Abstract
Obesity is a topic on which many views are strongly held in the absence of scientific evidence to support those views, and some views are strongly held despite evidence to contradict those views. We refer to the former as “presumptions” and the latter as “myths”. Here we present nine myths and ten presumptions surrounding the effects of rapid weight loss; setting realistic goals in weight loss therapy; stage of change or readiness to lose weight; physical education classes; breast-feeding; daily self-weighing; genetic contribution to obesity; the “Freshman 15”; food deserts; regularly eating (versus skipping) breakfast; eating close to bedtime; eating more fruits and vegetables; weight cycling (i.e. yo-yo dieting); snacking; built environment; reducing screen time in childhood obesity; portion size; participation in family mealtime; and drinking water as a means of weight-loss. For each of these, we describe the belief and present evidence that the belief is widely held or stated, reasons to support the conjecture that the belief might be true, evidence to directly support or refute the belief, and findings from randomized controlled trials, if available. We conclude with a discussion of the implications of these determinations, conjecture on why so many myths and presumptions exist, and suggestions for limiting the spread of these and other unsubstantiated beliefs about obesity domain.
Introduction
Obesity is inextricably connected to individual beliefs about work ethic1, beauty2,3, self-discipline vs. wanton indulgence4, environmental sustainability5,6, culture and family5-7, and even morality5-10. At a superficial level, all individuals in industrialized societies have some daily contact with aspects of obesity. Passionate interests and inherent human tendency to seek explanations for phenomena combined with daily exposure oftentimes provoke the development of beliefs about obesity that are held with zeal despite limited evidence. Unfortunately, when the general public, mass media, government agencies, and even some scientists hold and espouse potentially false beliefs, research to examine the veracity of the beliefs may be stifled. We thus suggest that it is useful to review some common beliefs that in our estimation are unsupported by current evidence. We use the term myth to refer to beliefs held true despite the existence of substantial refuting evidence. We use the term presumption to refer to beliefs that are held to be true but for which convincing evidence does not yet exist to confirm or to disconfirm their legitimacy. The distinction between myths and presumptions is important, because the overriding message is different. For myths, the key message is: let us accept the data, leave these myths behind, and move on to more productive uses of our investigative, clinical, and public health resources. In contrast, for presumptions, the message is: let us move forward vigorously to devote resources to examining the validity of these presumptions to better inform obesity intervention and treatment efforts. For each myth or presumption, we (1) describe the belief and present evidence that the belief is widely held or stated; (2) present reasons why the conjecture may be supported and/or propagated; (3) present evidence to directly support or refute the belief; and (4) where available present evidence from randomized controlled trials (RCTs).
Myth 1 - Losing weight quickly will predispose to greater weight regain relative to losing weight more slowly
Exposition of belief and support that the belief is widely held or stated
That gradual, slower weight loss results in better long-term outcomes than abrupt weight loss, is a belief that likely emerged as a reaction to the adverse effects of nutritionally insufficient very-low-calorie diets (VLCDs) in the 1960s. The propagation of the myth has been maintained over the past 50 years as evidenced by its inclusion in well-respected textbooks of nutrition and the recommendations of many health authorities. It is one of the basic rules dietitians apply to the management of obesity. For example, in an international textbook of nutrition11, the section “Reasonable treatment of obesity” starts with the statement, “In the 1990s, experts on obesity treatments … now embrace small changes, moderate losses, and reasonable goals, (pg. 266) …[with] at least six months for a 10% loss of initial weight, (pg. 267).” This corresponds to less than one pound (0.38 kg) per week for an obese person with an initial body weight of 220 pounds (100 kg). In a table in this same textbook titled “Weight-Loss Consumer Bill of Rights (pg. 231)” the following is highlighted: “WARNING: Rapid weight loss may cause serious health problems. Rapid weight loss is weight loss of more than 1½ to 2 pounds per week or weight loss of more than 1% of body weight per week after the second week of participation in a weight-loss program. Only permanent lifestyle changes, such as making healthful food choices and increasing physical activity, promote long-term weight loss.” Further, expert insights from physicians posted on websites state “To lose weight permanently, you must make a commitment to gradually adopt a healthier way of life,” and warn that rapid weight loss usually results in regaining lost weight12,13.
Reasons to support the conjecture that the belief might be true
These statements apparently arise from the plausible common belief that weight gain and obesity are a simple consequence of an inappropriate diet and lack of physical activity. It follows that if everyone were to comply with official recommendations for healthy diet composition and daily physical activity, no one would be obese. It also implies that more rapid weight loss is not only unnecessary but even counterproductive when it is not accomplished by the proposed lifestyle changes. Furthermore, the assertion is that gradual changes in diet and lifestyle are more likely to be permanent and thus better maintained long term.
Evidence directly refuting the belief
The results of many observational studies show that, in least in terms of association, the opposite is true. In fact, numerous post-hoc analyses of trials correlate greater initial weight loss with greater long-term success. Of course, it must be recognized that such analyses demonstrate correlation and not necessarily causation, because the greater initial weight loss is not the variable to which subjects are randomized. A comprehensive review of the literature conducted in 200014 consistently found a beneficial association of rapid weight loss on weight loss maintenance, and these findings have been further supported by studies published since that time. Using data from the 1999–2002 National Health and Nutrition Examination Survey (NHANES), Weiss et al. reported that among 1,300 adults who had lost 10% or more of their body weight, 59% maintained this weight loss and 7% lost more weight over the following year15. Losing a greater percentage of maximum weight (20% vs. 10%-15%) was additionally associated with improved long-term outcomes (odds ratio [OR]: 2.8; 95% confidence interval [CI]: 2.0–4.1). Jeffery et al. found that initial weight losses were positively, not negatively, related to weight loss after 30 months in patients following a behavior therapy program16. This finding was largely due to differences in initial weight loss. Finally, Björvell and Rössner found a significant correlation between weight loss after a 2-year treatment period and weight at 4 years of follow-up as well as weight loss at 10 to 12 years of follow-up17.
Evidence from randomized trials
To systematically investigate whether slow gradual weight loss facilitates improved long-term weight loss maintenance, trials that randomize obese patients to slow versus rapid initial weight loss have been conducted. For example, Toubro and Astrup randomized 43 obese adults to 8 weeks of a VLCD or 17 weeks of a conventional diet 1200kcal/day (5 MJ/day) to produce slower weight loss, in which the difference in duration was targeted to reach a similar weight loss18. After the weight loss phase, all patients were enrolled in a 1-year weight maintenance program with a 2-year follow-up after completion of weight loss. The weight loss achieved by patients in the 8-week and 17-week treatment programs was similar (13.6 kg vs. 13.6 kg), whereas the rate of initial weight loss in the VLCD group was twice that in the conventional group (1.6 kg vs. 0.8 kg/week). The weight loss maintained by the two groups was similar, with a tendency toward improved 2-year maintenance in the VLCD group (3.0 kg vs. 2.4 kg, NS)19. Studies have also compared smaller with greater initial weight loss over 1 year in obese patients with osteoarthritis. After 1 year, mean weight loss in the low-energy diet group was 10.9 kg (11%) compared with 3.6 kg (4%) in the control group (p<0.0001)20. To date, the totality of evidence does not support the myth that gradual weight loss improves long-term outcomes21.
Today, even authorities such as the Food and Drug Administration (FDA) acknowledge that those who do not lose weight initially are unlikely to do it in the long-term as reflected in the approval text for the weight loss drug Lorcaserin (Belviq): “The approved labeling for Belviq recommends that the drug be discontinued in patients who fail to lose 5 percent of their body weight after 12 weeks of treatment, as these patients are unlikely to achieve clinically meaningful weight loss with continued treatment.” 22 In a randomized trial 23, among 776 subjects (83%) who achieved at least an 8% reduction in their initial body weight, a greater weight loss at week 8 (>12.7 kg, i.e., above the upper quartile) was associated with lower attrition during the subsequent 6 month dietary intervention period as compared with a lower weight loss quartile (<8.6 kg; OR: 0.92; 95% CI: 0.88-0.97, P = 0.001). Dropout rate was also lower among subjects in the upper quartile of initial weight loss than among those in the lower quartiles.
Myth 2 - Setting realistic weight loss goals in obesity treatment is important because otherwise patients will become frustrated and lose less weight
Exposition of belief and anecdotal support that the belief is widely held or stated
Support for this recommendation can be found in general goal-setting theory and in some aspects of cognitive-behavioral theory 24. Cooper and Fairburn 25 suggest a cycle in which a decrease in the rate of initial weight loss is followed by a subsequent decline in patients' belief in their ability to attain their initial goal. This may lead to premature disengagement from treatment as well as abandonment of the key weight loss strategies necessary for future weight loss efforts as well as weight maintenance 26,27. Accordingly, the unified message from various major public health entities28-30 and statements from key professional organizations 31;32-35 is for overweight and obese patients to start gradually and pursue modest weight loss goals.
Reasons to support the conjecture that the belief might be true
First-generation weight loss interventions conducted in the 1980s targeted attainment of ideal body weight and thus embraced the more ambitious goals and expectations of patients 36, perhaps as a way of encouraging weight loss efforts. However, researchers discovered that ideal weight could only be achieved by a select few, and it was very rarely maintained over time 16,37,38. Thus, in the 1990s, the prevailing message from researchers and professional organizations began to change to promote more realistic weight loss goals favoring 5% to 10% reductions in weight 36. Findings from the general goal-setting literature suggest that unattainable goals lead to impaired performance and discontinuation of the goal-seeking behavior26.
Two studies reported that less realistic goals were predictive of higher rates of attrition39,40 or less success with weight loss39 among individuals seeking obesity treatment. Additionally, studies have shown that unrealistic goals were significantly related to negative psychological outcomes, including poorer body image41-44, lower self-esteem42, and more depressive symptoms45. Given this substantial discrepancy between patients' expectations and what is actually recommended and achievable, it is intuitive to conclude that patients' unrealistic weight loss goals will lead to disappointment, lack of engagement in treatment, and ultimately poorer weight loss outcomes.
Evidence directly supporting or refuting the belief
Whereas a handful of studies have suggested that unrealistic weight loss goals may be problematic, most findings on this topic indicate no meaningful or consistent association between patients' goals and program completion or subsequent weight loss43,46,47. Further, individuals seeking weight loss treatment often have much more ambitious goals or expectations. When asked to define their “dream” or “ideal” weights, patients report weights reflecting 24% to 38% reductions in body weight 51-57. When asked to identify “happy” or “acceptable” weights, weight loss goals typically range from 15% to 31%reductions in initial weight 51;53;54;56. Even weight loss goals described as “disappointing” by patients generally exceed 10% 36.
In fact, some studies have reported that less realistic goals were actually associated with better weight loss outcomes43,46,47. For instance, Fabricatore et al.46 reported that weight loss goals of individuals receiving pharmacotherapy, lifestyle modification, or combined therapy were unrelated to actual weight loss, although greater weight loss was associated with less realistic goals among patients receiving medication plus brief therapy from their physicians. In two separate studies, Linde et al.43,48 also found that unrealistically high goals were associated with greater weight loss, although this relationship varied by gender, the operational definitions of weight loss goals, and length of follow-up. In a systematic review including 13 observational studies, overall, no association between pretreatment goals and weight loss was observed 49. Conversely, lower expectations could prevent engagement in a program to lose weight 49. In a more recent meta-analysis of observational studies conducted between 1998-2012, encompassing eleven studies which were delivered a weight loss intervention in humans lasting at least six weeks, and assessed baseline weight loss goals as well as pre- and post-weight either in the form of body mass index or some other measure that could be converted to weight the overall correlation between goal weight and weight at intervention completion was small and statistically insignificant 50.
One single-group pilot study examined the effects of a modified behavioral weight loss intervention incorporating strategies to alter patients' unrealistic goals in order to potentially improve initial and long-term weight loss51. While the modified program produced significant changes in weight loss expectations (such that patients' goals became more realistic), the modified intervention did not result in greater weight loss, longer-term weight loss maintenance, or better psychological outcomes (e.g., depressive symptoms, self-esteem). Similarly, in a study among successful weight losers discrepancies between weight loss expectations and actual weight loss did not impact weight regain 52.
Evidence from randomized trials
One randomized pilot study compared the effects of a standard behavioral weight loss intervention with a modified intervention that targeted unrealistic weight loss expectations53. Similar to findings from the other behavioral treatment pilot study, the modified intervention resulted in more realistic weight loss expectations but did not influence weight loss itself. Results from such studies further support the conclusion that unrealistic goals are not meaningfully related to actual weight loss. In fact, it is possible that adjusting patients' goals to modest outcomes may undermine optimism and motivation for engaging in the behaviors required for successful weight loss 51. Therefore, the analytical evidence of existing studies, considered together, does not demonstrate that setting realistic goals leads to more favorable weight loss outcomes.
