Abstract
Interferons (IFNs) are cytokines with important anti-proliferative activity and exhibit key roles in immune surveillance against malignancies. Early work initiated over 3 decades ago led to the discovery of IFN receptor activated Jak-Stat pathways and provided important insights into mechanisms for transcriptional activation of interferon stimulated genes (ISGs) that mediate IFN-biological responses. Since then, additional evidence has established critical roles for other receptor activated signaling pathways in the induction of IFN-activities. These include MAPK pathways, mTOR cascades and PKC pathways. In addition, specific microRNAs (miRNAs) appear to play a significant role in the regulation of IFN-signaling responses. This review focuses on the emerging evidence for a model in which IFNs share signaling elements and pathways with growth factors and tumorigenic signals, but engage them in a distinctive manner to mediate anti-proliferative and antiviral responses.
Introduction
Because of their antineoplastic, antiviral, and immunomodulatory properties, recombinant interferons (IFNs) have been used extensively in the treatment of various diseases in humans (1). IFNs have clinical activity against several malignancies and are actively used in the treatment of solid tumors such as malignant melanoma and renal cell carcinoma; and hematological malignancies, such as myeloproliferative neoplasms (MPNs) (1). In addition, IFNs play prominent roles in the treatment of viral syndromes, such as hepatitis B and C (2). In contrast to their beneficial therapeutic properties, IFNs have been also implicated in the pathophysiology of certain diseases in humans. In many cases this involvement reflects abnormal activation of the endogenous IFN system, which has important roles in various physiological processes. Diseases in which dysregulation of the Type I IFN system has been implicated as a pathogenetic mechanism include autoimmune disorders such as systemic lupus erythematosous (3), Sjogren’s syndrome (3,4), dermatomyositis (5) and systemic sclerosis (3, 4). In addition, Type II IFN (IFNγ) overproduction has been implicated in bone marrow failure syndromes, such as aplastic anemia (6). There is also recent evidence for opposing actions of distinct IFN subtypes in the pathophysiology of certain diseases. For instance, a recent study demonstrated that there is an inverse association between IFNβ and IFNγ gene expression in human leprosy, consistent with opposing functions between Type I and II IFNs in the pathophysiology of this disease (7). Thus, differential targeting of components of the IFN-system, to either promote or block induction of IFN-responses depending on the disease context, may be useful in the therapeutic management of various human illnesses. The emerging evidence for the complex regulation of the IFN-system underscores the need for a detailed understanding of the mechanisms of IFN-signaling in order to target IFN-responses effectively and selectively.
It took over 35 years from the original discovery of IFNs in 1957 to the discovery of Jak-Stat pathways (8). The identification of the functions of Jaks and Stats dramatically advanced our understanding of the mechanisms of IFN-signaling and had a broad impact on the cytokine research field as a whole, as it led to the identification of similar pathways from other cytokine receptors (8). Subsequently, several other IFN receptor (IFNR)-regulated pathways were identified (9). As discussed below, in recent years there has been accumulating evidence that beyond Stats, non-Stat pathways play important and essential roles in IFN-signaling. This has led to an evolution of our understanding of the complexity associated with IFN receptor activation and how interacting signaling networks determine the relevant IFN response.
Interferons and their functions
The interferons are classified in 3 major categories, Type I (α, β, ω, ε, τ, κ, ν); Type II (γ) and Type III IFNs (λ1, λ2, λ3) (1, 9, 10). The largest IFN-gene family is the group of Type I IFNs. This family includes 14 IFNα genes, one of which is a pseudogene, resulting in the expression of 13 IFNα protein subtypes (1, 9). There are 3 distinct IFNRs that are specific for the 3 different IFN types. All Type I IFN subtypes bind to and activate the Type I IFNR, while Type II and III IFNs bind to and activate the Type II and III IFNRs, respectively (9–11). It should be noted that although all the different Type I IFNs bind to and activate the Type I IFNR, differences in binding to the receptor may account for specific responses and biological effects (9). For instance, a recent study provided evidence that direct binding of mouse IFNβ to the Ifnar1 subunit, in the absence of Ifnar2, regulates engagement of signals that control expression of genes specifically induced by IFNβ, but not IFNα (12). This recent discovery followed original observations from the 90s that revealed differential interactions between the different subunits of the Type I IFN receptor in response to IFNβ binding as compared to IFNα binding and partially explained observed differences in functional responses between different Type I IFNs (9).
A common property of all IFNs, independently of type and subtype, is the induction of antiviral effects in vitro and in vivo (1). Because of their potent antiviral properties, IFNs constitute an important element of the immune defense against viral infections. There is emerging information indicating that specificity of the antiviral response is cell type dependent and/or reflects specific tissue expression of certain IFNs. As an example, a recent comparative analysis of the involvement of the Type I IFN system as compared to the Type III IFN system in antiviral protection against rotavirus infection of intestinal epithelial cells demonstrated an almost exclusive requirement for IFNλ (Type III IFN) (13). The antiviral effects of IFNα have led to the introduction of this cytokine in the treatment of hepatitis C and B in humans (2) and different viral genotypes have been associated with response or failure to IFN-therapy (14).
Most importantly, IFNs exhibit important antineoplastic effects, reflecting both direct antiproliferative responses mediated by IFNRs expressed on malignant cells, as well as indirect immunomodulatory effects (15). IFNα and its pegylated form (peg IFNα) have been widely used in the treatment of several neoplastic diseases, such as hairy cell leukemia (HCL), chronic myeloid leukemia (CML), cutaneous T cell lymphoma (CTCL), renal cell carcinoma (RCC), malignant melanoma, and myeloproliferative neoplasms (MPNs) (1, 16). Although the emergence of new targeted therapies and more effective agents have minimized the use of IFNs in the treatment of diseases like HCL and CML, IFNs are still used extensively in the treatment of melanoma, CTCL and MPNs (1, 16, 17). Notably, recent studies have provided evidence for long lasting molecular responses in patients with polycythemia vera (PV), essential thrombocytosis (ET) and myelofibrosis (MF) who were treated with IFNα (16). Beyond their inhibitory properties on malignant hematopoietic progenitors, IFNs are potent regulators of normal hematopoiesis (9) and contribute to the regulation of normal homeostasis in the human bone marrow (18). Related to its effects in the central nervous system, IFNβ has clinical activity in multiple sclerosis (MS) and has been used extensively for the treatment of patients with MS (19). The immunoregulatory properties of Type I IFNs include key roles in the control of innate and adaptive immune responses, as well as positive and negative effects on the activation of the inflammasome (15). Dysregulation of the Type I IFN response is seen in certain autoimmune diseases, such as Aicardi-Goutières syndrome (20). In fact, self-amplifying Type I IFN-production is a key pathophysiological mechanism in autoimmune syndromes (21). There is also emerging evidence that IFNλ may contribute to the IFN signature in autoimmune diseases (3).
