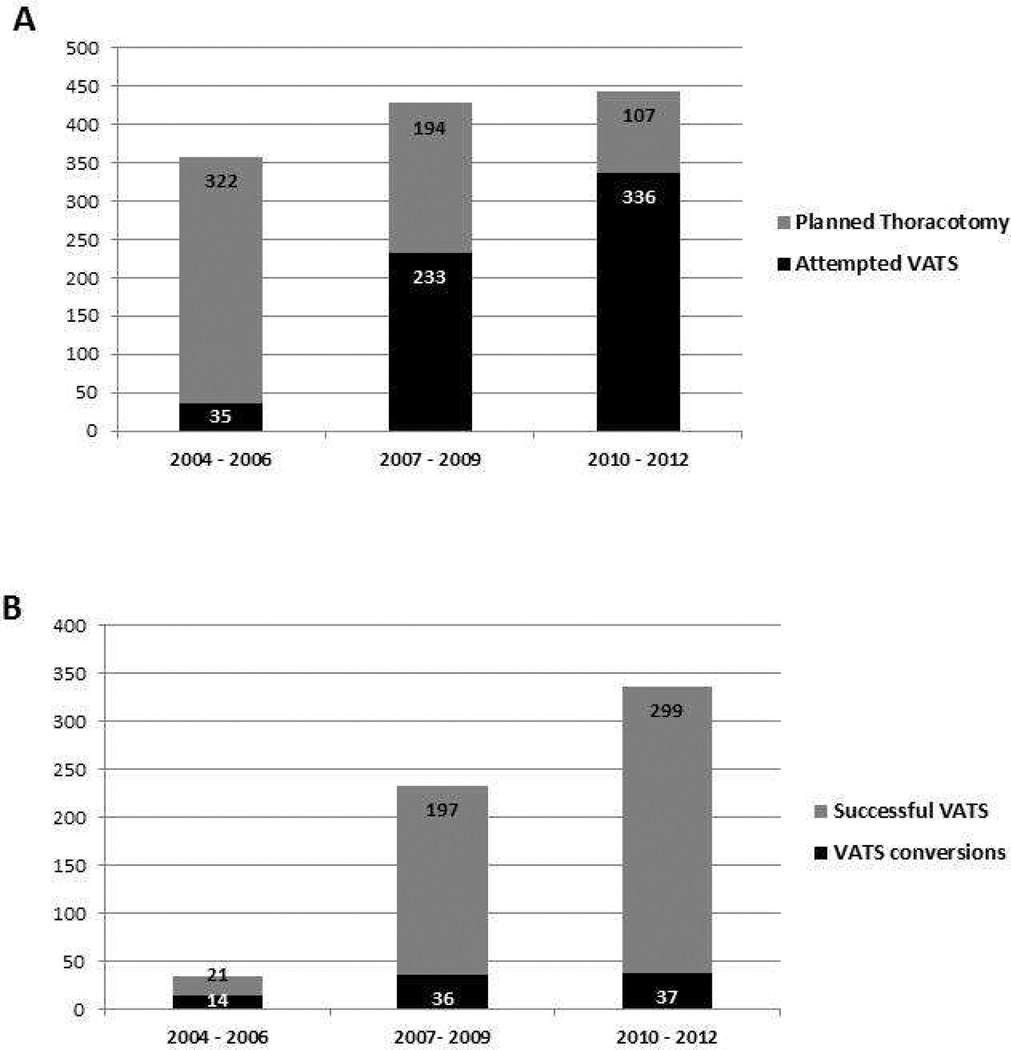
During the study period, the conversion rate for VATS lobectomy dropped from 21/74 (28%), to 29/194 (15%), to 37/336 (11%) (p<0.001) over 3-year intervals. (Figure 1A) Over the same time periods, the proportion of operations started VATS increased from 74/473 (16%), to 194/311 (62%), to 336/443 (76%) (p<0.001). (Figure 1B) Iin a multivariate Cox proportional hazard model evaluating patients with pathologic stage I disease, only increasing age, male gender, current smoking status, and lower carbon monoxide diffusing capacity (DLCO) percent predicted were associated with elevated risk of long-term mortality. Intraoperative conversion was not associated with greater hazard of long-term mortality. (Table 4)
Figure 1.