Myth 3 - Assessing “stage of change” or “readiness” to diet is important in helping patients who pursue weight loss treatment to lose weight
Exposition of belief and support that the belief is widely held or stated
The Transtheoretical Model (TTM) 54 has been applied to a wide variety of behavioral change interventions 55 under the auspice that an individual's readiness to change (i.e., to engage in the behaviors required, in this case, to lose weight) can be conceptualized in five stages: precontemplation, contemplation, preparation, action, and maintenance. Identifying the stage of readiness of a given individual is thought to be important to facilitate the delivery of more targeted treatment 56. The TTM is said to offer a theoretical framework for the assessment of readiness to change, which is considered vital for tailoring interventions according to the patient's motivation. Perhaps the most popular of these is the Weight Loss Readiness Test II 57, formerly the Diet Readiness Test 58. The 27 items answered on a 5-point Likert scale are said to measure 6 factors related to readiness: motivation, expectations, confidence, hunger and eating cues, binge eating and purging, and emotional eating. It is commonly believed that the answers to these 27 questions are predictive of weight-loss success. Thus, some health professionals, laypeople, and weight loss organizations espouse the view that readiness is a core concept and an important factor in helping individuals decide whether to pursue weight loss and whether their weight loss efforts will be successful. This is evident, for example, by an article on the Mayo Clinic Web site which states, “Your weight-loss success depends in large part on your readiness to take on the challenge” 59. Dozens of Web sites related to health and weight loss (e.g., Weight Watchers, Mayo Clinic, CNN) provide information on the importance of weight loss readiness, as well as interactive assessments purported to help an individual to determine his or her readiness. Successful assessment of readiness is also thought to be important in minimizing dropout from weight management programs or from individual weight loss efforts 60,61.
Reasons to support the conjecture that the belief might be true
Intuitively, it seems logical to conclude that if an individual believes that he or she is psychologically and motivationally prepared to take on a series of major behavioral changes to produce a desired outcome, that individual will experience greater success. For example, individuals in the pre-contemplative or contemplative stages may benefit from counseling on the risks of being overweight and the benefits of weight loss in order to assist them in progressing to the preparation stage, when they will begin to make plans to do something tangible to address their weight. Indeed, the underlying assumption of the TTM is that readiness translates into motivation and that motivation is critical for successful engagement in behavioral weight loss programs 54,55. By matching the level of readiness with the appropriate treatment (e.g., counseling for those in the pre-contemplative stage, a tangible diet and exercise plan for those in the action stage), it is thought that the chances of engaging in a process to ultimately produce successful long-term weight loss will be maximized 56,60-62.
Evidence directly supporting or refuting the belief
Overall, there are few data showing that readiness has an effect on weight loss outcomes. Some evidence indicates that readiness and its variants such as self-motivation, self-determination, and self-efficacy may play a small role in predicting weight outcomes, typically in the short term 61,63-70. Research conducted to date varies markedly in the way readiness and related motivational constructs are measured. However, the Dieting Readiness Test and the Weight Efficacy Lifestyle Questionnaire were not shown to be predictive of magnitude of weight loss or treatment attendance 58,71. Furthermore, a recent study 72 of 227 consecutive patients undergoing adjustable gastric banding surgery found that readiness was not associated with percentage reduction in BMI at 2 years or compliance or likelihood of complications. Finally, a Cochrane review 73 limited to five RCTs (3,910 participants, median length of 9 months) specifically evaluating the effect of randomization to interventions which incorporated stages of change (not exclusively readiness) from the TTM to controls that did not use TTM assessment found that the use of stages of change resulted in minimal overall weight loss (∼2 kg or less) and reported no conclusive evidence of sustained weight loss.
Evidence from randomized trials
Unfortunately, randomizing groups based on stage of change is not possible. However, while the available evidence indicates that TTM may not be predictive of weight loss outcomes, it may be of some value to assess readiness to assist individuals in determining whether they wish to engage in weight loss efforts.
Myth 4 – Physical education classes, as currently delivered, play an important role in reducing the prevalence of childhood obesity
Exposition of belief and support that the belief is widely held or stated
Many authorities have expressed concern that a secular decline in children's exposure to physical education (PE) classes over the last several decades has been concurrent with the increasing prevalence of obesity in school-aged youth74. The U.S. Surgeon General has also identified cutbacks in PE as a contributor to pediatric obesity75. In addition, numerous researchers and public health organizations have recommended that increased exposure to PE be adopted as an important public health strategy for reducing the prevalence of childhood obesity. For example, the Institute of Medicine's report, Preventing Childhood Obesity, Health in the Balance, included the recommendation that schools should “Expand opportunities for physical activity through physical education classes”76.
A recently released Institute of Medicine report, Educating the Student Body: Taking Physical Activity and Physical Education to School, recommends that “School districts should provide high-quality curricular physical education during which students should spend at least half of the class time engaged in vigorous- or moderate-intensity physical activity”77. However, the fact that exercise in general, in appropriate types and amounts, benefits children's health is not the focus of this myth. Rather, the issue is whether school PE specifically, in its typical form, provides sufficient doses of physical activity without subsequent energetic compensation to provide obesity prevention.
Reasons to support the conjecture that the belief might be true
Typically, the rationale for providing students with PE has been based on the health benefits of exercise for children. These benefits, including a positive impact on weight status, are well documented 78 and were summarized in the report of the Physical Activity Guidelines Advisory Committee 79. PE has often been criticized for providing students with very limited amounts of moderate-to-vigorous-intensity physical activity (MVPA)80, the type of physical activity that provides the greatest health benefits. On the other hand, a number of studies have shown that when PE classes are structured to prioritize providing MVPA, they do deliver substantial doses of physical activity81-83. In the context of the obesity epidemic, numerous authorities have recommended that PE programs be modified, expanded, or enhanced84-87. The reasonable premise underlying this recommendation is that high-frequency exposure to PE classes that emphasize MVPA can provide physiologically meaningful doses of physical activity that promote healthy body composition in youth.
Evidence directly supporting or refuting the belief
Studies examining the direct relationship between exposure to PE and students' weight status and/or body composition are fairly limited. Several cross-sectional observational studies have assessed the effects of federal or state PE policies (i.e. increased time in PE classes on BMI)88-91. These studies found that the relationship between time in PE class and BMI was weak, with two studies reporting no association91,90,92-96 and two reporting very small inverse associations89,88,97. One prospective observational study, the Early Childhood Longitudinal Study, evaluated the relationship between exposure to PE and changes in BMI as children transitioned from kindergarten to first grade98. PE exposure for most children in this study increased over time as the result of changes in the daily schedule between kindergarten and first grade. The study found that a 1-hour per week increase in PE between kindergarten and first grade resulted in a 0.31-point reduction in BMI for overweight/obese girls98 but no reduction in overweight/obese boys or normal weight girls or boys. It was unclear whether the relationships seen in this age group would apply to older children. However, a recent follow-up re-evaluating outcomes when the children were in fifth grade identified a “relatively small” effect on body fat effect and only in boys 97,99-101. Given the known decrease in body fat that occurs in boys with proximity to puberty an effect of PE on obesity is difficult to decipher.
Evidence from randomized trials
Two types of experimental RCTs, interventions to increase PE time and interventions to improve PE, have also examined the effects of PE on weight status and/or BMI. Of the studies that focused only on increasing time in PE, the majority increased the number of days children attended PE classes. The expanded time in PE resulted in modest attenuations in the increase in BMI over time 102-104; however, the results were not consistent across genders or age groups. Another option for improving children's health via exposure to PE is to improve the PE curriculum. Some intervention studies, for example, have focused on increasing the amount of time children spend in MVPA during PE class. Of the 10 studies that used this approach and measured weight status, two showed slight decreases in BMI105,106 or attenuated gains in BMI, while the majority showed no significant effects of the intervention81,107-113. Reviews and meta-analyses of school-based physical activity interventions, including those designed to modify PE, have concluded that interventions can increase physical activity during PE classes, but the increased time in physical activity does not result in significant improvements in children's BMI or weight status114-116. For example, increased PE has been associated with improved motor skills but not BMI93. Thus, on the basis of the literature to date, PE, as currently delivered in the typical school in the U.S., is not contributing importantly to prevention of overweight and obesity. A reasonable interpretation of the relevant literature is that PE, delivered in an ideal form, might have the potential to influence body weight status in children. However, in its current form, PE is probably not influencing BMI or obesity level in the population of school-aged children.
Myth 5 - Breastfeeding is protective against obesity in breastfed offspring
Exposition of belief and support that the belief is widely held or stated
That breastfeeding results in a reduction in the incidence of obesity compared to formula feeding is a belief that arose in all likelihood in the late 1800's, the advent of the first commercially available infant formula 117 (p. 122). The belief has been advanced the World Health Organization 118, US government agencies 119-123 and professional organizations 124,125 to name but a few. Support for breastfeeding as a first line of defense against obesity and is obesity prevention 126 has garnered wide appeal in the past 30 years. Statements such as “One of the most highly effective preventive measures a mother can make in protecting the health of her infant and herself is to breastfeed”123 (January 2011) and statements by first Lady Michelle Obama (February, 2011) citing evidence that breastfeeding reduces obesity throughout the life span bolster belief in this proposition.
Reasons to support the conjecture that the belief might be true
The protective effect of breastfeeding against obesity seems logically intuitive, in the context of numerous health benefits. The supposition follows that for millennia breast milk was the sole source of nutriture for an infant and therefore must promote optimal body composition trajectories. Infants who exclusively breastfeed weigh less and grow more slowly than their formula fed counterparts in the first 12 months of life127-130. However, an association with breastfeeding and obesity weakens after the first year of life 129,131-139. Nevertheless, in one of the largest studies, Gillman and colleagues actually reported a protective association of breastfeeding in early adolescence. The analyses included 15,000 children from participants in the Nurses' Health Study II and showed a significantly lower risk of being overweight at 9-14 years if exclusively or mostly breastfed compared to children who were exclusively or mostly fed formula 140. In a recent update, however, the author of that study acknowledges that “breastfeeding no longer appears to be a major determinant of obesity risk” 141.
Three systematic reviews and meta-analyses that have included 54 studies142-144 have also concluded breastfeeding is associated with obesity risk in breastfed offspring. Both the Agency for Healthcare Research and Quality (AHRQ) and the World Health Organization (WHO) have interpreted the evidence from these studies. The AHRQ concluded the evidence as ‘tentative’ where as the WHO concluded breastfeeding had a small ‘protective’ effect 145,146. Thus, the preponderance of the evidence suggesting that breastfeeding might be protective against obesity risk stems from epidemiological observational studies that have evaluated the association of breastfeeding initiation and/or duration with overweight in early childhood and in adolescence. The essential evidence brought to bear in favor of the belief is the purported observation that there is an association between breastfeeding and lower risk of obesity later in life.
Critical Evaluation of the Purported Association
Although it has been stated that there is an association between breastfeeding and offspring obesity later in life, there are multiple reasons to doubt both that any such association indicates a causal effect and even whether the purported association even exists.
Reasons to Doubt the Existence of the Association
There are two major reasons to doubt that an association between breastfeeding and later risk of obesity exists. First, meta-analysis show that studies of among persons who have reached adulthood, there is no significant association between having been breastfed as a child and obesity 147,148.
Second, the WHO's analysis revealed strong evidence of publication bias 148, implying that studies which did not find evidence of an association between breastfeeding and later obesity were more likely to be unpublished. Thus, meta-analysis of only the published literature may give the appearance of an association that is greater than the true population association. Thus, the association observed in the published literature, even among younger persons, may not be real.
Reasons to Doubt That Any Observed Association Represents Causation
The reason to doubt that an association between breastfeeding and obesity, if such association exists, represents causation, is that the association may be due to confounding 148. Although most epidemiologic analyses entail some statistical control for plausible confounding factors, such analyses can only control for confounding variables that are known, measured, measured well, and far which the functional form is correctly modelled. Because one can never guarantee that one has completely controlled for all possible confounding variables in any association study, all association findings must be viewed with skepticism as indicators of causal effects.
Beyond this general statement of principle, we can look to observational studies which more rigorously control for potential confounders. If such studies show equally strong associations, this might bolster the plausibility that this association represents causation. In contrast, if the studies with more rigorous control find evidence of association then this reduces the plausibility that any observed association represents.
Evidence from randomized trials
True RCTs of breastfeeding per se do not exist, due to practical and ethical constraints. Rather, there are RCTs of assignment to breastfeeding promotion programs. Using a cluster-randomized experimental intervention design where units/packets (i.e. hospitals and maternal/infant clinics) are randomized to a control intervention (breastfeeding practices and policies currently in place) or to an experimental intervention (based upon the Baby-Friendly Hospital Initiative developed by WHO and United Nations Children's Fund149) are as close to a true randomized trial that currently exists in the literature 150. This cluster design was utilized enrolling 31 Belarusian hospitals and clinics were randomized to either the experimental intervention (n=16) or to control (n=15) where the association between breastfeeding duration and exclusivity on BMI and adiposity (estimated by skinfold measurement) was studied in over 17,000 children 151. Results from this unique study design revealed at both 6.5 years 152 and 11.5 years 138 that the promotion of extending breastfeeding fidelity had no impact on reducing either adiposity or prevalence in obesity.
In conclusion, the actual empirical evidence supporting an effect of breast feeding on obesity is weak at best, making it difficult to defend any strong statements about the benefits of breastfeeding on obesity. The human RCTs suggest a lack of causal effect on average, under ordinary circumstances. That said, we cannot rule out that there are no effects for anyone under any circumstances.