Jak-Stat pathways
Jak kinases and DNA binding Stat-complexes
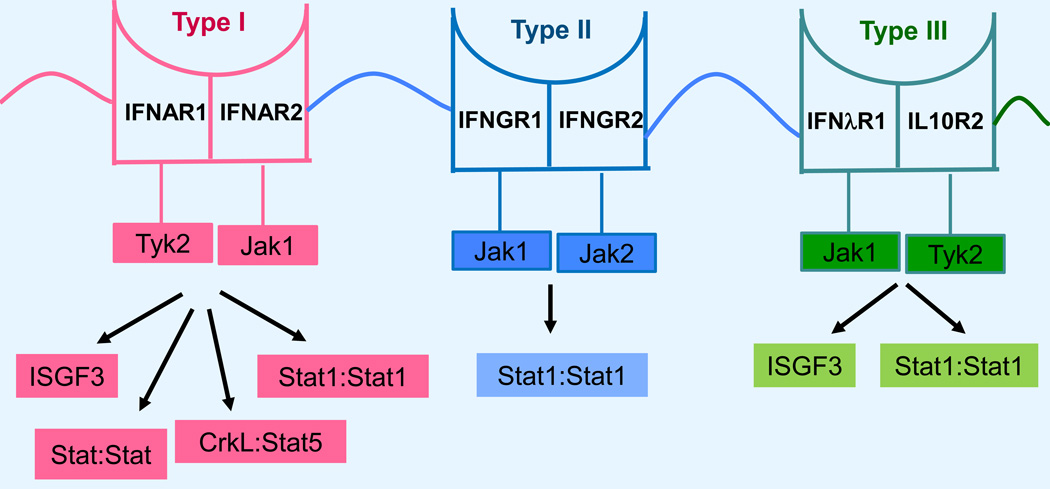
Tyrosine kinases of the Janus family (Jaks) are associated in unique combinations with different IFNRs and their functions are essential for IFN-inducible biological responses. Stats are transcriptional activators whose activation depends on tyrosine phosphorylation by Jaks (8, 9). In the case of the Type I IFN receptor, Tyk2 and Jak1 are constitutively associated with the IFNAR1 and IFNAR2 subunits, respectively (8, 9) (Fig. 1). For the Type II IFN receptor, Jak1 and Jak2 are associated with the IFNGR1 and IFNGR2 receptor subunits, respectively (8, 9) (Fig. 1). Finally, in the case of the Type III IFNR, Jak1 and Tyk2 are constitutively associated with the IFN-λR1 and IL-10R2 receptor chains, respectively (10) (Fig. 1). Upon engagement of the different IFNRs by the corresponding ligands, the kinase domains of the associated Jaks are activated and phosphorylate tyrosine residues in the intracellular domains of the receptor subunits that serve as recruitmenst sites for specific Stat proteins. Subsequently, the Jaks phosphorylate Stat proteins that form unique complexes and translocate to the nucleus where they bind to specific sequences in the promoters of ISGs to initiate transcription. A major Stat complex in IFN-signaling is the interferon stimulated gene factor 3 (ISGF3) complex. This IFN-inducible complex is composed or Stat1, Stat2 and IRF9 and regulates transcription by binding to IFN stimulated response elements (ISRE) in the promoters of a large group of IFN stimulated genes (ISGs) (8, 9). ISGF3 complexes are induced during engagement of the Type I and III IFN receptors, but not in response to activation of Type II IFN receptors (8–10) (Table 1). Beyond ISGF3, several other Stat-complexes involving different Stat homodimers or heterodimers are activated by IFNs and bind to IFNγ-activated (GAS) sequences in the promoters of groups of ISGs (8, 9). Such GAS binding complexes are induced by all different IFNs (I, II and III), although there is variability in the engagement and utilization of different Stats by the different IFN-receptors (Table 1). It should also be noted that engagement of certain Stats, such as Stat4 and Stat6, is cell type-specific and may be relevant for tissue specific functions (9). The significance of different Stat binding complexes in the induction of Type I and II IFN responses was in part addressed in a study in which Stat1 cooperative DNA binding was disrupted by generating knock-in mice expressing cooperativity-deficient STAT1 (22). As expected, Type II IFN-induced gene transcription and antibacterial responses were essentially lost in these mice, but Type I IFN-dependent recruitment of Stat1 to ISRE elements and antiviral responses were not affected (22), demonstrating the existence of important differences in Stat1 cooperative DNA binding between Type I and II IFN signaling.
Figure 1.
Type I, II, III interferon receptors subunits, associated kinases of the Janus family, and effector Stat-pathways. Note: Stat:Stat reflects multiple potential Stat:Stat compexes, as outlined in Table 2.
Table 1.
Different Stat-DNA binding complexes induced by Type I, II and III IFNs.
| IFN Type | Stat complexes |
|---|---|
| Type I | Stat1:Stat2:IRF9 Stat1:Stat1, Stat1:Stat2, Stat1:Stat3, Stat2:Stat3, Stat3:Stat3, Stat4:Stat4, Stat1:Stat4, Stat1:Stat5, Stat5:Stat5, Stat5:Stat6, Stat5:Stat6, CrkL:Stat5 |
| Type II | Stat1:Stat1, Stat1:Stat3, Stat3:3 |
| Type III | Stat1:Stat2:IRF9, Stat1:1 |
Serine phosphorylation of Stats
The nuclear translocation of Stat-proteins occurs after their activation, following phosphorylation on specific sites by Jak kinases (8, 9). It is well established that phosphorylation on tyrosine 701 is required for activation of Stat1 and phosphorylation on tyrosine 705 is required for activation of Stat3 (8, 9). Beyond tyrosine phosphorylation, phosphorylation on serine 727 in the Stat1 and Stat3 transactivation domains is required for full and optimal transcriptional activation of ISGs (8, 9). There is evidence that serine phosphorylation occurs after the phosphorylation of Stat1 on tyrosine 701 and that translocation to the nucleus and recruitment to the chromatin are essential in order for Stat1 to undergo serine 727 phosphorylation (23). Several IFN-dependent serine kinases for Stat1 have been described, raising the possibility that this phosphorylation occurs in a cell type specific manner. After the original demonstration that protein kinase C (PKC) delta (PKCδ) is a serine kinase for Stat1 and is required for optimal transcriptional activation in response to IFNα (24), extensive work has confirmed the role of this PKC isoform in the regulation of serine 727 phosphorylation in Stat1 and has been extended to different cellular systems (25–29) (Table 2). In the Type II IFN system five different serine kinases for the transactivation domain (TAD) of Stat1/phosphorylation on serine 727 have been demonstrated in different cell systems. These include PKCδ (30, 31), calmodulin dependent kinase II (CAMKII) (32), PKCε (33), PKCα (34), Erk (35) and cyclin dependent kinase 8 (CDK8) (36) (Table 2). Thus, it appears that in the Type I IFN system, PKCδ is the predominant kinase that regulates phosphorylation of Stat1 on serine 727 (Table 2), while in the Type II IFN system several serine kinases appear to play roles in different cell types (Table 2). It remains to be determined whether the diversity of serine kinases in the Type II IFN system reflects differences in cellular expression patterns or is context-dependent, possibly influenced by parallel signaling events. It should also be noted that it is likely that, beyond phosphorylation on serine 727 in the TAD, phosphorylation on other serine residues in Stats, may be important for transcriptional activity and the generation of IFN-responses. For instance, phosphorylation of serine 708 in Stat1 by the kinase IKKε is important for transcriptional activation of a subset of ISGs (37). Thus, it appears that our current understanding of the role of serine phosphorylation of Stats is incomplete and future studies may uncover other serine phosphorylation sites and corresponding kinases relevant to Stat activity.
Table 2.
Serine kinases demonstrated to regulate phosphorylation of Stat1 on serine 727 in response to different IFNs.
| IFN Type | Putative Kinases for TAD/Serine 727 |
|---|---|
| Type I | PKCδ |
| Type II | PKCδ, PKCε, PKCα, CAMKII, Erk, JNK, CDK8 |
| Type III | Unknown |
Regulatory effects of phosphatases on Jak-Stat signaling
After undergoing tyrosine phosphorylation by Jaks and translocation to the nucleus to regulate ISG transcription by binding to specific promoter elements, nuclear Stats are de-activated by de-phosphorylation. Several phosphatases have been identified as regulators of IFN-signaling pathways (Table 3). In the Type I IFN system, it has been shown that the tyrosine phosphatase TC-PTP modulates Stat1 activity in BCR-ABL transformed leukemia cells (38). TC-PTP is involved in dephosphorylation of Stat1 in the Type II IFN system and pharmacological inhibition of its activity has been shown to enhance IFNγ signaling (39). In the Type I IFN system, SHP1 is associated with the Tyk2 tyrosine kinase (40), while it has been shown to dephosphorylate IFNγ-tyrosine phosphorylated Stat1 in brain microglia and astrocytes (41). Additionally, interaction of tyrosine phosphorylated SHP-2 (pY-SHP-2) with cytosolic STAT1 has been shown to prevent recruitment of Stat1 to the Type II IFNR and to inhibit Stat1-mediated signaling (42). The protein tyrosine phosphatase PTPN1 has been implicated in both Type I (43) and Type II (44) IFN signaling. Moreover, there is evidence that PTPN1 and TC-PTP have non-overlapping roles in Type II IFN-signaling (44). Finally, recent studies have shown the negative regulator of JAK kinases, phosphatase CD45 exhibits regulatory effects on IFN-signaling (45). Thus, the coordinated function of distinct tyrosine phosphatases at different important check points of IFN-activated signaling cascades accounts for control of tyrosine phosphorylation-mediated signaling events and optimal balancing of IFN-responses.
Table 3.