A. Distribution of Attempted VATS vs. Planned Thoracotomies over 3-year intervals
B. Distribution of Successful VATS vs. VATS conversions over 3-year intervals
Table 4.
Cox regression analysis studying factors associated with long-term mortality in patients with pathologic stage I NSCLC (n=558)
| Variable | Hazard Ratio | 95% Confidence Interval |
P value |
|---|---|---|---|
| Age | 1.058 | 1.039 – 1.078 | <0.0001 |
| Body Mass Index | 0.979 | 0.946 – 1.013 | 0.216 |
| Female Gender | 0.684 | 0.494 – 0.946 | 0.021 |
| Caucasian race | 1.019 | 0.588 – 1.767 | 0.946 |
| Prior lung cancer | 1.348 | 0.699 – 2.597 | 0.391 |
| Current smoker | 1.535 | 1.081 – 2.182 | 0.019 |
| Hypertension | 1.153 | 0.816 – 1.629 | 0.416 |
| Study Group Converted, VATS to open Open incision VATS incision |
Reference 1.036 0.550 |
-- 0.577 – 1.860 0.290 – 1.042 |
-- 0.907 0.067 |
| FEV1 >50% percent predicted | 0.955 | 0.463 – 1.971 | 0.902 |
| DLCO >50% percent predicted | 0.573 | 0.367 – 0.895 | 0.021 |
Discussion
The main findings of our study are that with increasing experience a greater proportion of lobectomy operations can be performed via VATS and an unplanned conversion to an open approach does not carry any significant elevated risk over a planned open thoracotomy. Though conversions may be hard to predict, we also note that only a minority of conversions are emergent, and even those operations are not associated with an elevated risk of morbidity or mortality over non-emergent conversions or planned thoracotomy operations.
VATS lobectomy has consistently been shown to be equivalent or superior to conventional open lobectomy for objective perioperative outcomes. Several institutional series have demonstrated a lower incidence of pulmonary complications, transfusion requirement, and arrhythmias in propensity-matched populations of VATS patients compared to open operations.(2, 11, 12) VATS lobectomy has also been found to be less morbid in elderly patients and those with limited pulmonary function.(11, 13) These findings have been reconfirmed in a large STS database analysis that compared matched groups of patients undergoing VATS or open lobectomy and found a significantly lower incidence (24% vs. 35%) of complications in the VATS group.(4) Meta-analyses have confirmed these results. (14, 15) Our findings regarding perioperative complications are quite similar. Despite the aforementioned benefits, and the likely overall cost savings to the healthcare system, the penetrance of VATS lobectomy for stage I NSCLC remains below 29% nationally (source – National Cancer Database, 2006–10 statistics, personal communication from author AP).
In the face of this evidence, it is likely that inadequate exposure to, and concerns about intraoperative complications including conversion, prevent a wider acceptance of VATS lobectomy. Indeed, a study on more than 13,000 patients from the Nationwide Inpatient Sample database found that patients who underwent VATS lobectomy were 1.6 times more likely to have intraoperative complications than patients who underwent open lobectomy.(16) Prominent publications describe a low (1%) incidence of truly catastrophic intraoperative complications (17), yet these may also have the effect of dissuading surgeons comfortable with an open approach from attempting VATS lobectomy. New VATS lobectomy programs may also be concerned by the papers recommending the need for 100 or more cases to attain efficiency.(18)
Many centers describe conversion rates for attempted VATS lobectomy ranging from 10–20%.(6, 8, 19) We found that with increasing experience, our conversion rate dropped from 28% to 11%. Similarly, Gazala et al noted their conversion rate fell from 15% to 11% over a 3 year period.(8) Highly proficient VATS lobectomy programs are able to safely achieve conversion rates as low as 4%.(20, 21) While several authors have described causes for conversion (19, 20), these were formalized by Gazala et al into the parsimonious “VALT” classification system.(8) Though they described greater than 40% of conversions due to vascular causes, the majority (64%) of conversions in our cohort were due to anatomy (hilar or pleural adhesions/large tumor size). This is likely related to the nearly 15% incidence of prior cardiothoracic surgery, and greater than 25% likelihood of prior granulomatous disease on CT scan in our series. Samson et al have utilized a CT scan calcification score as a predictor of conversion.(6) Due to the pattern of referrals to our practice, a substantial proportion of radiographic images for our patients were from other facilities and could not be reviewed to assign calcification scores. Instead we utilized a binary variable of presence or absence of granulomatous disease, signified by intrapulmonary granulomas or mediastinal/hilar lymph node calcification, for our analysis. Though we did not find an association between granulomatous disease and the risk of conversion, it is possible that a dichotomous variable is not sensitive enough to detect a difference. The only variable significant for association with conversion in our study was male gender. No clear biologic explanation for this is available and our finding may be spurious.