Causation
Although this does not completely eliminate the possibility of confounding, studying sibling pairs, who presumably experience many of the same confounding maternal factors but vary in the duration or initiation of breastfeeding, should radically reduce it. Findings from these studies have been equivocal. Gillman et al.140 analyzed 5614 sib pairs and reported the adjusted odds ratio for overweight among siblings with longer breast-feeding duration, compared with shorter duration, was 0.92, but it was not statistically significant (95% confidence interval = 0.76–1.11). Metzger and McDade reported that breastfed siblings were less likely to be overweight or obese than their non-breastfed siblings 153. Similarly, in a sibling analysis drawn from the Helsinki Birth Cohort, 84% of which had been breastfed on average for 6 months, O'Tierney et al (2009) reported that longer periods of breast-feeding were associated with lower BMI at 1 y of age (p<0.05); however, this association with BMI had largely vanished and was not statistically significant by the age of 7 years (p=0.50). Further, in this cohort, among siblings who had objectively measured heights and weights, those who had been breast-fed for less than 2 months or greater than eight months had the highest BMI and percentage body fat. In contrast, Nelson and colleagues 154 reported that discordant breast-feeding of sibling pairs did not predict BMI Z-score differences or discordant overweight status. Interestingly, a study by Evenhouse and Reilly 155observed that the breastfed sibling is more likely to be overweight (p<0.10). Thus, sibling analyses which control for many maternal variables have not generally produced supportive results. Although sibling analyses provides the capacity to control for effects of some of the maternal confounding factors, they do not account for child confounders that may be related to the discordance in duration or initiation of breastfeeding. It was recently suggested that sibling analysis may represent greater confounding than the sorting across families156. Clearly, no observational epidemiological study is capable of removing all confounding. The only method that can control for both known and unknown confounding from all pre-randomization variables is randomization, provided one adheres to the intent-to-treat principle.
Myth 6 –Daily self-weighing interferes with weight loss
Exposition of belief and support that the belief is widely held or stated
Daily body weight fluctuations are highly variable due to large fluctuations in total body water content, glycogen content, and intestinal content, sometimes amounting to several pounds in 24 hours. Therefore it is believed that for a person trying to lose weight, seeing his or her weight go in the direction opposite to their efforts can be highly discouraging. Thus, dietitians and commercial weight loss groups are reluctant to have their clients weigh themselves daily as an adjunct to controlling their weight 157,158. Consequently, it is often held that self-weighing is detrimental to long-term weight loss.
Reasons to support the conjecture that the belief might be true
Daily weighing was advocated by the work of Stuart, who promoted Behavioral Modification as an alternative to dietary restriction as a means of weight reduction. As part of behavioral modification, Stuart suggested weighing oneself not once daily but four times a day. Stuart first reported success with a combination of daily weighing and other methods to modify behavior to cause significant weight loss159. The study stimulated considerable research to further test whether adding daily self-weighing to behavioral modification would facilitate weight loss. Unfortunately, numerous studies examining the effect of adding daily self-weighing to other behavioral treatments to promote weight loss failed to find an advantage of self-weighing160-163. In some cases, self-weighing was actually found to impair the rate of weight loss164.
Evidence directly supporting or refuting the belief
Three recent experimental studies, however, challenged this conclusion by asking a slightly different question. Rather than examine the effects of daily self-weighing as an adjunct to weight loss therapy, these studies examined the use of daily self-weighing, on its own, to produce a significant weight loss. Oshima et al. 165 observed a significant weight loss effect by having overweight participants weigh themselves and view a chart of their weight twice each day for 12 weeks. Consistent with these effects, Steinberg et al. 166 had participants weigh themselves daily and used Internet messaging to inform participants of their progress towards making their target weight. They observed a significant reduction in body weight in the self-weighing group compared with a control group by the end of 6 months without any further therapy. Similarly, Pacanowski167 demonstrated a significant effect of daily self-weighing and viewing a chart on weight loss by the end of 1 year. Even more importantly, this group maintained their weight loss for 1 year.
A second area in the weight loss literature that demonstrates the effectiveness of daily self-weighing for controlling weight analyzed the behavioral characteristics of people who were successful at losing or maintaining the lost weight. These studies consistently showed that people who weighed themselves daily were more likely to succeed at either losing weight or maintaining their weight after weight loss treatment168-174. Thus, it seems just as likely to conclude that people who weigh themselves daily are more motivated to lose or maintain their weight loss than are those who weigh themselves less frequently as it is to conclude that daily weighing enhances weight loss or weight maintenance.
A third area of support for the use of daily weighing in weight control involves the assessment of daily weighing as an intervention to prevent age-related weight gain or weight regain following weight reduction. Levitsky et al. 175 compared a group of first semester freshmen who were asked to weigh themselves daily and to view a chart of their weight with a matched group of students who were weighed only at the beginning and at the end of their first semester. The results are shown in Figure 1. In two successive tests over 2 years, daily self-weighing prevented significant weight gain in the self-weighing group, whereas the controls who did not weigh themselves daily gained between 2 and 3 kg over the course of the first semester.
Figure 1. Within Subject Weight Change.
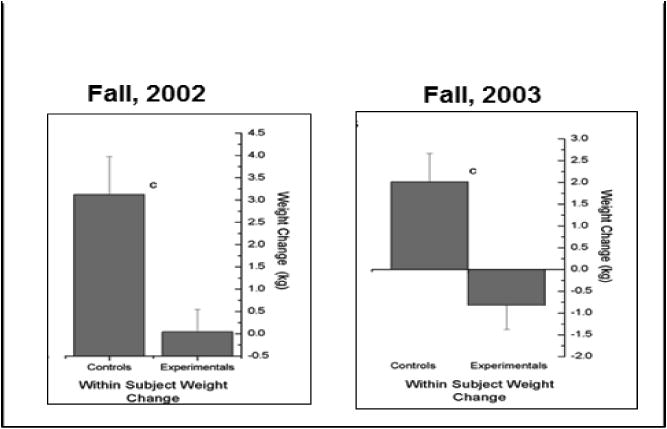
Other attempts at preventing weight gain in freshmen were not as successful. Gow et al. 176 did not observe a significant effect of daily weighing on weight gain prevention in freshmen asked to weigh themselves daily, perhaps because the control freshmen with whom they were compared failed to show a significant weight gain. Strimas and Dionne 177 also failed to find an effect of weight monitoring in a group of freshmen compared with a matched group of freshmen who were asked to monitor their heart rate instead. However, the authors did not instruct the weight monitoring group to maintain their weight.
Wing et al. 174 observed that daily self-weighing was effective in reducing the proportion of a sample of participants who regained their weight after having lost 10% of their body weight as the result of dietary restriction. However, daily self-weighing did not reduce the mean amount of weight regained. Such a finding suggests that daily self-weighing may prevent weight gain in most participants, but once weight regain occurs, further measures such as face-to-face meetings with a professional may be required.
Evidence from randomized trials
Gokee-LaRose et al. 169 randomly divided a group of people who had lost weight as the result of dietary restriction into a group who received behavioral treatment and weighed themselves only once each week and a group who weighed themselves daily and used other methods of self-regulation. The weight loss of the two groups did not differ at the end of the 10 weeks of treatment. However, after the 10-week follow-up period, the daily self-weighing group had lost more weight (-0.18 kg), whereas the group who weighed themselves only once per week had gained weight (+0.37 kg). The difference was not statistically significant. Thus, it appears that the idea that daily self-weighing is not advantageous for weight control is a myth. Published studies show that people who weigh themselves daily lose more weight, maintain the lost weight, and are better able to prevent gaining or regaining weight than are people who do not weigh themselves daily.
Myth 7 - Genes have not contributed to the obesity epidemic
Exposition of belief and support that the belief is widely held or stated
Although the specific mechanisms of the increase in prevalence of obesity since the early 1970s178, particularly in the extremely obese category (BMI > 40 kg/m2)179, remain unknown, existing explanations predominantly focus on certain societal factors which lead to increased energy intake180 or decreased physical activity181, often dismissing or under appreciating the contribution of other potential causes182. Genetic influences have been disregarded primarily because increasing BMI trends have been documented over a few decades and this is considered an insufficient period of time for a genetic effect183. For example, this belief is stated from research group mission statements184 to NIH funding announcements (e.g., NIH Program Announcement; PA-12-84 2012) and has been propagated by health officials through statements such as “the rapid changes in obesity prevalence over the past 30 years cannot be due to genetic changes, which take thousands of years to manifest” (New York State Department of Health. Early Recognition of Overweight and Obesity 119).
Reasons to support the conjecture that the belief might be true
Proponents of this view do not believe a genetic contribution is absent in the shaping of obesity. Rather, the point is that the recent epidemic has come in too short a time frame for a change in the population-based frequency of genetic predisposition to obesity: “Obesity like other complex diseases is caused by a complex interaction between genetic, behavioral and environmental factors. While there is certainly an important genetic component to obesity, the recent epidemic of obesity cannot be due to genetic changes in the population and therefore must be due to changes in environmental influences” (NIH Program Announcement). Therefore, because of the short time span, the cause must lie elsewhere.
Evidence directly supporting or refuting the belief
The argument that a genetic effect is impossible to have occurred over a few decades assumes that mate choice and the number of children born are both independent of BMI. However, a growing body of evidence suggests that these two assumptions are invalid183,185-193. Human mate choice has been observed to be nonrandom (i.e., the reproductive pairing of individuals who have more traits in common than would likely be the case if mating were random) for BMI, which is referred to as assortative mating.183 Stronger evidence was obtained in a retrospective analysis of the Copenhagen School Health Records Register186,191,193 which compared marriages with BMI. The study found assortative marriages between spouses whose BMI was high at age 13 years and the trend increased over time. Likewise, epidemiologic evidence consistently shows that women and couples with higher than average BMI produce more offspring (referred to as differential realized fertility)186,191,193. A recently developed mathematical model that incorporates the effects of assortative mating and differential realized fertility provided quantification of the potential contribution to obesity prevalence187. Through simulation and model analysis, the combined effects of assortative mating and differential realized fertility play a small but significant role in the recent rise in obesity prevalence in the U.S. In addition to assortative mating and differential realized fertility, other genetic mechanisms may also contribute to the obesity epidemic. For example, understanding how gene expression and gene-environment interaction impact obesity is a rapidly growing area of investigation194. Inquiries such as these will expose other means by which genetics have contributed to the obesity epidemic.
Myth 8 – The freshman year of college is associated with or causes 15 pounds of weight gain
Exposition of belief and support that the belief is widely held or stated
A recent Google search (June 2013) of the term “Freshman 15,” which yielded 617,000 hits. The degree to which this idea is believed to be a serious problem can be gleaned from the fact that another Google search for the term “Avoiding the Freshman 15” yielded 371,000 hits.
Reasons to support the conjecture that the belief might be true
As many as 35% of college students in the U.S. are overweight or obese 195,196. College students may experience a significant decrease in physical activity and exercise owing to increased time spent studying, which is largely a sedentary activity 197-199. In addition, the eating habits of many college students may be poor owing to a lack of cooking skills and inadequate cooking facilities 197, thus compromising diet quality. Furthermore, college students may consume more alcohol, contributing to higher energy intake because of new-found autonomy from parents or peer pressure 200,201. All may affect shifts in energy balance and thus lead to weight gain among college students197,202,203.
Evidence directly supporting or refuting the belief
About 35 studies have examined weight gain during the first year of college. The distribution of the amount of weight gained observed in these studies is displayed in Figure 2. To facilitate comparisons among studies, all weights were recalculated as weight gain per year. This wide disparity is due to differences in the length of the measurement period, retention rates, and the various colleges (big and small, public and private) from which the data were derived. However, despite these differences and the very wide range of weight gain reported in these studies, approximately a 6-pound gain as the modal yearly weight gain, not 15 pounds was shown. It is also evident from Figure 2 that the vast majority of published studies observe a positive weight gain204-208, although several studies failed to find a significant weight gain176,205,209,210.
Figure 2. Distribution of weight gain observed in college students. To facilitate comparisons between studies, all weights were recalculated as weight gain per year.
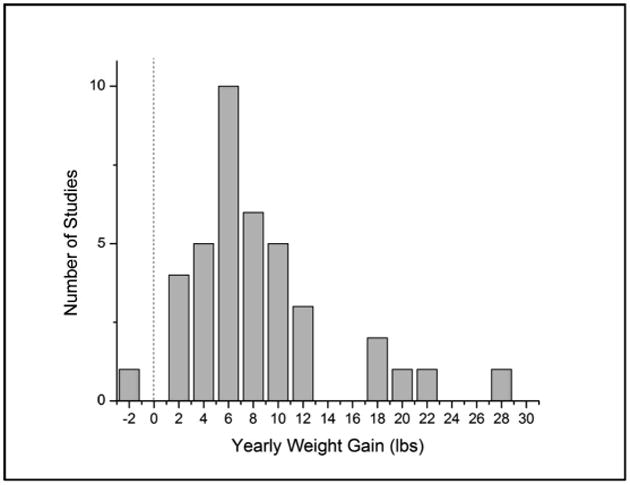
Still, a yearly increase in body weight of about 6 pounds is noticeable and appears to be greater than the rate of weight gain observed in the general population. This apparent high rate of increase in body weight is due to the extrapolation from initial weight gain to yearly weight gains. Several studies have shown, however, that the initial weight gain observed during the first semester (12 weeks) is considerably greater than that observed in the student's later college life210-216. Multiplying a 4-month (1 semester) weight gain by three to extend the weight gain to 12 months inflates the effect of the early and shorter observations.
Figure 3 displays data extracted from the 2011 Behavioral Risk Factor Surveillance System (BRFSS) data217. It is a plot of mean body weight as a function of age for Americans between the ages of 18 and 22 comparing those who attended at least one year of college to those who did not attend at least one year. The rate of gain in body weight of those who attended college (slope = 0.21 pounds per month) is about the same as observed in accumulated studies of weight gain in college. More importantly, however, is that both the absolute body weight and the rate of weight gain of the college students is significantly lower, not higher, than that of those who did not go to college as indicated by a significant interaction between group (college or no college) and age. (Note that the data from the BRFSS are cross-sectional. No attempt was made to infer longitudinal functions from these data. These data were used to compare weight gain data from published studies with large-scale epidemiologic survey data.)
Figure 3.