Protein tyrosine phosphatases with regulatory effects on Jak-Stat pathways in IFN-signaling.
| IFN Type | Tyrosine phosphatases |
|---|---|
| Type I | TC-PTP, PTPN1, SHP1, CD45 |
| Type II | TC-PTP, PTPN1, SHP1, SHP2, |
| Type III | Not known |
Other modifications of Stat activity
There has been accumulating evidence over the last decade that events unrelated to Stat-phosphorylation have important regulatory roles on the functions of Stats. IFN-inducible unphosphorylated Stat1 (U-Stat1) appears to increase or maintain the expression of a subset of ISGs independently of tyrosine phosphorylated Stat1 (46). Remarkably, this regulation can also occur by the formation of a complex with unphosphorylated Stat2 and IRF9, suggesting the existence of an unphosphorylated ISGF3 complex (8). Unphosphorylated Stat2 (U-Stat2) has also recently been shown to play important regulatory roles in transcriptional activation of a set of ISGs (47). Although the physiological and pathophysiological relevance of unphosphorylated Stat functions remain to be precisely defined, it has been suggested that some of the genes regulated by the unphosphorylated ISGF3 complex (8) may be mediators of resistance of tumor cells to DNA damage, to chemotherapy and/or radiation. Other events that appear to modulate IFN-dependent Stat activity include SUMO conjugation (48, 49) and interaction with histone deacetylases (HDACs) (50). A recent study demonstrated that a complex involving the ATP binding RVB proteins (RVB1 and RVB2) is required for Type I, but not Type II, IFN-dependent transcription of ISGs (51). In that study it was shown that RVB1 and RVB2 interact with the transactivation domain of STAT2 after Type I IFN-treatment (51), suggesting a mechanism by which their effects occur and underscoring the complexity of events required for optimal ISG transcriptional activation. It should be noted that IFN-inducible Jak-Stat signaling is tightly regulated by additional negative regulators. Beyond the factors described above, suppressors of cytokine signaling (SOCS) and protein inhibitors of activated Stats (PIAS) have been associated with negatively regulating IFN inducible Jak-Stat signaling (reviewed in 52). Additionally, the IFN-inducible ubiqutitin carboxy-terminal hydrolase 18 (USP18)/ubiquitin-specific protease, UBP43, can displace Jak1 from the associated IFNAR2subunit, thereby affecting Jak-Stat signaling (reviewed in 52).
Map kinase pathways
Mitogen activated protein (Map) kinases control key effector pathways in cytokine signaling and play important roles in the control of various important cellular processes (9). Map kinase pathways are involved in the regulation of innate immunity (53) and have important roles in the pathophysiology of malignancies, a fact that makes them attractive therapeutic targets for the treatment of certain tumors (54). All 3 major classes of Map kinases (p38 MAPK, Erk and JNK) have been shown to participate in the induction of IFN-responses via distinct cellular signaling events, as outlined below.
p38 Map kinase pathways
There is extensive evidence that the p38 Map kinase signaling pathway acts as an auxiliary cascade for Jak-Stat pathways and that its function is required for optimal transcriptional activation of ISGs (9, 18). This pathway has been shown to be engaged by the Type I (9, 55–60), Type II (60, 61) and Type III (62) IFN receptors. The functional relevance of this signaling cascade in the Type I IFN system was established some time ago, when it was demonstrated that p38 MAP kinase engagement is essential for Type I IFN-dependent suppression of normal and leukemic hematopoiesis (9, 60). There is also recent evidence implicating the p38 MAPK in the induction of Type I IFN-dependent antiproliferative and/or pro-apoptotic responses in T-cell leukemia cells (58) and primary malignant hematopoietic progenitors from patients with polycythemia vera expressing the JAK2-V617F mutation (63). Not surprisingly, as the function of the p38 MAP kinase pathway is required for IFN-inducible expression of ISG protein products, its engagement is essential for IFN- responses against different viruses (9, 57, 64). However, there is also evidence for selectivity in the IFN responses controlled by the p38 MAPK, suggesting differential regulation of target genes by p38 signals. For instance, the neuroprotective effect of IFNβ against mitochondrial toxicity occurs via modulation of Stat1 activity, but it seems to be p38 MAPK-independent (65).
Because of the importance of this pathway in the generation of IFN-responses, extensive work has been conducted to identify the mechanisms by which its activation is regulated and to define downstream signaling effectors. The engagement of the p38 MAPK by the Type I IFN receptor occurs via activation of an upstream cellular pathway that involves the Vav proto-oncogene and/or other guanine exchange factors, the small G-protein Rac1 and/or other GTPases, a yet to be defined MAPKKK and then MMK3/6 (9) (Fig. 2). Several downstream effectors of the Type I IFN activated p38 MAPK have been identified. These include the kinases MapKapK2/3 (55, 57), the nucleosomal kinase Msk1 (57), and the transcription factor ATF-2 (56). Although the precise mechanisms by which the p38 MAPK pathway regulates gene transcription in the IFN-system remain to be identified, it is possible that Msk1 plays a role by modifying nuclear histone phosphorylation (9). It is also possible that the involvement of p38 MAPK in the generation of Type I IFN growth inhibitory responses involves transcriptional regulation of members of gene families with growth inhibitory properties, such as Schlafen (Slfn) genes (66). Notably, malignant transformation by BCR-ABL has been shown to involve suppression of Type I IFN-dependent gene transcription, via inhibition of both p38 MAPK activity and Stat-activation (67), underscoring the relevance of the p38 MAPK in immune-surveillance against cancer. A novel function of the p38 MAPK was recently established by Fuchs and colleagues (68). Evidence was provided for ligand-independent PKR-like endoplasmic reticulum kinase (PERK) - mediated p38 MAPK activation that regulates priming phosphorylation of IFNAR1, facilitating ubiquitination and degradation of this receptor subunit (68).
Figure 2.
Map kinase pathways in Type I IFN signaling. GEF- Guanine exchange factor; RGT - Regulation of gene transcription; RMT - Regulation of mRNA translation; PTRGE-Post-transcriptional regulation of gene expression.
Although a functional p38 MAPK is required for Type I IFN transcriptional activation via ISRE or GAS elements (55, 57), it does not appear to play a major role in Type II IFN-induced Stat-dependent ISG transcription (57). However, the p38 MAPK pathway is activated by the Type II IFN receptor and/or plays important roles in the generation of Type II IFN-biological responses in several different cell types (34, 60,61, 69). There is also evidence for what appears to be cell-type specific regulation of expression of genes required for the innate immune response (70). As the p38 MAPK is a mediator of the suppressive effects of IFNγ on normal hematopoiesis (60), its pharmacological inhibition may be relevant in diseases in which there is overproduction of IFNγ–associated suppressed hematopoiesis. Indeed, there is evidence that inhibition of p38 MAPK activity can enhance hematopoietic progenitor colony formation in vitro from bone marrows of patients with aplastic anemia (60), anemia of chronic disease (71) and myelodysplastic syndromes (71, 72).
The roles of the p38 Map kinase in the Type III IFN system is less well-defined compared to the Type I and II IFN systems, primarily because of the relatively recent discovery of the IFNλ family compared to other IFNs. Nevertheless, it has been shown that IFNλ induces activation of the p38 MAPK pathway and that, as is the case for Type I IFN signaling, p38 activity is required for ISG transcription (62). Future studies should define the effectors of the p38 MAPK pathway in the Type III IFN system and determine whether there are Type III IFN-specific effectors or unique Type III IFN-dependent engagement of downstream pathways. Viewed altogether, the p38 MAPK pathway plays a prominent role in signaling for all 3 different classes of IFNs and its function is central for the biological effects of IFNs.
Erk kinase pathways
There is considerable evidence that the Mek/Erk kinase pathway is also activated by IFNs. Since the original demonstration that Type I IFNs activate Erk (reviewed in 9) a substantial amount of data have defined the relevance of Mek/Erk signaling to the generation of IFN-responses. This activation appears to be Jak1-dependent and to involve upstream Raf1 activation (9) (Fig. 2). Recent evidence reveals that Type I IFN-dependent activation of Erk occurs in several different cell types, including hapatocytes (73), endometrial epithelial cells (74); gastric carcinoma cells (75) and adrenal chromaffin cells (76). Type I IFN-activated Erk has been shown to regulate phosphorylation of tyrosine hydroxylase on serine 31 (76) and to mediate IFNα-induced apoptosis (77). Importantly, a recent study demonstrated that engagement of the MEK/Erk pathway is critical for IFNα-induced phosphodiesterase 4 (PDE4) activation and repression of cAMP in Treg cells (78). This study provided evidence for a novel Type I IFN-induced, Erk-mediated, function, involving inhibition of the suppressive effects of Tregs on CD4+ T cells and NK cells (78).