The impact of conversion on perioperative morbidity has been variably reported. A study from the UK showed no difference in postoperative complications between successful VATS and conversions; however the data were not reported in a standardized format like the STS database, and hence are harder to interpret.(9) Others have shown a higher incidence of arrhythmias and pulmonary complications, greater estimated blood loss, and a longer length of stay with conversions.(6, 7) This follows general clinical principles and we feel that the comparison group for converted VATS operations should comprise those undergoing a planned open thoracotomy. Our study did not find any difference in either the overall postoperative complication rate or the incidence of specific complications between these 2 groups. Similarly, Samson et al had noted identical postoperative complications between converted and planned thoracotomy patients with the exception of a slightly higher incidence of atrial arrhythmias in the converted group.(6)
We did not note a difference in long-term survival in patients with pathologic stage I NSCLC who underwent successful VATS lobectomy in comparison to those who were converted or underwent planned open operations on multivariate analysis. Though this was not the focus of our study, others have reported potentilaly longer survival in patients undergoing VATS lobectomy (compared to open) for clinical stage I NSCLC.(22, 23) The reasons for any potential long-term survival advantage for VATS are unclear. It is possible that a more invasive operation (thoracotomy) may disrupt cancer immune-surveillance to a greater degree orthat patients who undergo VATS are better able to tolerate adjuvant treatment if needed. (24)
Limitations to our study include its retrospective design that makes it difficult to control for selection bias in treatment allocation between the open and VATS approaches. Our study also cannot account for variations in practice patterns of individual practitioners, and their level of comfort in proceeding and continuing with a VATS approach in a specific circumstance, though all the surgeons have been trained at our own program and have a similar technical approach to pulmonary surgery. Lastly, with the cohort drawn from a single institution, sample size limitations introduce the possibility of type II error, where true differences between groups may not be detected due to sample size constraints. We have tried to avoid misclassification bias in the analysis with 2 reviewers independently analyzing each conversion and a panel discussing any ambiguities. Additionally, with our study, we could not objectively categorize specific cases as “unsuitable for VATS lobectomy”. However we noted that pulmonary arterial bleeding or hematomas were responsible for the majority of emergent conversions and were much more likely to be transfused. Hence we have altered our group’s practice to electively convert cases where we are experiencing significant difficulty in exposure or dissection of the pulmonary artery. Hence, we conclude that with increasing experience, a high proportion of lobectomy operations can be safely completed thoracoscopically and VATS should be strongly considered as the initial approach for the majority of patients undergoing lobectomy.
Supplementary Material
Acknowledgments
Grant support
Varun Puri -NIH K07CA178120, K12CA167540-02 (Paul Calabresi award)
Appendix 1
Major perioperative morbidities as defined by the Society of Thoracic Surgeons General Thoracic Database.
| Pulmonary |
| Atelectasis requiring bronchoscopy |
| Pneumonia |
| ARDS/Respiratory Failure |
| Bronchopleural Fistula/Empyema |
| Pulmonary Embolus |
| Initial Ventilator support >48 hours |
| Reintubation |
| Tracheostomy |
| Cardiovascular |
| Atrial Arrhythmia requiring treatment |
| Ventricular Arrhythmia requiring treatment |
| Myocardial Infarction |
| Deep Vein Thrombosis requiring treatment |
| Infection |
| Surgical Site infection, specify: |
| Sepsis |
| Neurology |
| New Central Neurological Event |
| Miscellaneous |
| New Renal Failure per RIFLE criteria |
| Other events requiring OR with General Anesthesia, specify |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 94th Annual Meeting of the American Association for Thoracic Surgery, Toronto, Ontario, Canada. April 29, 2014.