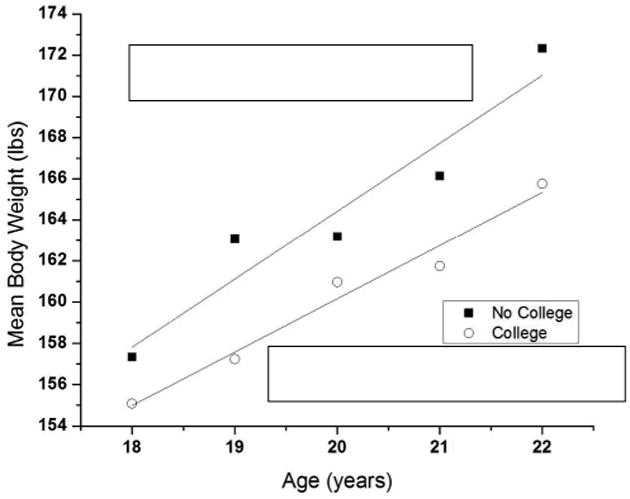
Mean body weight of college-age young adults as a function of age and whether the participants ever attended college. Both the effect of age and the interaction between age and whether the participants attended college were statistically significant (p < 0.001). Data were taken from the BRFSS 216.
This small increase in the rate of weight gain in the people who did not attend college relative to those who did attend does not stop after leaving college, but continues until about the age of 40. After about the age of 50, the mean body weight of those who did not attend college appears to decrease, eventually crossing that of those who did attend college at about the age of 65. The weight gain of those who attended college continues but reaches an asymptote at about the age of 40. The mean body weight of everyone decreases after the age of 70 years. Current data indicate that freshmen do not gain 15 pounds on average, but they do gain weight (approximately 6 pounds on average). This weight gain, however, is not restricted to those attending college, but is related to a more profound phenomenon of age-related weight gain, a process that continues until about the age of 40. Moreover, these data suggest that the college environment, although it may promote weight gain, is not more powerful than forces in the non-college environment in the promotion of weight gain. The curves relating weight gain to age appear to be unaffected by attending college and display a rather smooth function until reaching an asymptote at about the age of 40. Therefore, it is probably not the stress of college, the alcohol drinking, the lack of exercise, the late nights, or the anxiety caused by leaving home that is the source of the freshman weight gain, but rather a cumulative effect of various factors in the environment related to the life changes occurring in young adults moving through late adolescence 218.
Evidence from randomized trials
None.
Myth 9 - Food deserts (areas with little or no access to stores offering fresh and affordable healthy foods, including produce) lead to higher obesity prevalence
Exposition of belief and support that the belief is widely held or stated
Many policymakers and academics suggest that one reason for the higher prevalence of obesity among some populations is that such populations are more likely to live in food deserts, namely, areas or neighborhoods that are economically deprived and where there is limited access to fresh and affordable healthy foods. First lady Michelle Obama has advocated for elimination of food deserts as a key component in her Let's Move initiative to combat childhood obesity 219.
As part of this initiative, the U.S. Department of Agriculture (USDA) launched a project that created a Food Desert Locator (currently modified to a Food Access Research Atlas) 220. Although substantial heterogeneity exists in the scientific literature in how food access and food deserts are measured, for the purposes of the USDA Food Desert Locator map, a food desert is defined as a census tract that has a poverty rate of 20% or higher and in which at least 33% of the population live more than a mile from a supermarket or large grocery store (if a metropolitan census tract) or more than 10 miles if a nonmetropolitan census tract221. The Healthy Food Financing Initiative launched by the USDA and the U.S. Department of Health and Human Services is using federal tax credits, below-market-rate loans, loan guarantees, and grants to attract grocery stores or other small-scale initiatives to bring fresh produce to areas identified as food deserts222. Several foundations, including the Robert Wood Johnson Foundation, the Kellogg Foundation, and Kaiser Permanente, are also awarding many grants to improve access to healthy foods in food deserts222.
Reasons to support the conjecture that the belief might be true
One hypothetical contextual driver of obesity risk is the lack of access to healthy foods and the easy access to calorically dense fast food and convenience store food. There is evidence that the communities in the U.S. with the highest prevalence of obesity, namely, low-income and minority (primarily African American and Latino) communities, live in neighborhoods, census tracts, and zip code areas with fewer supermarkets or with more fast-food outlets and convenience stores 223-228. This can lead to the conjecture that the food environment has a causal effect on the obesity risk of low-income and minority populations.
Evidence directly supporting or refuting the belief
The counterargument is that food outlets may simply reflect the food preferences of the communities, because businesses are likely to choose locations on the basis of expected demand and expected profits.
The USDA's year report provides good reason to be skeptical about the importance of food deserts in influencing a healthy diet229. The USDA reports that only 2.3% of all households in the U.S. (and 3.6% of households in low-income areas) lack access to a vehicle and do not have a supermarket within 1 mile of their homes. On average, those living in food deserts spent 19.5 minutes traveling one way to a grocery store, compared with the national average of 15 minutes. Although the difference is statistically significant, one might debate its practical significance. Whereas food deserts are defined on the basis of the lack of a supermarket within 1 mile of the residence, the report states that, on average, families participating in Supplemental Nutrition Assistance Program (SNAP) shopped at grocery stores located 4.9 miles away.
Other recent studies provide good reason to be skeptical about the assumption that residing in food deserts has a causal effect on obesity risk. Lee 230 performed a national-level longitudinal study and found that children residing in low-income and minority neighborhoods have greater access to fast-food outlets and convenience stores but also to large-scale supermarkets. More importantly, Lee 230 found that food outlet exposure has no independent relationship to child weight gain over time. An and Sturm 231 used data on children and adolescents from California and found no relationship between consuming a healthy diet and the food environment as measured by type of food outlets within 1.5 miles of the respondent's home or school. Using 15 years of longitudinal data from CARDIA Boone-Heinonen et al. found that supermarket availability near the home is unrelated to diet quality or produce intake232.
Evidence from randomized trials
Ideally, the question of whether eliminating food deserts could improve diets and reduce obesity risk would be settled via RCTs, but RCTs have not been done on this research topic. However, results are available from two quasi-experiments in the United Kingdom where a major supermarket opened in an underserved neighborhood. Both studies used a simple pre-post design and a pre-post comparison with a control group and evidence of a statistically significant increase in fruit and vegetable intake that could be attributed to the opening of the supermarket was lacking233,234. This leads to further skepticism about the role of food deserts in obesity risk. Of course, one might argue against generalizing results from studies in the United Kingdom to the U.S. Although similar quasi-experimental studies being launched in the U.S. (for example, PHRESH by the RAND Health Organization) should provide additional information down the road, the current scientific evidence does not support that food deserts independently increase obesity 235,236.
Presumption 1 - Regularly eating (versus skipping) breakfast is protective against obesity
Exposition of belief and support that the belief is widely held or stated
The consumption of breakfast is thought to result in fewer total calories consumed in a day. By causing satiety earlier in the day, the desire to consume food later would be sufficiently lower, possibly because of changes in hunger-related hormones such as ghrelin, leptin, and insulin.
“Breakfast is the most important meal of the day” is a widely touted maxim both in general and in relation to weight loss. A WebMD Feature titled “Lose Weight: Eat Breakfast” declares that “making breakfast a daily habit can help you lose weight – and keep it off,” citing opinions from dietitians, medical doctors, researchers, and a couple of observational studies237. The Academy of Nutrition and Dietetics also has several pages on Eatright.org with phrases such as “[Breakfast] can also help to promote a healthy weight and good behavior”238; “Want to trim your waist? Try eating breakfast!”239; and “With two thirds of Americans overweight, a morning meal may just be the best kept waist-trimming secret” 239. The Mayo Clinic also states the belief, with an article titled, “Why does eating a healthy breakfast help control weight?”240. The Mayo Clinic article was in turn cited by the Lance Armstrong Foundation, claiming, “If you skip breakfast while trying to cut calories and lose weight, you may actually be setting your weight loss back.”241. In response to a paper investigating the scientific merit of the presumed effect of breakfast and obesity, an article on WebMD responded, “Hogwash, I say… Do we really need to have people question if they should eat breakfast?… Perhaps the scientific evidence on breakfast and weight is mixed. I don't care242.”
Reasons to support the conjecture that the belief might be true
The “breaking the fast” concept is thought to move an individual from a hypometabolic, fasted state toward one of energy dependence. According to the hypothesis, eating shortly after waking and hypothetically early in the day is thought to give an individual all day to metabolize the energy, as opposed to consuming calories later (e.g., before sleep) when energy utilization may be low. Numerous observational studies have shown associations between breakfast consumption and lower BMI. For example, a review of 58 studies and 88 study groups found that the OR of being overweight or obese among those skipping breakfast compared to those consuming breakfast was 1.55 (95% CI: 1.46, 1.65; p<10-42) 243.
Further, in one-day studies, subjective and hormonal measurements of appetite differed between individuals who skipped breakfast and those who consumed breakfast, thus indicating an acute link between breakfast and appetite, and by extrapolation that this change in appetite may influence body weight244,245. Moreover, two studies that redistributed an isocaloric diet so calories were consumed predominantly in the morning or evening resulted in improved weight loss in the group whose calories were predominantly consumed at breakfast 246,247. However, these studies did not look specifically at the influence of breakfast alone.
Evidence directly supporting or refuting the belief
Beyond observational and single-meal studies, very little evidence directly supports or refutes the belief that breakfast eating affects weight. Shorter, single-meal, controlled studies have investigated the links between breakfast consumption and factors related to weight. Some evidence indicates that skipping breakfast results in partial compensation during subsequent meals244,245,248, although this is not necessarily associated with an increase in total energy intake245,249 and in some cases results in decreased total energy intake248,250,251. Importantly, in anobservational analysis of absolute versus relative breakfast calories, Schusdziarra et al. observed252 that increasing the amount of calories consumed at breakfast was associated with greater overall caloric intake in normal weight and obese subjects.
Evidence from randomized trials
Few randomized studies have directly investigated the effects of breakfast consumption on weight loss. In a randomized study of 791 Jamaican grade school children (mean age, 9.0 ± 1.2 years), half of whom were ≤ -1 SD of weight-for-age, children who were assigned to consume breakfast for one school year gained more weight than did those who were not assigned to consume breakfast, pooled across weight-for-age status253. In another randomized study of obese women, regular breakfast eaters lost more weight when they did not eat breakfast, but those who regularly skipped breakfast lost more weight when they did eat breakfast 254. There was no main effect of breakfast consumption. In studies in which energy was consumed in single daily meals over 1 or 3 weeks, individuals lost more weight consuming only breakfast relative to those consuming only dinner255. In a comparison of ready-to-eat cereals versus ready-to-eat cereals plus nutritional advice versus no breakfast, only the ready-to-eat cereals plus nutritional advice group showed greater weight loss compared with the no breakfast group; thus, the weight loss could not be attributed solely to the consumption of breakfast256. In summary, although there is fairly consistent observational evidence that breakfast consumption versus breakfast skipping is associated with a lower BMI, the available evidence from RCTs is insufficient to make causal claims about skipping breakfast itself independently affecting obesity.
Presumption 2: Eating close to bedtime contributes weight gain
Exposition of belief and support that the belief is widely held or stated
The directive to not eat before bed presumes that evening calories play a unique causative role in obesity. This concept has been eloquently expressed by Adelle Davis, who quipped, “Eat breakfast like a king, lunch like a prince, and dinner like a pauper” 257. This presumption takes one of two forms. In one, individuals are discouraged to eat after a specific time of day [e.g., 6 PM 257-260,261]. The other is that individuals should not eat a certain number of hours before going to sleep [e.g., 3 to 4 hours 258,262]. For some, the first form is a specific example of the second, in which it is assumed that people will be going to sleep within 3 to 4 hours of 6 to 7 PM. At least one diet suggests not eating after 5, although it goes beyond the current presumption in that it restricts all eating to between 9 AM and 5 PM 263.
Several web sites promote the idea that restricting the time one eats before going to sleep has benefits for weight. DietAdvices.com posted an article entitled, “Not eating after 6pm actually helps your diet,” and notes that “this weight loss idea is very widespread” 258. Livestrong.com has an article entitled, “How to Lose Weight By Not Eating After 6PM,” citing another source as saying that “food eaten after 6PM is metabolized too slowly”259. The Web site “Health is Wealth,” authored by a Nobel Laureate in Medicine and his colleague, has a post titled, “Eating after 8 PM may increase risk of obesity,” where they say the answer to the question, “Do you snack before bedtime?” may be critical to weight loss 264. An excerpt from Jillian Michael's book Master Your Metabolism posted on CanadianLiving.com states, “Eating more calories during the evening will pack more fat around your belly” 261. The popularity of this presumption is further supported by the number of questions and discussions on this topic in weight loss forums.
Reasons to support the conjecture that the belief might be true
Some studies have associated eating more in the evening with increased weight. For instance, late-shift workers reported eating their final meal later than day-shift workers and also had a greater self-reported weight gain since starting their job (4.2 vs. 0.9 kg, respectively) 265. In addition, those who become obese later in life tend to eat at night 266. Individuals with night eating syndrome, a disorder including a shift in caloric consumption to late in the day with eating episodes throughout the night and disrupted sleep, tend to be obese, although disrupted sleep itself is also associated with obesity 267. Furthermore, individuals who ate ≥33% of their daily calories between 5 PM and midnight were more likely to be overweight or obese, although this was not seen in a subgroup of individuals with objectively measured energy expenditure 268. In rodents, the timing of food availability influences weight gain: mice given 24-hour free access to either a high fat or control diet gained more weight than mice only given access to the same diets during the 12 hour active period 269. Finally, human participants who were told to eat more at dinner lost less weight than did those told to eat more at breakfast, with both groups being told to eat the same amount throughout the day 247.