In addition to effects on transcriptional activation of ISGs, the Mek/Erk pathway has an important role in mRNA translation of ISGs via activation of at least 2 distinct effectors. One pathway involves engagement of the kinase Mnk1 and downstream phosphorylation of the eukaryotic initiation factor 4E (eIF4E) (79). Although Mnk kinases can be activated downstream of either p38 MAPK or Erk in response to stress signals (80), it appears that in the Type I IFN system, this activation occurs selectively downstream of the Mek/Erk, but not the p38 MAPK pathway (79). Studies using cells from mice with targeted deletion of Mnk1 and Mnk2 have established that the Mnk pathway is essential for mRNA translation of the Isg15 and Isg54 genes and that its function is required for the generation of the inhibitory effects of Type I IFNs in normal hematopoietic progenitors (79). A recent study (81) showed that the Mnk pathway is an essential mediator of the antineoplastic effects of IFNα on malignant hematopoietic progenitors from patients with myeloproliferative neoplasms (MPNs). The key role for Mnk in these responses (81) has provided important clues on the mechanisms by which Type I IFNs generate their antitumor effects against Jak2V617F malignancies. Other studies have shown that Sprouty (Spry) proteins are stabilized/upregulated downstream of Type I IFN-dependent Mnk kinases and exert negative feedback regulatory roles on the activation of the p38 MAPK and Erk kinase pathways (82). These proteins have negative regulatory roles in the generation of antiviral and antileukemic effects of Type I IFNs (82). Another effector of the MEK/Erk pathway in the Type I IFN system is the kinase RSK1 (83). This kinase regulates Type I IFN-dependent eIF4B phosphorylation in hematopoietic cells and is required for induction of Type I IFN-dependent antileukemic responses (83).
The Mek/Erk pathway is also activated during engagement of the Type II IFN receptor (70, 84–86) and is required for optimal gene transcription via IFNγ-activated site (GAS) elements (84) and IFNγ-activated transcriptional elements (GATE) (87). Effectors of the pathway, such as Mnk1, are also engaged downstream of MEK/Erk activation by the Type I IFN receptor (88). The MEK/Erk pathway has been shown to mediate diverse responses following engagement of the Type II IFN receptor, including suppressive effects on normal hematopoiesis (88); IFNγ-dependent death of oligodendroglial progenitor cells (85); and bacterial internalization by gut epithelia (86). There is also evidence that the MEK/Erk pathway is activated by the Type III IFN receptor and mediates IFNλ-dependent activation of the kinase RSK1 and downstream upregulation of p21WAF1/CIP1, suggesting a mechanism for the generation of IFNλ- inducible growth inhibitory responses (89).
JNK kinase pathways
The JNK family of Map kinases is composed of 3 distinct isoforms (JNK1, JNK2 and JNK3) (53). In recent years, there has been accumulating evidence that the members of this family participate directly in IFN-signaling and mediate IFN-biological responses. Type I IFNs have been reported to activate JNK1 (27, 56, 77), although this activation appears to be weaker than the activation of other MAPK pathways by the Type I IFNR. Two reports (27, 77) have suggested a unique mechanism of activation of the JNK pathway by the Type I IFN receptor, involving PKCδ-dependent activation of JNK1 (Fig. 2). This sequential activation was found to be essential for Type I IFN-induced apoptosis of malignant cells (27, 77). Other studies have shown that sequential IFNα-dependent activation of PKCδ and JNK is required for IFN-induced expression of IFIT4 (90) and induction of phospholipid scramblase 1 (PLSCR1) which promotes pro-apoptotic and antiviral activities (26).
The JNK kinase pathway is also activated during engagement of the Type II IFN receptor (70, 91, 92) and mediates important biological and biochemical responses, including transcriptional activation of genes involved in antigen presentation (70), differentiation of neural progenitor cells (92), upregulation of B7-DC and antitumor immunity (91). Similarly, the JNK pathway is engaged by the Type III IFN receptor, albeit in a cell type restricted-manner, and appears to participate in Type III IFN-gene induction (62).
mTOR pathways
The ability of the mammalian target of rapamycin (mTOR) pathway to regulate initiation of mRNA translation is critical for important functions in normal cells, including cell proliferation and survival, cell division and motility, lipid synthesis, glycolysis and autophagy (93). Because of these roles in important cellular functions, dysregulation of the mTOR pathway has been implicated in the pathogenesis and/or pathophysiology of diverse diseases and syndromes, including malignancies, obesity, diabetes, neurodegenerative diseases, cognitive defects and depression (93). As dysregulation of mTOR signaling is particularly important in promoting malignant transformation and neoplastic cell proliferation, there has been an intense interest leading to extensive efforts to target mTOR pathways for the treatment of cancer (94).
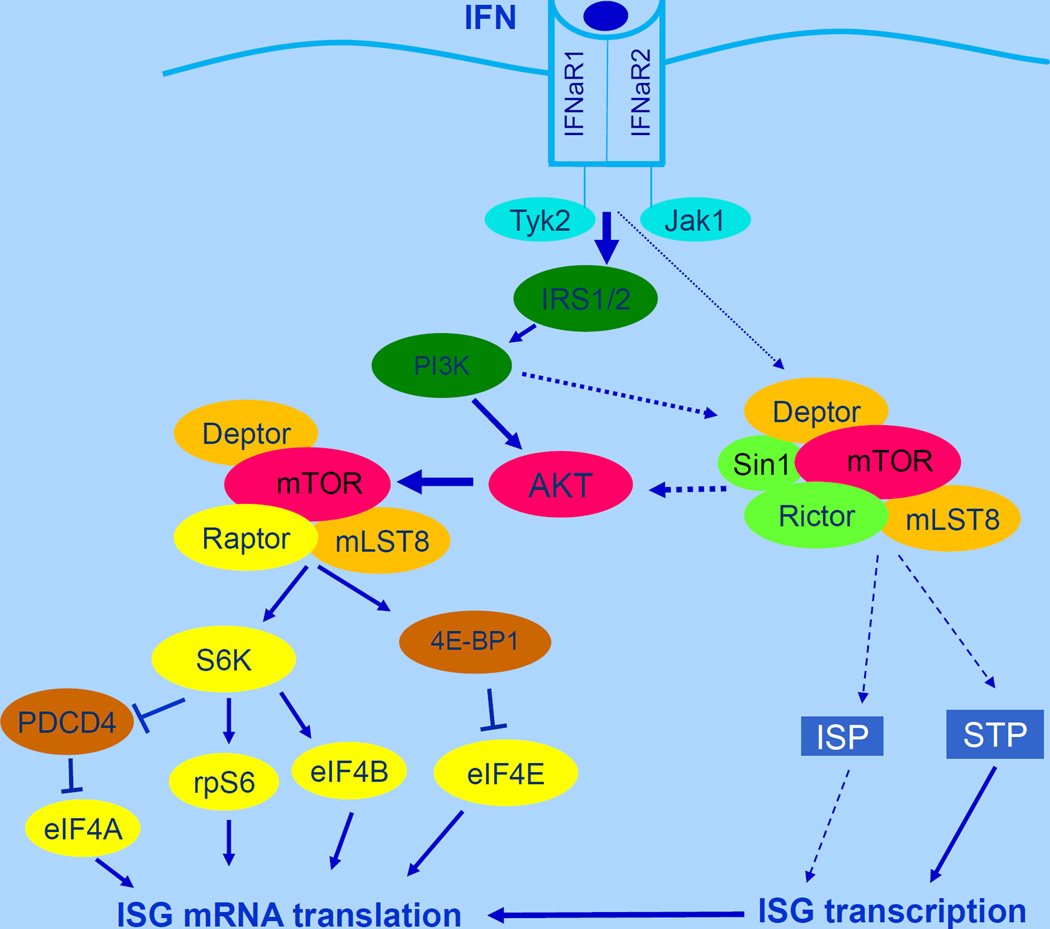
mTOR exists in at least 2 distinct complexes with unique elements and downstream effectors, namely mTORC1 and mTORC2 (93, 94). mTORC1 is a protein complex of mTOR with Deptor, mLST8 and Raptor, while the mTORC2 complex includes Deptor, mLST8, Sin1 and Rictor (93, 94) (Fig. 3). mTORC1 signals are important for the initiation of mRNA translation, while mTORC2 is critical for the activation of survival cellular pathways via engagement of the AGC family of kinases, which include AKT, SGK and PKCα (93, 94). mTOR pathways are activated during engagement of the Type I and II IFN receptors and play important roles in mRNA translation of ISGs (9). The first evidence implicating mTOR in IFN-signaling emerged about 10 years ago, when it was demonstrated that Type I IFN treatment of cells results in phosphorylation/activation of the p70 S6 kinase and its downstream effector, S6 ribosomal protein, as well as the translational repressor, 4E-BP1 (95). At that time it was also shown that engagement of mTORC1-dependent signals is defective in cells with targeted disruption of the p85α and p85β regulatory subunits of the PI 3’ kinase (95). In subsequent studies, evidence was also provided that mTOR pathways are activated during engagement of the Type II IFN (IFNγ) receptor (96). Later studies identified upstream effectors and regulators of the mTOR pathway in the IFN-system as the PI3’kinase (97) and the AKT kinase (98). These kinases are sequentially activated in an IFN-dependent manner and act as positive upstream effectors of mTORC1 activity (97, 98) (Fig. 3). On the other hand, TSC1/2 act as negative upstream effectors of IFN-activated mTORC1 (99). Several of the downstream effectors of the mTOR pathway during engagement of the IFN receptors have been identified and their functions defined. The translational repressor 4E-BP1 is phosphorylated on multiple sites by IFN-activated mTOR, resulting in its dissociation from the eukaryotic initiation factor 4E (eIF4E), to allow for initiation of cap-dependent mRNA translation (95, 99). Induction of expression of Type I and II IFN-inducible proteins and IFN-antiviral responses are enhanced in cells with targeted disruption of the 4e-bp1 gene (99). Other studies have shown a key role for the S6K effector, eIF4B, in the generation of IFN-responses (83). IFN-dependent phosphorylation of eIF4B promotes the interaction of the protein with eIF3A (p170/eIF3A) and results in increased associated ATPase activity (83). In addition, the IFN-activated form of S6K was shown to phosphorylate the tumor suppressor protein, programmed cell death 4 (PDCD4), on Ser67, resulting in the interaction of PDCD4 with the ubiquitin ligase β-TRCP (β-transducin repeat-containing protein) and its subsequent degradation (100). This degradation of PDCD4 results in increased IFN-induced eukaryotic translation initiation factor 4A (eIF4A) activity and binding to translation initiation factor eIF4G and increased cap-dependent translation (100). Other studies have shown that mTORC2 complexes are engaged by the Type I IFN receptor and regulate expression of IFN-stimulated genes (ISGs) (101). Remarkably, these complexes appear to selectively regulate an Akt/mTORC1 axis in response to engagement by the Type I IFN receptor, but not in response to growth factor receptors or oncogenic signals, suggesting the existence of an IFN-specific mTORC2/mTORC1 modification and function (101). Altogether, the mTOR pathway exerts important roles in Type I IFN-dependent responses (97–99,102, 103) and antineoplastic effects (77). Importantly, the mTOR pathway is also required for Type I IFN production by plasmacytoid dendritic cells (pDCs) (104), suggesting the existence of a positive feedback regulatory loop for the induction of IFN-responses. There is also some evidence for engagement of mTOR by the Type III IFN receptor (10), suggesting important roles for this signaling cascade in responses to each of the IFN Types.