References
- 1.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg. 2006;81:421–425. doi: 10.1016/j.athoracsur.2005.07.078. discussion 425–426. [DOI] [PubMed] [Google Scholar]
- 2.Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezu036. [DOI] [PubMed] [Google Scholar]
- 3.Swanson SJ, Herndon JE, 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25:4993–4997. doi: 10.1200/JCO.2007.12.6649. [DOI] [PubMed] [Google Scholar]
- 4.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–378. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg. 2012;256:487–493. doi: 10.1097/SLA.0b013e318265819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samson P, Guitron J, Reed MF, Hanseman DJ, Starnes SL. Predictors of conversion to thoracotomy for video-assisted thoracoscopic lobectomy: a retrospective analysis and the influence of computed tomography-based calcification assessment. J Thorac Cardiovasc Surg. 2013;145:1512–1518. doi: 10.1016/j.jtcvs.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg. 2009;36:487–490. doi: 10.1016/j.ejcts.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Gazala S, Hunt I, Valji A, Stewart K, Bedard ER. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg. 2011;12:962–964. doi: 10.1510/icvts.2010.259663. [DOI] [PubMed] [Google Scholar]
- 9.Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes? Ann Thorac Surg. 2008;86:235–239. doi: 10.1016/j.athoracsur.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 10.Iedema RA, Jorm C, Long D, et al. Turning the medical gaze in upon itself: root cause analysis and the investigation of clinical error. Soc Sci Med. 2006;62:1605–1615. doi: 10.1016/j.socscimed.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg. 2008;85:231–235. doi: 10.1016/j.athoracsur.2007.07.080. discussion 235–236. [DOI] [PubMed] [Google Scholar]
- 12.Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg. 2009;138:419–425. doi: 10.1016/j.jtcvs.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Yendamuri S, Demmy TL. Lobectomy for patients with limited lung function. Semin Thorac Cardiovasc Surg. 2011;23:191–195. doi: 10.1053/j.semtcvs.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Cao C, Manganas C, Ang SC, Yan TD. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg. 2012;1:16–23. doi: 10.3978/j.issn.2225-319X.2012.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–2562. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 16.Gopaldas RR, Bakaeen FG, Dao TK, et al. Video-assisted thoracoscopic versus open thoracotomy lobectomy in a cohort of 13,619 patients. Ann Thorac Surg. 2010;89:1563–1570. doi: 10.1016/j.athoracsur.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg. 2011;142:1412–1417. doi: 10.1016/j.jtcvs.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Wang J, Ferguson MK. Competence versus mastery: The time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg. 2014;147:1150–1154. doi: 10.1016/j.jtcvs.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Wang J, Yang F, et al. Indications for conversion of thoracoscopic to open thoracotomy in video-assisted thoracoscopic lobectomy. ANZ J Surg. 2012;82:245–250. doi: 10.1111/j.1445-2197.2011.05997.x. [DOI] [PubMed] [Google Scholar]
- 20.Villamizar NR, Darrabie M, Hanna J, et al. Impact of T status and N status on perioperative outcomes after thoracoscopic lobectomy for lung cancer. J Thorac Cardiovasc Surg. 2013;145:514–520. doi: 10.1016/j.jtcvs.2012.10.039. discussion 520-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg. 2011;35:590–595. doi: 10.1007/s00268-010-0913-6. [DOI] [PubMed] [Google Scholar]
- 22.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–2016. doi: 10.1016/j.athoracsur.2008.07.009. discussion 2016-2008. [DOI] [PubMed] [Google Scholar]
- 23.Chen FF, Zhang D, Wang YL, Xiong B. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol. 2013;39:957–963. doi: 10.1016/j.ejso.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Jiang G, Yang F, Li X, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for administration of adjuvant chemotherapy after lobectomy for non-small cell lung cancer. World J Surg Oncol. 2011;9:170. doi: 10.1186/1477-7819-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.