Evidence directly supporting or refuting the belief
Few studies have looked specifically at calories consumed before going to sleep. In 2011, for instance, Kong and others conducted a secondary analysis of weight loss study data and showed no association between evening (9 PM to 12 AM) snacking as a binary variable and percentage weight loss 270. Most of the studies used to justify this presumption were confounded by other factors. In the shift-worker study mentioned above, for instance, the evening and night shifts were pooled 265. Shift-workers present two conceptual issues with this presumption: if using the first definition of this presumption (i.e., that one should not eat after a specified time of evening), then night-shift workers would be prohibited from eating during their entire work shift; conversely, if they are not to eat after a specified time before going to sleep, then they may not be allowed to eat in the morning, which would contradict temporal definitions of breakfast (and therefore the breakfast presumption). Therefore, we assert that this assumption must be limited to individuals with a fairly normal diurnal pattern because night-shift work comprises a complete chronobiological disruption. Similarly, night eating syndrome describes a clinical diagnosis, confounded with poor sleep, eating between sleep episodes, and a shift in calorie intake throughout the day, and can co-occur with anxiety and substance abuse 267.
Some proponents of this presumption conflate sleep quality with eating before bedtime 259, using evidence of associations between inadequate sleep and weight as evidence for not eating after 6 PM. In addition, associating evening energy intake with obesity includes wide timespans, such as the 7-hour definition of evening energy consumption described above 268. In turn, energy consumption in a broadly defined evening might violate the first expression of this presumption (i.e., the individuals may have consumed food more than 4 hours before bedbut after 6 PM) or the second (i.e., eating before 7 PM but less than 4 hours before bed) 268.
Evidence from randomized trials
Waller et al. in 2004 randomized individuals concerned about their evening snacking to either eat an evening snack of cereal and milk or to continue their dietary patterns 271. In the total sample, there were no differences in evening calories or weight, and in the self-reported compliant group, there was a significant decrease in evening calories with no significant decrease in weight (p=0.06 in favor of the cereal group). In another study of nighttime snacking, women were randomized to eat a snack either at 10 AM or 11 PM in a crossover design for 13 days with a 1-day room-calorimetry test day. No differences were seen in weight, total energy expenditure, or total energy intake, but there was a decrease in fat oxidation with non-significant increases in carbohydrate and protein expenditure in the nighttime snacking group 272. Two studies that redistributed an isocaloric diet so calories were consumed predominantly in the morning (6-9 AM) or evening (6-9 PM) resulted in greater weight in the evening calorie group 246,247. In an earlier, similar weight loss study in which 70% of calories were consumed between breakfast or lunch versus 70% between dinner and evening snack (2000-2030 h), those in the PM pattern lost less weight, but maintained more lean mass 273. However, these studies did not look exclusively at evening calorie intake.
Together, these studies demonstrate little direct evidence to support or refute a unique obesogenic effect of calories consumed in the evening. However, the alternate viewpoint that there is no influence of chronobiology on weight is also widely conclusively stated 274,275, despite insufficient evidence of no effect. If the association between eating before bed and weight is simply a function of increased calories, then the dietary advice to not eat before bed is just a heuristic to decrease energy consumption. However, whether changes in substrate utilization and the efficiency of energy absorption when eating close to bedtime impact weight gain have been insufficiently examined. As Bray and Young review 276, it is unclear whether obesity causes disruptions in daily rhythms, disrupting daily rhythms causes obesity, or both.
Presumption 3 - Eating more fruits and vegetables will lead to weight loss or less weight gain, regardless of whether one intentionally makes any other changes to one's behavior or environment
Exposition of belief and support that the belief is widely held or stated
Perhaps more than any of the other beliefs addressed here, careful exposition of exactly what the belief is and is not is critical. It is the belief that consuming more fruits and vegetables (F&V) will lead to weight loss or less weight gain regardless of whether one intentionally makes any other changes to one's behavior or environment that we are labeling a presumption. It is also important to note that we are examining increased consumption of F&V with regard to body weight, not other aspects of health or well-being.
Many web sites, magazines, news segments, infomercials, and organizations state the value of increasing F&V consumption to prevent and decrease overweight and obesity. The statements made are as varied as they are numerous. Some statements are responsible and point out that the idea is speculative and not necessarily supported by data. For example, the Produce for Better Health Organization states, “It is often assumed that increasing fruit and vegetable intake would almost automatically result in a decrease in the intake of other foods, such as unhealthy snacks. … [but] lack of compensation could lead to a higher calorie intake and contribute further to unnecessary weight gain” 277. Although in general one will not see explicit statements that eating more F&V will cause weight loss even if there is no decrease total energy intake, statements that imply this are common. Some typical statements are: “There is convincing evidence that fruits and vegetables decrease the risk for obesity” 278, “Increased fruit and vegetable consumption is considered a potential strategy to prevent weight gain or reduce obesity” 279, and “If you're confused about nutrition for weight loss, try to increase your fruit and vegetable intake – and you'll notice a real difference” 280.
Reasons to support the conjecture that the belief might be true
Because F&V contain metabolizable energy, if no other aspect of energy intake of expenditure is changed, adding more F&V to the diet should cause weight gain, not weight loss 281. However, if when eating more F&V, people spontaneously reduce their intake or other foods, particularly those higher in energy density, to a sufficient degree to more than compensate for the F&V consumed, then weight loss could result. Indeed, a number of studies have shown that reducing dietary energy density by substituting F&V for more energy-dense components of the diet is associated with lower daily energy intake 282,283. Such reductions in energy density along with the increase in fiber intake that can accompany an increase in F&V intake might enhance satiety and lower energy intake, thus promoting weight loss or less weight gain 282,283. The 2010 Dietary Guidelines committee reported that “strong and consistent evidence in adults indicates that dietary patterns that are relatively low in energy density improve weight loss and weight maintenance” 284. Furthermore, “there was moderately strong evidence from methodologically rigorous longitudinal cohort studies in children and adolescents to suggest that there is a positive association between dietary energy density and increased adiposity.” Thus, advising people to eat more F&V instead of foods higher in energy density coupled with other actions such that an overall reduction in the energy density of their diets occurs may facilitate weight management.
In 2004, two comprehensive reviews evaluated the evidence from both intervention and epidemiologic studies on the relationship between F&V consumption and body weight 282,285. Both reviews concluded that few studies had been designed to specifically address the relationship and that those available vary in methodology and provide inconsistent results. A 2014 review also found that the evidence for an association between vegetable intake and weight loss appeared inconclusive286.
Evidence directly supporting or refuting the belief
Observational studies have demonstrated an inverse association between F&V intake and weight status 287. For example, two large prospective studies from Europe found weak associations between F&V intake and weight gain 288,289. However, because observational studies alone cannot determine causality, we will focus on the effects of randomized interventions designed to increase F&V intake that have been conducted since the 2004 reviews 282,285.
Evidence from randomized trials
Short-term studies indicate that adding more vegetables to a meal has little impact on energy intake, whereas substituting them for more energy-dense meal components significantly reduces energy intake 283. Although dietary guidance from many health organizations stresses substitution, this approach has had little systematic investigation for weight loss. One 3-month randomized trial tested the impact of increased F&V consumption but also emphasized substituting F&V for high-fat, high-energy foods. This study did find that the intervention was associated with reduced dietary energy density and the change in vegetable intake was a significant correlate of the change in body weight 290.
Simply advising people to eat more F&V has not been found to reduce body weight. In two 8-week-long randomized studies 291,292, participants were provided with F&V that they were required to consume. Advice on how to consume this extra food was not provided and little is known about preparation methods, timing of intake, or what else was eaten. Participants showed no weight change 292 or even weight gain 291. Consuming the extra produce in liquid or dried forms appeared to facilitate overconsumption 291. In a recent year-long weight loss trial, advice to double portions of vegetables consumed was associated at 3 months with greater hunger satisfaction and an improvement in weight loss compared to an energy reduced healthy diet; however, the difference in weight loss was not sustained over the year293.
Two small RCTs were designed to compare advice to increase vegetables 294 or vegetables and fruit 295 with restrictive messages to limit high-fat foods. In both studies the low-fat group lost more weight over the course of the trial. In the trial that focused on vegetables only, the increase in intake was not sustained over the 18 months of observation294 and the energy density of the diet was not assessed. In the other trial, which lasted 6 months, it was found that an increase in F&V intake, a decrease in fat intake (although they were not instructed to eat less fat), and a decrease in dietary energy density were associated with weight loss 295. Other studies have found that advice to eat more F&V as part of a weight loss program facilitates weight loss related to the reduction in energy density 296,297.
In summary, there are few randomized experiments with body weight as an outcome and in which subjects were instructed to simply eat more F&V without any other changes being imposed. Perhaps the best direct test was offered by Whybrow et al.292, who found no significant effect. Thus, it appears that increased F&V intake can be part of a healthy weight loss diet if people are taught how to effectively substitute F&V for more energy-dense foods. There is little evidence that simply advising or requiring consumers to eat more, or providing them more F & V leads to weight loss or less weight gain.
Presumption 4 - Weight cycling (i.e., yo-yo dieting) increases the mortality rate
Exposition of belief and support that the belief is widely held or stated
Much is written and discussed in the general public and scientific literature suggesting that repetitive weight loss and weight regain (also called weight cycling or yo-yo dieting) increases morbidity and mortality. Fitness experts have warned of these purported dangers of yo-yo dieting and have suggested alternative methods to lose and keep weight off to avoid the harm of weight cycling. Under the heading “How Yo-Yo Dieting Can Kill You,” health and fitness experts at Ezinearticles.com warn, “even more importantly, is the elevated risk of heart disease, heart attack, and stroke among yo-yo dieters …. Numerous cardiologists have found … aggressive yo-yo diet[ers] are … prone to restricted blood flow to the heart …[which] could be an indication of blockages in the coronary arteries, which ultimately results in heart attack or stroke. Now, just because you are thin and haven't had to diet in 5 years, your cardiovascular system has still taken a beating and you could pay for it with your life. So, if you plan on dieting, really plan it and plan it well. Dieting is similar to owning a pet” 298. Considering the propensity to regain lost body weight, and the large population of overweight and obese individuals, this raises the question of whether it is advisable to encourage individuals to embark on a weight loss regimen in the first place. The messages are not unique to media outlets directed towards consumers. The FDA has made statements like “sustained, long-term weight loss in an obese or overweight person is a much more important benefit than short-term weight loss because long-term weight loss in these individuals reduces the risk of … mortality …, while short-term weight loss does not” 299. Such a statement suggests at best no benefit on mortality from short-term weight loss and regain. The vast majority of obese individuals who lose substantial weight will subsequently recover most of the lost weight, potentially repeating the pattern multiple times throughout life. First linked in the 1980s, a discussion of weight cycling in relation to mortality became prevalent in the scientific literature during the 1990s. Since then a pervasive view found in many media outlets encourages caution when embarking on weight loss regimens owing to the dangers of weight cycling. Although many of these reports focus on health and disease patterns rather than mortality directly, the topic is of high interest and is a concern for those hoping to improve their health through weight reduction 300. Catchy headlines decry the “dangerous cycle of yo-yo dieting” and point to potential metabolic and health implications 301. Celebrities, friends, and families are constant reminders of the ups and downs of body weight changes, with the typical dieter trying multiple diets during his or her lifetime. Examples of individuals who have a history of weight cycling and increased mortality seem to reinforce the notion of the negative consequences of yo-yo dieting 302-305.
Reasons to support the conjecture that the belief might be true
Although few would dispute the proposed link between excess body weight, particularly body fatness, and disease and mortality, there is also an observed association of low body weight with mortality, as well as acute weight loss. When comparing individuals who are weight stable with those who lose or gain weight, weight-stable individuals have a lower relative risk of chronic disease than do either losers or gainers 306. One might hypothesize that those who lose weight and subsequently regain weight have the combined risk of each weight change, which would be worse than either loss or gain alone. Furthermore, weight loss and regain cycles may evoke allostatic physiologic, hormonal or behavioral responses counterproductive to the desired body weight modification, with the overall allostatic load (cumulative wear and tear of physiological responses to stressors) of multiple weight cycling events increasing morbidity and mortality risk 307-310. Older studies demonstrated that weight loss is indeed associated with mortality 311. However, weight loss is not unusual for individuals suffering from occult disease and is a consistent marker of impending mortality in various species.
Evidence directly supporting or refuting the belief
Attributed primarily to the lack of clearly defined outcomes and the inability to randomize persons to levels of weight variability, recent epidemiological studies have challenged the contribution of weight cycling to mortality312,313. One of the primary motivations of these studies was to focus on weight cycling in terms of intentional weight loss (IWL) followed by regain (as opposed to more general weight variability). Intentionality of weight loss was assessed by the individual's response about expressing intent to lose weight. While intention to lose weight assumes some form of intervention by that individual to lose weight, unintentional weight loss is often associated with sickness and occult diseases and can occur spontaneously absent any obvious intervention or lifestyle change. Stevens et al.313 and Field et al. 312, both of which were based on a large sample size, offer no compelling evidence to suggest that weight cycling, defined in terms of intentional weight loss followed by regain, is associated with excess mortality risk.
Although some of these recent individual epidemiologic studies account for factors that may have confounded earlier findings314,315, they continue to be confined by the limits of observational research and methodological limitations. First, various definitions of weight cycling have been used across studies. Examples include loss and regain of 10 pounds or 5% to 10% changes in body weight, with efforts to categorize the number of weight cycling events, which must be determined during a given period of time. Given the rapid weight regain that can follow weight loss and dietary constraint relief (even if less than 1 year), it is questionable whether most cycles are even captured in the majority of these epidemiologic studies.