Figure 3.
mTOR pathways in Type I IFN signaling. ISP-signals regulating transcription independent of Stat pathways; STP-Stat pathways.
MicroRNAs (miRs) and the IFN response
IFN-inducible JAK-STAT, MAPK and mTOR signaling cascades are also regulated potentially by microRNAs (miRs). miRs are important regulators of post-transcriptional events, leading to inhibition of mRNA translation or mRNA degradation (105). In recent years it has become apparent that the direct regulation of STAT activity by mIRs has profound effects on consequent gene expression, specifically in the context of cytokine-inducible events (106). Pertinent for this review of IFN-inducible STAT activation, miR-145, miR-146A and miR-221/222 target STAT1 and miR-221/222 target STAT2 (106). Numerous studies describe different miRs that target STAT3: mIR-17, miR-17-5p, mIR-17-3p, mIR-18a, miR-19b, mIR-92-1, miR-20b, Let-7a, miR-106a, miR-106-25, miR-106a-362 and miR-125b (106) (Fig. 4). mIR-132, miR-212 and miR-200a have been implicated in negatively regulating STAT4 expression in human NK cells (107) and miR-222 has been shown to regulate STAT5 expression (108). In addition, JAK-STAT signaling is affected by miR targeting of suppressors of cytokine signaling (SOCS) proteins. miR-122 and miR-155 targeting of SOCS1 releases the inhibition of STAT1 (and STAT5a/b) (109–111), and mIR-19a regulation of SOCS1 and SOCS3 effectively prolongs activation of both STAT1 and STAT3 (112). There is also evidence that miR-155 targets the inositol phosphatase SHIP1, effectively prolonging/inducing IFN-γ expression (113). Much of the evidence associated with miRs prolonging JAK-STAT activation relates to cancer studies, where tumor-secreted miRs promote cell migration and angiogenesis by prolonging JAK-STAT activation (114). miR-145 targeting of SOCS7 affects nuclear translocation of STAT3 and has been associated with enhanced IFNβ production (115). Beyond inhibition of SOCS proteins, miRs may influence the expression of other inhibitory factors associated with JAK-STAT signaling, and miR-301a and miR-18a have been shown to inhibit PIAS3, a negative regulator of STAT3 activation (116). There is also the potential for STATS to directly regulate miR gene expression. STAT5 suppresses expression of miR15/16 (117) and there is evidence that there are potential STAT3 binding sites in the promoters of about 200 miRs (118). Viewed altogether, there is compelling evidence for miR-STAT interactions, yet few studies have considered the contributions of miRs to IFN-inducible JAK-STAT signaling.
Figure 4.
Targeting and regulation of various proteins known to be involved in IFN-signaling by different miRNAs.
Given the accumulating evidence for a miR network that regulates JAK-STAT activation, additional miR networks that directly contribute to signaling output and biological responses induced by the different IFNs, associated with the other IFN signaling cascades, must operate. Analogous to miR networks that may affect IFN-inducible JAK-STAT signaling, there is a paucity of direct data linking miRs to IFN-inducible mTOR or MAPK signaling cascades. As above, we will identify miRs that potentially may interact with IFN-inducible signaling effectors. A number of studies have focused on miR inhibition of PI3K/mTOR signaling. Similar to investigations related to miR interactions with STATs, the majority of the miR-PI3K/mTOR studies have been conducted in the context of cancers. The catalytic subunit of PI3K, p110, is the target of miR-124 (119). mTOR is a direct target for miR-199a, miR-99a, miR-144, miR-100, miR-520c and miR-373 (120–123) (Fig. 4). When considering MAPK signaling, studies reveal that miR-124 targets and miR-128 suppress p38a transcripts (124). Moreover, there is some evidence that IFN-β inducible miR-431 targets intermediates in the MAPK pathway in cells sensitive to its growth inhibitory effects (125).
Not surprisingly, miRs have been identified that target IFNs and IFN-inducible genes. The expression of multiple IFNα species can be directly inhibited by miR-466I, through their 3’ untranslated region (126). miR-22, associated with the transition from cell quiescence to proliferation, suppresses type I IFN gene expression by targeting high mobility group box-1 and IRF5, preventing IRF3 and NFκB activation (127). This effectively blocks the antiproliferative response of type I IFNs. miR-203, an IFN-inducible miR, targets IFIT1, effectively providing an inhibitory feedback loop following IFN gene activation (128). miR-548 targets the 3’ untranslated region of IFNλ1, affecting IFNλ1 expression and the expression of a number of ISGs, including the human MxA protein and 2’5’-oligoadenylate synthetase-1 (OAS1) (129). SOCS1 targeting by miR-122 in liver Huh7 cells significantly increases type I IFN expression (109). miR-29 targets IFNγ mRNA and the transcription factors T-bet and Eomes, inhibiting IFNγ production (130, 31). Additionally, using miR databases, at least 17 miRs have been identified that potentially target IFNγ, including miR-29 (132). Undeniably, the contributions of miRs to an IFN response are profound, contextual, and involve a network of interactions, affecting both the production of IFNs and the sustainability of the signaling responses they invoke.
Evolution of our understanding of IFN-signals and future perspectives
A substantial amount of knowledge has accumulated since the original discovery of the Jak-Stat pathway in the early 90s. It is now clear that several key signaling cascades are essential for the induction of Type I, II and III IFN-responses. The original view that IFN-signals can be transmitted from the cell surface to the nucleus in two simple steps involving tyrosine phosphorylation of Stat proteins (8) now appears somewhat simplistic, as it has been established that modifications of Jak-Stat signals by other pathways and/or simultaneous engagement of other essential complementary cellular cascades is essential for induction of ISG transcriptional activation, mRNA translation, protein expression and subsequent induction of IFN-responses. Such pathways include PKC and MAP kinase pathways and mTORC1 and mTORC2-dpendent signaling cascades.
Over the next decade our understanding of the mechanisms by which IFN-signals are induced will likely continue to evolve, with the anticipated outcome that it will be possible exploit this new knowledge for translational-therapeutic purposes. For instance, selective targeting of kinase-elements of the IFN-pathway with kinase inhibitors may be useful in the treatment of autoimmune diseases where dysregulated/excessive Type I IFN production contributes to the pathophysiology of disease. On the other hand, efforts to promote the induction of specific IFN-signals, may lead to novel, less toxic, therapeutic interventions for a variety of viral infectious diseases and neoplastic disorders.