Furthermore, weight loss (at times quite rapid) often accompanies sickness or disease, influenced by alterations in the sense of smell and appetite, energy expenditure alterations and imbalances in homeostatic regulation of the body habitus. If present this unintentional body weight loss might be a predictor of underlying health-compromising conditions. Further complicating this relationship, it was previously observed that weight loss in an individual expressing intent to lose weight consists of both true intentional weight loss and unintentional weight loss (occult conditions), thus confounding the attribution of observed weight variability regarding intent 316. To our knowledge none of the existing epidemiologic studies have accounted for this statistical adjustment. Added to this, the recall and self-reported nature of the body weight data, even when including assessment of personal intent, is often a challenge for interpreting findings. Considered as a whole, the loss of body weight independent of intention might be a predictor of increased mortality 317,318. Similarly, age at which weight cycling occurs may be an important consideration when evaluating the question of weight cycling and excess mortality as compared to stable obesity in addition to any sex, race, and ethnic differences in the association 319 Hence, it will be important to support these findings through animal models in which adequate controls and true randomization can be implemented to improve estimations.
Evidence from randomized trials
Given the lack of long-term, RCTs of weight cycling in humans, and the improbability and questionable ethics of performing such a study, animal models may provide the most direct route of testing. One recent report moving in that direction used a small sample of male mice that were cycled between a low-fat diet and a high-fat diet every 4 weeks over the course of life. The authors reported no significant difference in survival of cycling mice compared with the continuously low-fat-fed controls and improved survival versus continuously high-fat-fed animals 320. Given the prevalence of weight cycling and the suggested association with increased morbidity or mortality risk, future research should carefully consider this in the application of larger-scale randomized controlled designs with animal models, particularly given the known beneficial health and longevity effects of alternate day fasting (or every other day feeding) in rodents which could be considered a chronic model of weight cycling given the percentage body weight changes that are observed 321-323.
Considering the present published data and studies currently underway using model organisms, bearing in mind the difficulties and limitations of exploring this association in human populations, there is inconsistent evidence to present a compelling case in support of weight cycling increasing mortality risk in the general population. Thus, although weight regain following successful weight loss remains one of the most challenging aspects of body weight regulation, there appear to be insufficient reasons to dissuade individuals from multiple attempts to lose weight given the critical impact of obesity on disease risk and mortality.
Presumption 5 - Snacking contributes to weight gain and obesity
Exposition of belief and support that the belief is widely held or stated
Adding snacks between meals is thought to simply add to total energy intake that is not appropriately compensated for at subsequent meals, thereby leading to a positive energy balance and weight gain. Often, the word snack evokes imagery of calorically dense, micronutrient poor foods that are sometimes described as ‘junk food’ or ‘empty calories;’ this imagery is reinforced by the predominance of such foods in image searches in popular search engines (Google and Bing images searched for ‘snack’ on 2013 DEC 12). Several media sources to which consumers turn regularly for dietary advice have pointed to snacking as a major culprit in the obesity epidemic. A WebMD video, for example, suggests that “snack attacks” lead to loss of appetite control and can promote the ingestion of an additional 700 to 1000 kcal/d 324. Excessive snacking has also been reported as a leading cause of the rising rates of childhood obesity and other childhood health problems according to Livestrong.com 325. A recent Yahoo Health title claims “Snacking, not portion size, largely driving U.S. overeating” 326. Many other health-related organizations, programs, web sites, and blogs 324,325,327,328 contend that snacking can contribute to weight gain, especially if the snacks consumed are high in energy density or low in nutritional value.
Reasons to support the conjecture that the belief might be true
The role of snacking and eating frequency among adolescents in relation to weight has been considered for decades,329 and subsequent research demonstrated a higher snack meal consumption frequency in obese women 330. The proportion of daily energy coming from snacking has increased in all adult age groups in the U.S. from 1977 to 2006, with both energy density and portion sizes of snacks increasing 331. It was estimated that the number of eating occasions in U.S. adults contributed an increase of 39 kcal/d to caloric intake yearly between 1994-1998 and 2003-2006 332. Yet, energy consumption at subsequent meals is not always reduced or compensated 333 which could lead to weight gain 334,335.
Evidence directly supporting or refuting the belief
Studying the influence of snacking on obesity can be difficult because of various definitions of what constitutes snacking, such as conflating snacking and eating frequency. For instance, Mesas et al. 336 present disparate definitions of snacking across several studies, including such constructs as eating between meals or consuming small portions of food, packaged food, or specific foods such as sweets and desserts. An earlier review considered snacks as unstructured eating episodes separate from the three standard meals 337. Other authors have also noted the challenges in measuring snacking behaviors in free-living populations in dietary databases 331,332. A review of the observational evidence on the relation between snacking behaviors and obesity among children and adolescents, which included mostly cross-sectional studies, likewise concluded that the results were conflicting and that more prospective studies were needed 338.
Even so, prospective studies have also yielded equivocal results. In a Spanish cohort of middle-aged men and women, self-reported usual snackers had a higher mean weight gain per year and were at a higher risk of substantial weight gain (OR: 1.66; 95% CI: 1.17, 2.35) than were those who did not usually snack 339. Similarly, more frequent snacking was associated with a higher BMI in a Finnish cohort 340. A case control study of obese children in Japan showed that children who regularly versus irregularly consumed snacks had a lower odds of obesity341, and a longitudinal study published in 2012 reported that a lower frequency of snacking behaviors was related to greater increases in BMI over time 342. Kong et al. showed no statistically significant associations in percent weight loss by snacking frequency in a secondary analysis of a diet and exercise RCT.343
Evidence from randomized trials
Palmer and others in 2009 reviewed the association between eating frequency and body weight 335, from which we identified 6 experiments that looked directly at snacking and weight271,292,330,344-346. In Table 1, we include those 6 studies, plus three more that were reported after their review 347-349. Studies were as short as 9 days344 to as long as 1 year 330, and include several different study designs, snacking patterns, and types of snacks. In none of these studies was a difference detected between the snacking versus the non-snacking study arms with respect to measurements of obesity including weight, weight change, BMI, body fat percentage, waist circumference, or waist to hip ratio.
Table 1.
RCTs investigating snacking and weight.
| Study | Design | Results |
|---|---|---|
| Bertéus Forslund 2008 | 1 year two arm trial of 3 meals/day or 3 meals + 3 snacks/day | No effect on weight loss |
| Johnstone 2000 | 9 day cross-over | No effect on body weight |
| Poston 2005 | 24 week CR diet for weight-loss; snacker and non-snackers were assigned to meal replacement or meal replacement plus snacks | No effect on weight loss |
| Tey 2011 | Hazelnuts, chocolate, potato crisps, or no snacks (1100 kJ/d) for 12 weeks to be eaten daily | No effect on body weight, BMI, fat mass, body fat%, waist fat %, or waist circumference |
| Vander Wal 2006 | 8 weeks standardized meals with or without post dinner snack | No effect on body weight, BMI, BF%, or WC |
| Viskaal van Dongen 2010 | High or low energy density snacks with or apart from meals (25% of energy) for 8 weeks | No effect on body weight or changes in body fat |
| Waller 2004 | 4 weeks cereal snack at least 90 minutes after dinner. | No significant effect on weight change for compliant and total samples (p>0.05) |
| Whybrow 2007 | Partial cross-over, 14 d, high fat, high carb, or mixed snack at 0, 1.5, or 3.0 MJ/d | No effect on weight change |
| Zaveri 2009 | Almond or cereal bar snack or neither for 12 weeks | No effect on body weight, BMI, BF%, WC, or waist:hip |
Presumption 6– The built environment, in terms of sidewalks and park availability, influences obesity
Exposition of belief and support that the belief is widely held or stated
Modifying the built environment to be more conducive to active lifestyles is widely advocated as a way to reduce obesity in communities350. The Department of Health and Human Services' Guide to Community Preventive Services identifies community designs in which residents can walk or bicycle to nearby destinations as effective ways of promoting overall health351. The Institute of Medicine has stated that improving the walkability of neighborhoods and increasing access to recreation facilities are essential strategies for preventing childhood obesity352. A recent headline in the New York Daily News read, “Fast-food Zoning Limits and More Walkable Neighborhoods Key to Fighting Obesity”353.
Reasons to support the conjecture that the belief might be true
Sedentary lifestyles are not simply attributed to personal choice but also the built environment, which refers broadly to the collective availability of green spaces, sidewalks, parks, trails, recreational facilities, traffic safety, neighborhood safety, and other neighborhood characteristics that may promote recreational physical activity as well as functional physical activity, such as active transport to work, school, or errands 354-359. Evidence suggests that physical-activity-friendly facilities are much less common in predominantly minority and economically disadvantaged neighborhoods (relative to white, or more affluent neighborhoods) where there is also greater obesity prevalence360-364. Reports have emphasized that sprawl, or the number of people per unit of developed land, is associated with greater commuting times, less physical activity, and lesser accessibility to physical activity resources 360,365. Together, these ideas lead to the conjecture that disparities in the built environment directly contribute to disparities in physical activity and thereby to disparities in obesity risk.
The major empirical support for this hypothesis stems from observational evidence. Glass et al. found that adult residents of neighborhoods ranking high in psychosocial hazards (measured by using indicators of social disorganization, public safety, physical disorder, and economic deprivation) had higher BMI, less physical activity, and less healthy diets than did their peers in neighborhoods ranking lower in psychosocial hazards366. A systematic review of 169 articles found that most studies showed a positive association between built environments that promoted physical activity with increased physical activity and, to a lesser extent, a lower obesity risk 367.
Evidence directly supporting or refuting the belief
Virtually all of the studies on the built environment and obesity risk have been observational, most are cross-sectional, which limits the capacity for causal inference367,368. Yet, several investigators overstate study findings and use causal language in their conclusions. For example, review of a set of studies stated that it was “evident” that the built environment “impacted” obesogenic behaviors in children and that “interventions that are designed to provide safe, walkable neighborhoods with access to necessary destinations will be effective in combating the epidemic of obesity” 369. Conversely, the authors of a recent report that showed a small, protective (albeit nonsignificant) effect of green space for those living in the greenest areas, with markers of total physical activity not attenuating the observed associations. The authors concluded that, “better evidence for the utility of greenspace in the prevention of weight gain is required before greenspace interventions are developed370.”
Evidence from randomized trials, or other intervention designs
The biggest challenge for researchers is to employ study designs that address the underlying confounders of selection into more physical-activity-friendly communities. Whereas experimental study designs with randomization to intervention and control groups would ideal, such studies are difficult to perform and certain observational designs (e.g., quasi-experimental) can also help to advance the science. For example, two studies used a match-control design at the neighborhood level to examine physical activity among children, in which one neighborhood received access to a new playground or a trail and the other did not371,372. Beyond the equivocal results particularly across age groups, the utility and validity of inclusion of self-reported screen time must be questioned (see presumption 7). Notably, an increase in physical activity does not necessarily lead to a decrease in obesity risk, as caloric compensation (i.e., increased energy intake) due to increased energy expenditure can prevent weight loss. Given the paucity of either RCTs or quasi-experimental studies alongside the weak observational data, the hypothesis regarding the built environment and obesity is a presumption.
Presumption 7: Reducing screen time will decrease obesity in children
Exposition of belief and support that the belief is widely held or stated
In 1984, the American Academy of Pediatrics (AAP) issued a statement regarding the potential of television viewing, a screen-time behavior, to promote obesity in children373. In 1985, a study published by Dietz and Gortmaker, examined the relationship between television viewing and weight status in children and adolescents by using data from the National Health Examination Survey374. The results indicated that television viewing was positively related to the prevalence of obesity, and a dose-response relationship between the variables was proposed in response to this evidence. In 1990, the AAP issued its first recommendations regarding limiting television viewing time in children to no more than 2 hours per day with headlines reading “The Small Screen Looms Large in the Obesity Epidemic” 375.
Since 1985, observational research has continued to show a positive relationship between television viewing and weight status in children 376-378. As changes in leisure-time activities have occurred in children, investigations began to combine television watching with video game playing and computer use, termed collectively as “screen time” or “electronic media use,” 379-381. Screen time has increased among youth, with reports now indicating that children engage in approximately 7 hours per day of screen time (e.g., television, videos, DVDs, video games, and/or computers) 382. In 2001, the AAP broadened its television viewing recommendations so that other screen-based activities, such as video game playing, were included in the recommendations383, and the most current guidelines, published in 2011, recommended limiting non-educational screen time to less than 2 hours per day 384. Limiting screen time is now widely recommended as a behavior to target for preventing and treating pediatric obesity. Assessment of and anticipatory guidance for screen time, as outlined by Bright Futures 385, is encouraged to be part of every well-child visit owing to its relationship to obesity, and limiting screen time is part of a list of recommended interventions in the AAP-adopted guidelines for treating pediatric obesity in primary care settings386.
Reasons to support the conjecture that the belief might be true
When the relationship between television viewing and pediatric obesity was reported in 1985, the initial mechanisms proposed for the relationship involved both sides of the energy balance equation374. Simply speaking, sitting in front of a screen is sedentary behavior; sedentary behavior contributes to obesity; thus, screen time equates to obesity. Further, it has been suggested that watching TV exposes one to cues, via commercials advertising energy-dense foods and other food cues in television programs, that may prompt eating374,387-389. Also, when television watching is repeatedly paired with eating, television viewing may become a cue, even when hunger is not present, for eating387-389. Eating when watching television may be especially problematic because television viewing may also distract awareness of how much is being eaten or may inhibit signals of satiation. Indeed, basic eating research has shown that eating while watching TV slows down or disrupts the development of habituation to food cues390, a proposed mechanism by which satiation occurs. On the energy expenditure side, television watching may displace time from engaging in activities of higher energy expenditure, particularly MVPA, or even lower metabolic rate391. Thus, highlevels of television watching may result in lower overall energy expenditure, contributing to excess weight gain374,387-389,392.
More recently, a third mechanism has been offered regarding the relationship between screen time and obesity in children384. Greater amounts of screen time, particularly in the evening, may disturb sleep. Engaging in a screen time activity may delay bedtime or may make falling asleep more challenging393. As observational research has also reported a relationship between shorter sleep time and obesity in children394, if greater screen time produces shorter sleep, then shorter sleep, through factors that are believed to increase energy intake and decrease energy expenditure, could increase weight status in children393. Little research has been conducted examining the pathway of screen time, sleep, and obesity.
Evidence directly supporting or refuting the belief
For the proposed mechanisms regarding the relationship between screen time and pediatric obesity, observational research has found small to moderate positive associations between screen time and unhealthy dietary behaviors (e.g., lower F&V consumption; higher intake of energy-dense snacks, drinks, and fast food; and greater overall energy intake)395. However, when cross-sectional laboratory studies have examined overall intake in children when consuming a meal or snack while watching television or while sitting quietly, the results have not consistently found that eating while watching television increases intake 396-398.
Observational research examining the relationship between physical activity and screen time in children has found that these behaviors may be independent of one another 379,389,399,400. In the two experiments referenced above, 387,392 both studies found decreases in physical activity374,389; when screen time was decreased, however, only one trial found that physical activity increased in nonobese youth 392. Thus, there is little research to suggest that decreasing screen time leads to increased physical activity in and of itself.
Evidence from randomized trials
Intermediary Endpoints
Experimental studies in children investigating the effect on consumption of watching food advertisements vs. watching nonfood advertisements have consistently found greater consumption when food advertisements are watched401-403. Two experimental field trials manipulated screen time in children by increasing and decreasing the amount of screen time engaged in by 25% to 50% from baseline levels during 3-week periods and examined self-reported dietary intake387,388. One trial found that during the decreased screen time phase adolescents decreased their energy intake, but there was no change in energy intake during the increase phase388. The second trial found that school-aged children did not change their energy intake when screen time was decreased but increased their energy intake when screen time was increased 387. Thus, whereas observational studies consistently show a relationship between screen time and dietary intake, experimental findings are equivocal. This discrepancy may be a consequence of the differing ways screen time, particularly television viewing, may influence eating or residual confounding.
RCTS with weight loss as an endpoint
Two systematic reviews examining the impact of interventions designed to reduce screen time in youth on weight status have been published. Wahi and colleagues404 included 13 studies in their review of RCTs, whereas Schmidt and colleagues405 review included 36 RCTs, four quasi-experimental trials, five pre-post design studies, one matched comparison trial, and one nonrandom controlled study. The screen-based interventions included in both reviews contained interventions that focused solely on reducing screen time as well as interventions that reduced screen time along with changes in other behaviors such as diet and physical activity. Additionally, some of the studies were testing obesity treatment interventions, whereas others were examining obesity prevention programs. Both reviews reported mixed outcomes: a nonsignificant effect between the intervention and control studies for reducing screen time was reported in one review404, whereas the other review reported that less than half of the included studies reported significant reductions in screen time in the screen time interventions compared with control comparisons405. When only RCTs that focused on reducing screen time were examined, results were strengthened, such that slightly more than half of these trials demonstrated greater screen time reductions in the intervention compared to control 404,405.
Regarding anthropometric outcomes, the meta-analysis by Wahi and colleagues404 reported a nonsignificant effect of interventions to reduce screen time on BMI. In the review by Schmidt and colleagues405, for the RCTs that showed a greater reduction in screen time in the intervention than in the control comparison and also measured anthropometric outcomes, 45.5% showed significant reductions in anthropometrics in the intervention compared with the control condition. When only trials with interventions that focused solely on reducing screen time were examined, for those RCTs that did significantly reduce screen time, Wahi and colleagues reported that 50% significantly reduced anthropometric variables and Schmidt and colleagues reported that 60% significantly did so.
In summary, although screen time has been defined as three behaviors—television watching, video game playing, and computer use—very little research has been conducted regarding the independent effects of video game playing and computer use on pediatric obesity or the proposed mechanisms between the relationship of these screen time behaviors and obesity. Television viewing may impact consumption via exposure to food cues on television, as noted, but “screen time” as such may be less related to physical activity than initially theorized. Most RCTs examining the relationship between screen time reduction and anthropometrics in children were not able to successfully reduce screen time and, in those that did reduce screen time, changes in anthropometrics were inconsistent. Thus, areas for future research include enhancing our understanding of the independent effect of video game playing or computer use on energy intake, energy expenditure, and anthropometrics in children. Research needs to more clearly examine the influence of each screen time behavior separately on the outcomes, rather than classifying them as one behavior. This will help to ascertain whether video game playing and computer use impact obesity and if altering them would help with reducing obesity in children. Although research suggests that television watching influences consumption, an enhanced understanding of the mechanisms of this relationship would be useful. Finally, interventions that are more effective at reducing screen time need to be developed to better understand whether reducing screen time can potentially reduce obesity in children.
Presumption 8: Decreasing the portion sizes served leads to less food intake without people being told to reduce their food intake or presumably intending to do so, even when the total food available is not limited
Exposition of belief and support that the belief is widely held or stated
Over an extended period of time, in the absence of any compensating factors, decreasing portion sizes of food would decrease calorie intake and therefore cause weight loss or less weight gain 406. Health professionals and dieting websites (e.g. 407,408) presume that people will inadvertently consume more calories when given larger portions regardless of their hunger 409. As a result, it is believed that smaller portions will lead them to eat less than they otherwise would, and therefore be a successful dietary weight-loss strategy.
Reasons to support the conjecture that the belief might be true
People infer how much is appropriate to eat from the size of the portion of food they are served 409. There is considerable evidence that smaller serving sizes significantly decrease consumption 410-413. These studies have shown that the decrease in energy intake due to downsizing can often reach 30% change in calorie intake without generating within-meal calorie consumption 414. For instance, smaller portions in restaurants have cut intake and maintained satisfaction 415, and smaller portions of packaged food – such as 100-calorie packs – have been shown to do the same 416. Smaller packages, smaller restaurant portions, and smaller dinnerware all have one thing in common. They all perceptually suggest to us that it is more appropriate, typical, reasonable, and normal to eat less food than larger versions 417.
It was recently found that the 104 calorie decrease in McDonald's Happy Meal did not result in any corresponding within-meal increases in the selection of more caloric options or additional purchases 417. Experimentally, Rolls, Roe, and Meengs (2006)418 found no differences in hunger when people were served 100% or 50% of usual servings, although their consumption had decreased by 16% and 26%, respectively. Indeed, a recent meta-analysis of 67 studies estimates that consumption quantity can decrease by 22% when serving size is cut in half but total intake is still unconstrained 419.
Reasons for Skepticism
Interestingly, one key reason as to why decreased portion sizes appear to unknowingly decrease intake is because individuals – even experts, such as nutrition science professors and registered dietitians – are frequently not aware they were served or ate a decreased size 409. Yet if people were aware they were being served less, as they conventionally would be with long-term use in non-laboratory settings, they might compensate by requesting a second portion or by eating extra food. Some of the most effective portion size studies also simultaneously decreased the size of the plate on which the food was served, thereby giving the illusion that one was still being served a full portion of food because in both cases they had a full plate of food. Hence, it may be important that people not perceive their portion size to be smaller than a regular-size portion. For instance, even simply changing the label of a 8-oz serving from regular to half-size lead patrons to eat 41% more 409,420.
In addition, although decreasing portion sizes can appear to robustly decrease same-meal or within-meal food intake without other forms of calorie compensation, there are two criticisms that may limit its effectiveness to promote unknowing weight loss over an extended period of time. First, many of the initial portion size studies appear to have used smaller portions that were binding or artificially limiting. That is, a sizable percentage of participants may have eaten less, simply because they ran out of food. In such cases, the small portion condition would have artificially – not naturally – limited how much they ate. Under normal circumstances, they would have responded by either serving themselves more or by compensating later on by eating extra food. Most of these studies did not examine compensation except for two that showed that a reduced calorie meal replacement did not trigger calorie compensation over a 10-day and an 11-day period 406,421, and even these did not study expenditure or weight422.
Perhaps the most essential limitation of all the supportive data is that they pertain only to effects on one component of energy balance (food intake) for very short periods of time (typically one day or less). Our opening phrases pertaining to this belief were “Over an extended period of time, in the absence of any compensating eating factors…”. Thus, any data pertinent to this belief must examine an indicator on integrated energy balance (e.g., body weight or composition).
Evidence from randomized controlled trials with weight outcomes
Whereas some RCTs have suggested that decreasing food portions can lead to weight loss 423, participants were aware that weight loss was an objective of the study. Since this presumption examines occasions when people are unaware of portions being reduced or of weight loss being an objective, such RCTs are not included as evidence.
Since an inclusion criterion is that individuals be unaware that they are reducing their portions, one way this has been accomplished in the past is by varying the size of dinnerware. There is wide-spread evidence that one robust driver of self-served portion sizes is the size of the plate upon which the food is served. Within a normal American plate size range of 24-30 cm, the smaller plates have been shown to consistently – and unknowingly – decrease how much food one serves by up to 22% 417,424. (Importantly, when plates become too small the cease to have this intended effect, partly because of refills and reactance)425,426. Randomized trials have been conducted with dinnerware size, and this could provide an imperfect but conservative proxy for portion size. That is, if smaller plates are randomly given to participants and if they subsequently lose weight, we could assume the mediating explanation was because these plates led them to serve smaller portions.
One such NIH trial 427 investigated the impact of decreased plate size on the food intake families. Although the pilot study did not measure weight, it showed that decreasing plate sizes remarkably decreased intake. Small plates decreased average meat intake by 34% for adults and 5% for children, and smaller bowls also significantly decreased both cereal and soup intake for both groups.
A second NIH study investigated 216 households in Syracuse, New York, who had been randomly assigned to receive either 25 or 30.5-cm plates 428. Average weight loss among those receiving smaller plates equaled 2.2 pounds (p<0.05), whereas those using the larger dishes experienced a 0.3 pound increase in weight (p>0.05) which was small and statistically insignificant. Overall, participants using the smaller plates lost nearly 2.5 more pounds (p<0.05) and experienced a 1.2% (p<0.05) reduction in BMI compared to individuals using the larger plates. Similarly, in 2007, Pederson et al conducted a six-month trial with Type 2 diabetics who were given portion-controlling dinner plates and cereal bowls. Although participants were aware of the manipulation, those receiving smaller dishes lost 4.6 pounds (p<0.05) compared to a decrease of 0.2 pounds (p>0.05). As a result, participants using the smaller dishes lost 4.4 more pounds (p<0.05) than those in the control group. Analyses in both studies complied with the intention-to-treat methodology.
Conclusion
Although there are many short-term studies that suggest reduced portion size can lead to an unknowing reduction in intake, there is much less evidence as to how sustainable such effects might be and whether they might generate unexpected compensation in another form. Benton summarizes our current state of knowledge well. “There is a need to establish that varying portion size does not lead to compensatory changes at either a psychological or physiological level. Although the portion size of many food items have increased, and laboratory studies find that more is eaten when more is on offer, this does not establish the real world importance of the phenomenon. …There is an urgent need for intervention studies that show that changing portion sizes reduce weight in those consuming a freely chosen diet, rather than in those in a laboratory situation that prescribes or limits the nature of consumption. Without such data we cannot be sure that the response to portion size is more than a laboratory phenomenon of limited practical significance.”422.
Presumption 9 - Participation in family mealtimes reduces obesity
Exposition of belief and support that the belief is widely held or stated
Partaking in meals with the family on a regular basis may help to reduce obesity risk in children and adolescents because it gives parents scope to provide their children with healthy fare and monitor their intake of calorically dense foods, develop healthy eating habits that might persist over the long term, and provide better family communications that can be protective against depression and psychosocial problems that are linked to obesity risk 429. The American Medical Association Expert Committee on the Assessment, Prevention, and Treatment of Child and Adolescent Overweight and Obesity included “encourage family meals on most, and preferably all, days of the week” as a key component of their four-stage approach to treat childhood obesity.
Reasons to support the conjecture that the belief might be true
Current literature finds that the frequency of family meals is positively related to overall diet quality and negatively related to consumption of fried food, trans-fat and saturated fat, and soda430,431. Moreover, adolescents express greater confidence in making healthful food choices when eating with families than otherwise. Further, family meal frequency is negatively related with the prevalence of eating disorders among adolescent females and with the prevalence of low self-esteem, depressive symptoms, and suicidal ideation among adolescents431-433.
Evidence directly supporting or refuting the belief
Among preschool children, exposure to at least two of three regular household routines—family dinners more than five nights per week, adequate sleep on weeknights, and limited screen-time—was associated with lower odds of obesity434. High frequency of family meals is also associated with lower risk of obesity among adolescents 435-437. A meta-analysis of 17 studies from six countries found that children and adolescents partaking in family meals three or more times per week are more likely to be in a normal weight range, have healthier dietary patterns, and be less likely to engage in disordered eating than are those eating fewer than three family meals438. However, all of these studies are observational and subject to confounding from unmeasured parental, child, and other contextual factors surrounding family mealtimes and obesity.