Footnotes
Conflict of Interest Disclosure: None
References
- 1.Borden EC, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann WP, Zeuzem S. A new standard of care for the treatment of chronic HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2011;8:257–264. doi: 10.1038/nrgastro.2011.49. [DOI] [PubMed] [Google Scholar]
- 3.Rönnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Curr. Opin. Rheumatol. 2013;25:248–253. doi: 10.1097/BOR.0b013e32835c7e32. [DOI] [PubMed] [Google Scholar]
- 4.Wahren-Herlenius M, Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 5.Baechler EC, Bilgic H, Reed AM. Type I interferon pathway in adult and juvenile dermatomyositis. Arthritis Res. Ther. 2011;13:249. doi: 10.1186/ar3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teles RM, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339:1448–1453. doi: 10.1126/science.1233665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 10.Kotenko SV. IFN-λs. Curr. Opin. Immunol. 2011;23:583–590. doi: 10.1016/j.coi.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur S, Platanias LC. IFNβ-specific signaling via a unique IFNAR1 interaction. Nat. Immunol. 2013;14:884–885. doi: 10.1038/ni.2686. [DOI] [PubMed] [Google Scholar]
- 12.de Weerd NA, et al. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nat. Immunol. 2013;14:901–907. doi: 10.1038/ni.2667. [DOI] [PubMed] [Google Scholar]
- 13.Pott J, et al. IFN-{lambda} determines the intestinal epithelial antiviral host defense. Proc. Natl. Acad. Sci. USA. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong VW, Sung JJ. Diagnosis and personalized management of hepatitis B including significance of genotypes. Curr. Opin. Infect. Dis. 2012;25:570–577. doi: 10.1097/QCO.0b013e328357f2f8. [DOI] [PubMed] [Google Scholar]
- 15.González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stauffer Larsen T, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk. Res. 2013;37:1041–1045. doi: 10.1016/j.leukres.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman HL, et al. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat. Rev. Clin. Oncol. 2013;10:588–598. doi: 10.1038/nrclinonc.2013.153. [DOI] [PubMed] [Google Scholar]
- 18.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 19.Killestein J, Polman CH. Determinants of interferon β efficacy in patients with multiple sclerosis. Nat. Rev. Neurol. 2011;7:221–228. doi: 10.1038/nrneurol.2011.22. [DOI] [PubMed] [Google Scholar]
- 20.Rice GI, et al. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat. Genet. 2012;44:1243–1248. doi: 10.1038/ng.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall JC, Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 2010;6:40–49. doi: 10.1038/nrrheum.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begitt A, et al. STAT1 cooperative DNA binding distinguishes between type-1 and type-2 interferon signaling. Nat. Immunol. 2014;15:168–176. doi: 10.1038/ni.2794. [DOI] [PubMed] [Google Scholar]
- 23.Sadzak I, et al. Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. Proc. Natl. Acad. Sci. USA. 2008;105:8944–8949. doi: 10.1073/pnas.0801794105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uddin S, et al. Protein kinase C-δ (PKC-δ) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J. Biol. Chem. 2002;277:14408–14416. doi: 10.1074/jbc.M109671200. [DOI] [PubMed] [Google Scholar]
- 25.Kaur S, et al. Role of protein kinase C-delta (PKC-delta) in the generation of the effects of IFN-alpha in chronic myelogenous leukemia cells. Exp. Hematol. 2005;33:550–557. doi: 10.1016/j.exphem.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Zhao KW, et al. Interferon-alpha-induced expression of phospholipid scramblase 1 through STAT1 requires the sequential activation of protein kinase Cdelta and JNK. J. Biol. Chem. 2005;280:42707–42714. doi: 10.1074/jbc.M506178200. [DOI] [PubMed] [Google Scholar]
- 27.Yanase N, Hayashida M, Kanetaka-Naka Y, Hoshika A, Mizuguchi J. PKC-δ mediates interferon-α-induced apoptosis through c-Jun NH2-terminal kinase activation. BMC Cell Biol. 2012;13:7. doi: 10.1186/1471-2121-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, et al. Hepatitis B virus polymerase impairs interferon-α-induced STAT activation through inhibition of importin-α5 and protein kinase C-δ. Hepatology. 2013;57:470–482. doi: 10.1002/hep.26064. [DOI] [PubMed] [Google Scholar]
- 29.Hald A, et al. STAT1 expression and activation is increased in lesional psoriatic skin. Br. J. Dermatol. 2013;168:302–310. doi: 10.1111/bjd.12049. [DOI] [PubMed] [Google Scholar]
- 30.Deb DK, et al. Activation of protein kinase C δ by IFN-γ. J. Immunol. 2003;171:267–273. doi: 10.4049/jimmunol.171.1.267. [DOI] [PubMed] [Google Scholar]
- 31.Kwon MJ, Yao Y, Walter MJ, Holtzman MJ, Chang CH. Role of PKCdelta in IFN-gamma-inducible CIITA gene expression. Mol. Immunol. 2007;44:2841–2849. doi: 10.1016/j.molimm.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nair JS, et al. Requirement of Ca2+ and CaMKII for Stat1 Ser-727 phosphorylation in response to IFN-γ. Proc. Natl Acad. Sci. USA. 2002;99:5971–5976. doi: 10.1073/pnas.052159099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhury GG. A linear signal transduction pathway involving phosphatidylinositol 3-kinase, protein kinase C-ε, and MAPK in mesangial cells regulates interferon-γ-induced STAT1α transcriptional activation. J. Biol. Chem. 2004;279:27399–27409. doi: 10.1074/jbc.M403530200. [DOI] [PubMed] [Google Scholar]
- 34.Hardy PO, Diallo TO, Matte C, Descoteaux A. Roles of phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase in the regulation of protein kinase C-alpha activation in interferon-gamma-stimulated macrophages. Immunology. 2009;128(1 Suppl):e652–e660. doi: 10.1111/j.1365-2567.2009.03055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, et al. ERK is integral to the IFN-γ-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J. Immunol. 2010;185:3041–3048. doi: 10.4049/jimmunol.1000993. [DOI] [PubMed] [Google Scholar]
- 36.Bancerek J, et al. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity. 2013;38:250–262. doi: 10.1016/j.immuni.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenoever BR, et al. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama-Fujita Y, Shimizu T, Sagawa M, Uchida H, Kizaki M. The role of TC-PTP (PTPN2) in modulating sensitivity to imatinib and interferon-α in CML cell line, KT-1 cells. Leuk. Res. 2013;37:1150–1155. doi: 10.1016/j.leukres.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, et al. Wedelolactone, a naturally occurring coumestan, enhances interferon-γ signaling through inhibiting STAT1 protein dephosphorylation. J. Biol. Chem. 2013;288:14417–11427. doi: 10.1074/jbc.M112.442970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yetter A, et al. Association of the interferon-dependent tyrosine kinase Tyk-2 with the hematopoietic cell phosphatase. J. Biol. Chem. 1995;270:18179–18182. doi: 10.1074/jbc.270.31.18179. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, et al. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: A novel anti-inflammatory function of DJ-1. Neurobiol. Dis. 2013;60:1–10. doi: 10.1016/j.nbd.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, et al. Selective sequestration of STAT1 in the cytoplasm via phosphorylated SHP-2 ameliorates murine experimental colitis. J. Immunol. 2012;189:3497–3507. doi: 10.4049/jimmunol.1201006. [DOI] [PubMed] [Google Scholar]
- 43.García-Ruiz I, et al. Protein-tyrosine phosphatases are involved in interferon resistance associated with insulin resistance in HepG2 cells and obese mice. J. Biol. Chem. 2012;287:19564–19573. doi: 10.1074/jbc.M112.342709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heinonen KM, Bourdeau A, Doody KM, Tremblay ML. Protein tyrosine phosphatases PTP-1B and TC-PTP play nonredundant roles in macrophage development and IFN-gamma signaling. Proc. Natl. Acad. Sci. USA. 2009;106:9368–9372. doi: 10.1073/pnas.0812109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porcu M, et al. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood. 2012;119:4476–4479. doi: 10.1182/blood-2011-09-379958. [DOI] [PubMed] [Google Scholar]
- 46.Cheon H, Stark GR. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl. Acad. Sci. USA. 2009;106:9373–9378. doi: 10.1073/pnas.0903487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Testoni B, et al. Chromatin dynamics of gene activation and repression in response to interferon alpha (IFNα) reveal new roles for phosphorylated and unphosphorylated forms of the transcription factor STAT2. J. Biol. Chem. 2011;286:20217–20227. doi: 10.1074/jbc.M111.231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tahk S, et al. Control of specificity and magnitude of NF-kappa B and STAT1-mediated gene activation through PIASy and PIAS1 cooperation. Proc. Natl. Acad. Sci. USA. 2007;104:11643–11648. doi: 10.1073/pnas.0701877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Begitt A, Droescher M, Knobeloch KP, Vinkemeier U. SUMO conjugation of STAT1 protects cells from hyperresponsiveness to IFNγ. Blood. 2011;118:1002–1007. doi: 10.1182/blood-2011-04-347930. [DOI] [PubMed] [Google Scholar]
- 50.Au-Yeung N, Mandhana R, Horvath CM. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. JAKSTAT. 2013;2:e23931. doi: 10.4161/jkst.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gnatovskiy L, Mita P, Levy DE. The human RVB complex is required for efficient transcription of type I interferon-stimulated genes. Mol. Cell Biol. 2013;33:3817–3825. doi: 10.1128/MCB.01562-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nature Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 54.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 55.Uddin S, et al. Activation of the p38 Map kinase by type I interferons. J. Biol. Chem. 1999;274:30127–30131. doi: 10.1074/jbc.274.42.30127. [DOI] [PubMed] [Google Scholar]
- 56.Zhao LJ, Hua X, He SF, Ren H, Qi ZT. Interferon alpha regulates MAPK and STAT1 pathways in human hepatoma cells. Virol J. 2011;8:157. doi: 10.1186/1743-422X-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, et al. Role of p38alpha Map kinase in Type I interferon signaling. J. Biol. Chem. 2004;279:970–979. doi: 10.1074/jbc.M309927200. [DOI] [PubMed] [Google Scholar]
- 58.Lee WH, Liu FH, Lee YL, Huang HM. Interferon-alpha induces the growth inhibition of human T-cell leukaemia line Jurkat through p38alpha and p38beta. J. Biochem. 2010;147:645–650. doi: 10.1093/jb/mvp213. [DOI] [PubMed] [Google Scholar]
- 59.Verma A, et al. Activation of the p38 mitogen-activated protein kinase mediates the suppressive effects of type I interferons and transforming growth factor-beta on normal hematopoiesis. J. Biol. Chem. 2002;277:7726–7735. doi: 10.1074/jbc.M106640200. [DOI] [PubMed] [Google Scholar]
- 60.Verma A, et al. Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J. Immunol. 2002;168:5984–5988. doi: 10.4049/jimmunol.168.12.5984. [DOI] [PubMed] [Google Scholar]
- 61.Matsuzawa T, et al. IFN-γ elicits macrophage autophagy via the p38 MAPK signaling pathway. J. Immunol. 2012;189:813–818. doi: 10.4049/jimmunol.1102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Z, et al. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu M, et al. Interferon-alpha targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp. Hematol. 2010;38:472–480. doi: 10.1016/j.exphem.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorzitto J, Galligan CL, Ueng JJ, Fish EN. Characterization of the antiviral effects of interferon-alpha against a SARS-like coronoavirus infection in vitro. Cell Res. 2006;16:220–229. doi: 10.1038/sj.cr.7310030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Filippo M, et al. Interferon-β1a protects neurons against mitochondrial toxicity via modulation of STAT1 signaling: Electrophysiological evidence. Neurobiol. Dis. 2013 doi: 10.1016/j.nbd.2013.09.022. pii: S0969-9961(13)00281-00287. [DOI] [PubMed] [Google Scholar]
- 66.Katsoulidis E, et al. Role of Schlafen 2 (SLFN2) in the generation of interferon alpha-induced growth inhibitory responses. J. Biol. Chem. 2009;284:25051–25064. doi: 10.1074/jbc.M109.030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katsoulidis E, et al. Suppression of interferon (IFN)-inducible genes and IFN-mediated functional responses in BCR-ABL-expressing cells. J. Biol. Chem. 2008;283:10793–10803. doi: 10.1074/jbc.M706816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhattacharya S, et al. Role of p38 protein kinase in the ligand-independent ubiquitination and down-regulation of the IFNAR1 chain of type I interferon receptor. J. Biol. Chem. 2011;286:22069–22076. doi: 10.1074/jbc.M111.238766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seo JY, Kim DY, Lee YS, Ro JY. Cytokine production through PKC/p38 signaling pathways, not through JAK/STAT1 pathway, in mast cells stimulated with IFNγ. Cytokine. 2009;46:51–60. doi: 10.1016/j.cyto.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Valledor AF, et al. Selective roles of MAPKs during the macrophage response to IFN-gamma. J. Immunol. 2008;180:4523–4529. doi: 10.4049/jimmunol.180.7.4523. [DOI] [PubMed] [Google Scholar]
- 71.Katsoulidis E, et al. Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes. Cancer Res. 2005;65:9029–9037. doi: 10.1158/0008-5472.CAN-04-4555. [DOI] [PubMed] [Google Scholar]
- 72.Navas TA, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108:4170–4177. doi: 10.1182/blood-2006-05-023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chai Y, et al. IL-29 and IFN-α regulate the expression of MxA, 2',5'-OAS and PKR genes in association with the activation of Raf-MEK-ERK and PI3K-AKT signal pathways in HepG2.2.15 cells. Mol. Biol. Rep. 2011;38:139–143. doi: 10.1007/s11033-010-0087-1. [DOI] [PubMed] [Google Scholar]
- 74.Banu SK, Lee J, Stephen SD, Nithy TK, Arosh JA. Interferon tau regulates PGF2alpha release from the ovine endometrial epithelial cells via activation of novel JAK/EGFR/ERK/EGR-1 pathways. Mol. Endocrinol. 2010;24:2315–2330. doi: 10.1210/me.2010-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu J, et al. Interferon-α sensitizes human gastric cancer cells to TRAIL-induced apoptosis via activation of the c-CBL-dependent MAPK/ERK pathway. Cancer Biol. Ther. 2011;12:494–502. doi: 10.4161/cbt.12.6.15973. [DOI] [PubMed] [Google Scholar]
- 76.Douglas SA, Bunn SJ. Interferon-alpha signalling in bovine adrenal chromaffin cells: Involvement of signal-transducer and activator of transcription 1 and 2, extracellular signal-regulated protein kinases 1/2 and serine 31 phosphorylation of tyrosine hydroxylase. J. Neuroendocrinol. 2009;21:200–207. doi: 10.1111/j.1365-2826.2009.01821.x. [DOI] [PubMed] [Google Scholar]
- 77.Panaretakis T, et al. Interferon alpha induces nucleus-independent apoptosis by activating extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase downstream of phosphatidylinositol 3-kinase and mammalian target of rapamycin. Mol.Biol.Cell. 2008;19:41–50. doi: 10.1091/mbc.E07-04-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bacher N, et al. Interferon-α Suppresses cAMP to Disarm Human Regulatory T Cells. Cancer Res. 2013;73:5647–5656. doi: 10.1158/0008-5472.CAN-12-3788. [DOI] [PubMed] [Google Scholar]
- 79.Joshi S, et al. Type I interferon (IFN)-dependent activation of Mnk1 and its role in the generation of growth inhibitory responses. Proc. Natl. Acad. Sci. USA. 2009;106:12097–12102. doi: 10.1073/pnas.0900562106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joshi S, Platanias LC. Mnk Kinases in Cytokine Signaling and Regulation of Cytokine Responses. Biomol. Concepts. 2012;3:127–139. doi: 10.1515/bmc-2011-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mehrotra S, et al. Essential role for the Mnk pathway in the inhibitory effects of type I interferons on myeloproliferative neoplasm (MPN) precursors. J. Biol. Chem. 2013;288:23814–23822. doi: 10.1074/jbc.M113.476192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma B, et al. Sprouty proteins are negative regulators of interferon (IFN) signaling and IFN-inducible biological responses. J. Biol. Chem. 2012;287:42352–42360. doi: 10.1074/jbc.M112.400721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kroczynska B, et al. Interferon-dependent engagement of eukaryotic initiation factor 4B via S6 kinase (S6K)- and ribosomal protein S6K-mediated signals. Mol. Cell Biol. 2009;29:2865–2875. doi: 10.1128/MCB.01537-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li N, et al. ERK is integral to the IFN-γ-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J. Immunol. 2010;185:3041–3048. doi: 10.4049/jimmunol.1000993. [DOI] [PubMed] [Google Scholar]
- 85.Horiuchi M, Itoh A, Pleasure D, Itoh T. MEK-ERK signaling is involved in interferon-gamma-induced death of oligodendroglial progenitor cells. J. Biol Chem. 2006;281:20095–20106. doi: 10.1074/jbc.M603179200. [DOI] [PubMed] [Google Scholar]
- 86.Smyth D, et al. Interferon-gamma signals via an ERK1/2-ARF6 pathway to promote bacterial internalization by gut epithelia. Cell Microbiol. 2012;14:1257–1270. doi: 10.1111/j.1462-5822.2012.01796.x. [DOI] [PubMed] [Google Scholar]
- 87.Roy SK, et al. MEKK1 plays a critical role in activating the transcription factor C/EBP-β-dependent gene expression in response to IFN-γ. Proc. Natl Acad. Sci. USA. 2002;99:7945–7950. doi: 10.1073/pnas.122075799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joshi S, et al. Essential role for Mnk kinases in type II interferon (IFNgamma) signaling and its suppressive effects on normal hematopoiesis. J. Biol. Chem. 2011;286:6017–6026. doi: 10.1074/jbc.M110.197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kroczynska B, et al. Regulatory effects of ribosomal S6 kinase 1 (RSK1) in IFNλ signaling. J. Biol. Chem. 2011;286:1147–1156. doi: 10.1074/jbc.M110.183566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang X, et al. Interferon-induced protein IFIT4 is associated with systemic lupus erythematosus and promotes differentiation of monocytes into dendritic cell-like cells. Arthritis Res. Ther. 2008;10:R91. doi: 10.1186/ar2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deng J, et al. Involvement of ERK and JNK pathways in IFN-γ-induced B7-DC expression on tumor cells. J. Cancer Res. Clin. Oncol. 2011;137:243–250. doi: 10.1007/s00432-010-0876-x. [DOI] [PubMed] [Google Scholar]
- 92.Kim SJ, et al. Interferon-gamma promotes differentiation of neural progenitor cells via the JNK pathway. Neurochem. Res. 2007;32:1399–1406. doi: 10.1007/s11064-007-9323-z. [DOI] [PubMed] [Google Scholar]
- 93.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 95.Lekmine F, et al. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J. Biol. Chem. 2003;278:27772–27780. doi: 10.1074/jbc.M301364200. [DOI] [PubMed] [Google Scholar]
- 96.Lekmine F, et al. Interferon-gamma engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp. Cell Res. 2004;295:173–182. doi: 10.1016/j.yexcr.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 97.Kaur S, et al. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J. Immunol. 2008;181:7316–7323. doi: 10.4049/jimmunol.181.10.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaur S, et al. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl. Acad. Sci. USA. 2008;105:4808–4813. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaur S, et al. Regulatory effects of mammalian target of rapamycin-activated pathways in type I and II interferon signaling. J. Biol. Chem. 2007;282:1757–1768. doi: 10.1074/jbc.M607365200. [DOI] [PubMed] [Google Scholar]
- 100.Kroczynska B, et al. Regulatory effects of programmed cell death 4 (PDCD4) protein in interferon (IFN)-stimulated gene expression and generation of type I IFN responses. Mol. Cell Biol. 2012;32:2809–2822. doi: 10.1128/MCB.00310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaur S, et al. Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses. Proc. Natl. Acad. Sci. USA. 2012;109:7723–7728. doi: 10.1073/pnas.1118122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsumoto A, et al. Interferon-alpha-induced mTOR activation is an anti-hepatitis C virus signal via the phosphatidylinositol 3-kinase-Akt-independent pathway. J. Gastroenterol. 2009;44:856–863. doi: 10.1007/s00535-009-0075-1. [DOI] [PubMed] [Google Scholar]
- 103.Burke JD, Sonenberg N, Platanias LC, Fish EN. Antiviral effects of interferon-β are enhanced in the absence of the translational suppressor 4E-BP1 in myocarditis induced by Coxsackievirus B3. Antivir. Ther. 2011;16:577–584. doi: 10.3851/IMP1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 106.Kohanbash G, Okada H. MicoRNAs and STAT interplay. Semin. Cancer Biol. 2012;22:70–75. doi: 10.1016/j.semcancer.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang Y, et al. MicroRNA regulation of STAT4 protein expression: rapid and sensitive modulation of IL-12 signaling in human natural killer cells. Blood. 2011;118:6793–6802. doi: 10.1182/blood-2011-05-356162. [DOI] [PubMed] [Google Scholar]
- 108.Dentelli P, et al. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler. Thromb. Vasc. Biol. 2010;30:1562–1568. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 109.Li A, et al. MiR-122 modulates type I interferon expression through locking suppressor of cytokine signaling 1. Int. J. Biochem. Cell Biol. 2013;45:856–865. doi: 10.1016/j.biocel.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 110.Su C, Hou Z, Zhang C, Tian Z, Zhang J. Ectopic expression of microRNA-155 enhances innate antiviral immunity against HBV infection in human hepatoma cells. Virol. J. 2011;8:354. doi: 10.1186/1743-422X-8-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yao R, et al. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7:e46082. doi: 10.1371/journal.pone.0046082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Collins AS, McCoy CE, Lloyd AT, O’Farrelly C, Stevenson NJ. miR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signalling. PLoS One. 2013;8:e69090. doi: 10.1371/journal.pone.0069090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Trotta R, et al. miR-155 regulates IFN-g production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhuang G, et al. Tumor-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noguchi S, et al. ssocs7, a target gene of microRNA-145, regulates interferon- induction through STAT3 nuclear translocation in bladder cancer cells. Cell Death Dis. 2013;4:e482. doi: 10.1038/cddis.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mycko MP, et al. microRNA-301a regulation of a T-helper 17 immune response controls autoimmune demyelination. Proc. Natl. Acad. Sci. USA. 2012;109:E1248–E1257. doi: 10.1073/pnas.1114325109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li G, et al. STAT5 requires the N-domain for suppression of miR15/16, induction of bcl-2, and survival signaling in myeloproliferative disease. Blood. 2010;115:1416–1424. doi: 10.1182/blood-2009-07-234963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rozovski U, et al. Signal transducer and activator of transcription (STAT)-3 regulates microRNA gene expression in chronic lymphocytic leukemia cells. Mol. Cancer. 2013;12:50. doi: 10.1186/1476-4598-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lang Q, Ling C. miR-124 suppresses cell proliferation in hepatocellular carcinoma by targeting PI3KCA. Biochem. Biophys. Res. Commun. 2012;426:247–252. doi: 10.1016/j.bbrc.2012.08.075. [DOI] [PubMed] [Google Scholar]
- 120.Oneyama C, et al. Micro-RNA-mediated downregulation of mTOR/FGFR3 controls tumor growth induced by Src-related oncogenic pathways. Oncogene. 2011;30:3489–3501. doi: 10.1038/onc.2011.63. [DOI] [PubMed] [Google Scholar]
- 121.Liu P, Wilson MJ. miR-520c and miR-373 upregulate MMP9 expression by targeting mTOR and SIRT1, and activate the Ras/Raf/MEK/Erk signaling pathway and NF-kB factor in human fibrosarcoma cells. J. Cell Physiol. 2012;227:867–876. doi: 10.1002/jcp.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Torres A, et al. Deregulation of miR-100, mir-99a and miR-199b in tissues and plasma coexists with increased expression of mTOR kinase in endometrioid endometrial carcinoma. BMC Cancer. 2012;12:369. doi: 10.1186/1471-2407-12-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iwaya T, et al. Downregulation of miR-144 is associated with colorectal cancer progression via activation of mTOR signaling pathway. Carcinogenesis. 2012;33:2391–2397. doi: 10.1093/carcin/bgs288. [DOI] [PubMed] [Google Scholar]
- 124.Lawson SK, Dobrikova EY, Shveygert M, Gromeir M. p38a mitogen-activated protein kinase depletion and repression of signal transduction to translation machinery by miR-124 and-128 in neorons. Mol. Cell. Biol. 2013;33:127–135. doi: 10.1128/MCB.00695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanka T, et al. Inhibition of cell viability by human IFN-β is mediated by microRNA-431. Int. J. Oncol. 2012;40:1470–1476. doi: 10.3892/ijo.2012.1345. [DOI] [PubMed] [Google Scholar]
- 126.Li Y, et al. MicroRNA-466I inhibits antiviral innate immune response by targeting interferon-alpha. Cell. Mol. Immunol. 2012;9:497–502. doi: 10.1038/cmi.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Polioudakis D, et al. A Myc-micro network promotes exit from quiescence by suppressing the interferon response and cell-cycle arrest genes. Nucleic Acids Res. 2013;41:2239–2254. doi: 10.1093/nar/gks1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Buggele WA, Horvath CM. MicroRNA profiling of Sendai-virus infected A549 cells identifies miR-203 as an interferon-inducible regulator of IFIT1/ISG56. J. Virol. 2013;87:9260–9270. doi: 10.1128/JVI.01064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, et al. MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-λ1. Protein Cell. 2013;4:130–141. doi: 10.1007/s13238-012-2081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma F, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-g. Nature Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 131.Steiner DF, et al. MicroRNA-29 regulates T-box transcription factors and interferon-g production in helper T cells. Immunity. 2011;35:169–181. doi: 10.1016/j.immuni.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamada M, et al. Interferon-g production by neutrophils during bacterial pneumonia in mice. Am. J. Respir. Crit. Care Med. 2011;183:1391–1401. doi: 10.1164/rccm.201004-0592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]