Evidence from randomized trials
An RCT currently underway is evaluating the effectiveness of promoting three household routines (family meals, adequate sleep, and limiting screen time) in reducing childhood obesity risk439, and did not find an effect of family meals. Until the evidence from that and other similarly designed studies are forthcoming, we believe it is appropriate to classify this as a presumption.
Presumption 10 – Drinking more water will reduce energy intake and will lead to weight loss or less weight gain, regardless of whether one intentionally makes any other changes to one's behavior or environment
Exposition of belief and support that the belief is widely held or stated
Adequate hydration is essential for health, and there is a common belief that water is a better choice for weight management than are caloric beverages 440,441. The belief addressed here is whether water consumption reduces hunger, enhances satiety, reduces energy intake, and thus promotes weight loss or prevents weight gain. Drinking extra water to enhance weight loss is a popular diet strategy in the media. Messages in the media identify water as the “miracle” 442, “or secret weapon” 443 in weight loss. In a national survey of weight-control practices, “drinking plenty of water” was reported to be one of the most consistently used practices for both weight loss and weight loss maintenance 444. A recent report from the Robert Wood Johnson Foundation445 states that “water consumption is associated with a number of health benefits including preventing obesity.” A team of experts from Australia gives physicians this specific advice: “Drinking 500 ml of water 30 minutes before each meal can be used in conjunction with a hypocaloric diet to lead to greater weight loss in overweight or obese middle-aged and older adults” 446.
Reasons to support the conjecture that the belief might be true
Although scientific evidence is lacking, the reason most commonly ascribed to the belief is that water has no calories and fills the stomach, initiating proprioceptors in the stomach that trigger satiety messages to the brain thus reducing caloric intake. Another purported reason is centered on displacement of caloric beverages, leading to lower overall energy intake.
Evidence directly supporting or refuting the belief
Dieters are sometimes told that they are eating because they are thirsty and not hungry and that if they drink water their hunger will diminish. It is unlikely however, that thirst would be mistaken for hunger. Thirst signals a need for fluid and is sensed as a dry, unpleasant-tasting mouth 447. Hunger signals a need for nutrients and is sensed primarily as stomach rumblings and growling. Furthermore, in a controlled study, when people were fluid-deprived and thirsty, they voluntarily reduced their food intake such that the greater their thirst, the greater the reduction in food intake 448. Although it is unlikely that thirst would be interpreted as hunger, it is nonetheless possible that drinking water could help fill people up and reduce hunger, which could reduce energy intake.
To determine whether water consumption enhances satiety, studies in adults have compared the effects on meal energy intake of drinking water, noncaloric beverages, or no beverage 449. When beverage consumption was varied within a meal, one early study found an effect of water on satiety. Two extra glasses of water consumed at breakfast reduced ratings of hunger and enhanced satiety during the meal but the effect did not persist and the effect on intake was not measured 450. Since that early study, however, increased water consumption during a meal has not been found to influence hunger ratings or energy consumed at the meal 451-453. Although such studies were conducted in a homogeneous population of young, non-dieting men and women, the parameters of the studies varied. For example, the required amounts of water and the comparison beverages (diet soda, noncaloric lemonade) varied, as did the foods offered at the meal. Thus, in these controlled studies there was little evidence that water consumed with a meal has effects on meal intake that differ from those of other noncaloric beverages or from having no beverage to consume.
The results are more variable in studies in which the effects on satiety of increased water consumption before a meal were assessed. In a thorough study conducted in young (21 to 39 years of age), non-dieting males, food intake was similar at lunch 30 or 60 minutes after drinking 8 or 16 ounces of water, a noncaloric lemonade, or no beverage 453. In another study of obese individuals aged 20 to 50 years, intake of 500 ml of water or noncaloric cola had similar effects on ratings of hunger, satiety, and fullness as well as measured ghrelin and incretins, over the next four hours. When food was offered after that delay, energy intake did not differ between the water and noncaloric cola 454. The lack of an effect of pre-meal water intake on subsequent food intake in young individuals was reinforced by a study in which no differences in lunch intake were found in 21- to 35-year-olds when a 500-ml water preload was compared with no preload 455. However, in that same study, the water preload did significantly reduce lunch energy intake in participants 60 to 80 years of age. The same research group went on to show a similar effect at breakfast in obese participants aged 55 to 75 years 456.
Overall, there is little evidence that drinking water with a meal or before a meal enhances satiety or reduces energy intake more than other noncaloric beverages or no beverage. If water were to influence satiety, it could be through gastrointestinal effects such as distension or transit time. However, when water is consumed as a beverage, it empties rapidly from the stomach and has a relatively small effect on satiety compared with water incorporated into food 457,458. These mechanistic studies have been conducted in younger individuals and it is possible that changes in the gastric handling of water known to occur with age could influence satiety459.
In children, the majority of the reports on the benefits of water intake on weight have been derived from studies that contend that increased water intake reduced intake of caloric beverages. Although increased caloric beverage consumption is often associated with an increase in total energy intake, an association with BMI is seldom observed. Further, whereas studies demonstrate that interventions such as increasing water fountains or giving water bottles increased reported water intake, weight effects have not been observed 460.
Evidence from randomized trials
A recent systematic review evaluated studies related to water consumption and body weight 460. Among the RCTs in that review, a secondary analysis of diet recall data from the A to Z Trial designed to test four popular diets, reported increases in drinking water were associated with weight and fat loss over the year-long intervention 461. Of note, the most significant weight loss was in the group following the Atkins diet, which is known to induce dehydration, and the selected sample included only women who reported drinking <1 L/day of water at baseline.
Only one RCT designed to test the effects on weight loss of consuming extra water has been conducted 440,455. Older adults were tested (N = 48; 55-75 years of age), because this research group found previously that only older individuals reduce their meal energy intake after drinking 500 mL of water 455,456. The participants who were overweight or obese were randomized to a reduced-calorie diet alone or with the instruction to drink 500 mL of water before each meal for 12 weeks. Both groups lost weight and the rate of decline in weight loss was greater in the pre-meal water group. Also, this group lost more fat mass. However, there were no significant differences between the treatments in percentage of initial weight lost or in the reduction in percentage body fat, BMI, or waist circumference 462.
Following the 12 weeks on the weight loss diet 462 the participants were invited to continue in their treatment group to determine the effects of pre-meal water on weight loss maintenance over the next 12 months. Drinking water did not affect laboratory measures of body weight change, but there was a significant improvement in weight loss maintenance in the pre-meal water group with daily self-reported weights (-0.67 vs. +1.00 kg) 463. Thus, the data from these 42 older individuals represent the only direct test of the effect of drinking extra water on body weight. The intervention was likely underpowered to show clear effects, although some of the analyses show some support for the presumption that drinking extra water affects body weight. However, the experimental design does not allow us to determine if there is anything special about water. The pre-meal water group was actively adding an extra activity (drinking water) to their weight management program while the control group had no extra activity. It also seems likely that the water group had heard that drinking water aids weight loss, because this is a topic frequently in the media. The belief that drinking water before each meal can affect hunger and food intake could be at least part of an explanation for any effects seen, but no studies have tested this.
Where do we go from here?
In Table 2, we reiterate the myths and presumptions addressed herein, and provide suggestions for moving forward. The recommendations can be summarized as “abandon,” “move the needle as far as we can towards measuring outcomes as methodologically rigorously as possible,” or “conduct the definitive study.” We generally suggest not studying the myths further, as it is not likely that additional studies will meaningfully add to our understanding. Presumptions, by definition, are insufficiently or incompletely studied and recommendations for moving forward vary depending on the potential for meaningful investigation. If conducting the definitive study is not feasible, creative and probative studies should be conducted that increase our understanding of the question. It must be acknowledged, though, that after completing multiple non-definitive studies we are still left with the possibility of a pervasive confounder464, and thus the assertion may become at best a strongly supported or refuted presumption. For example, a long-term RCT on weight cycling and mortality is not likely to be conducted, and therefore objective anthropometric measurements over time in humans and well-designed animal models may help us better inform the presumption, but we may never get a definitive answer. For most of the presumptions, however, definitive study designs exist that are eminently doable, obviating the need to extrapolate from observational or intermediate-endpoint studies 465. Studies should address questions that have not already been sufficiently answered, and be designed in such a way that they meaningfully advance our scientific knowledge about the question 243. Conducting and reporting mediocre, redundant, or non-probative science is an inefficient use of time, funds, and political capital. A quick litmus test of the probative value of a study may be to ask, “Does this (proposed) study really have the power to advance our knowledge about what works rather than merely raising attention or emotion?”
| Statement | Category | Recommendation |
|---|---|---|
| Losing weight quickly will predispose to greater weight regain relative to losing weight more slowly | Myth | Abandon and move on. |
| Setting realistic weight loss goals in obesity treatment is important because otherwise patients will become frustrated and lose less weight | Myth | Abandon and move on. |
| Assessing “stage of change” or “readiness” to diet is important in helping patients who pursue weight loss treatment to lose weight | Myth | Abandon and move on. |
| Physical education classes, as currently delivered, play an important role in reducing the prevalence of childhood obesity | Myth | Abandon and move on. |
| Breastfeeding is protective against obesity in breastfed offspring | Myth | Abandon and move on. |
| Daily self-weighing interferes with weight loss | Myth | Abandon and move on. |
| Genes have not contributed to the obesity epidemic | Myth | Abandon and move on. |
| The freshman year of college is associated with or causes 15 pounds of weight gain | Myth | Abandon and move on. |
| Food deserts (areas with little or no access to stores offering fresh and affordable healthy foods, including produce) lead to higher obesity prevalence | Myth | Abandon and move on. |
| Regularly eating (versus skipping) breakfast is protective against obesity | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
| Eating close to bedtime contributes weight gain | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
| Eating more fruits and vegetables will lead to weight loss or less weight gain, regardless of whether one intentionally makes any other changes to one's behavior or environment | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
| Weight cycling (i.e., yo-yo dieting) increases the mortality rate | Presumption | The definitive study may never be conducted for practical or ethical reasons. Continue studying only while such investigations are probative. At best this may rise to the level of a strongly supported or refuted presumption. |
| Snacking contributes to weight gain and obesity | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
| The built environment, in terms of sidewalks and park availability, influences obesity | Presumption | The definitive study may never be conducted for practical or ethical reasons. Continue studying only while such investigations are probative. At best this may rise to the level of a strongly supported or refuted presumption. |
| Reducing screen time will decrease obesity in children | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
| Decreasing the portion sizes served leads to less food intake without people being told to reduce their food intake or presumably intending to do so, even when the total food available is not limited | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
| Participation in family mealtimes reduces obesity | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
| Drinking more water will reduce energy intake and will lead to weight loss or less weight gain, regardless of whether one intentionally makes any other changes to one's behavior or environment | Presumption | Truly probative, causal tests of this question can ostensibly be completed. Let's do them. |
Discussion
We presented 19 beliefs that are common in the obesity field. This is by no means an exhaustive list and we recognize that there is no absolute certainty in empirical science and therefore, some necessary subjectivity in the selection of which myths and presumptions to include as well as in the distinction of the belief as either myth or presumption. Nevertheless, we believe that elucidating these myths and presumptions is important for several reasons. First, as these beliefs are themselves often the subject of decisions about obesity treatment, public health policies, public health recommendations, or future research, it is critically important to clarify the lack of substantiation for these beliefs to prevent a misallocation of resources to address this serious public health problem. Considering these beliefs may also help us to think about why such unsubstantiated beliefs are so common and, in some cases, so fervently held in the obesity field. Here we offer conjecture on why these beliefs may be so widely held to create windows of opportunity to limit the spread and dissemination of unsubstantiated beliefs going forward.
In the current research environment, we often believe things to be true and demonstrated when they are not true or not proven because we permit ipse dixit statements in the scientific literature rather than demanding empirical support. In a philosophical dialogue between Roman and Cicero 466 referring to Pythagoras's students debating, Cicero declares ipse dixit, that is, he said it himself, speaking of Pythagoras, whose authority they considered strong even without reason. This begs the question, why? Perhaps, some ideas seem so sensible that we never stop to ask for the data. Psychologists are well acquainted with the mere exposure effect467, which states that the more we are exposed to something, the more we come to like and believe it. Is it possible that if we are simply repetitiously exposed to discussion of obesity with the commonly used, vague words of implicated and linked that we will come to believe that the proposition has been proven? Conceivably, some ideas are so precious to us that we cannot bear to let them go despite evidence to the contrary. Or, we allow ourselves to be satisfied with weak data. Of course, there is also the more malign possibility that some people are deliberately distorting the available evidence to match their preconceived notions.
We agree that some of the myths are not amenable to RCTs. We also agree that simply saying there is no reason to believe a proposition is true because no RCT may exist to test its truth is misleading. There is a clear and important distinction between having a reason to believe a proposition is true and having shown it is true or false. One can reasonably believe something to be true without evidence to conclude that it is true and those are things we call presumptions. We have now tried to adjust our language to reflect this distinction more appropriately. Moreover, while we agree that many propositions are not amendable to testing in RCTs, many are. Our main emphasis is that where evidence is lacking we need to generate the evidence. For many of the beliefs presented, we (society) ought to know the answer, the RCTs would not be unduly difficult or expensive, and yet they are often not done. It is too often accepted that observational studies which sometimes justifiably increase belief in a proposition, but do not create conclusory knowledge are the only option, whereas the probative RCTs may indeed be possible. Finally, we wish to clarify, as we have in many other writings, that asking for honest unvarnished presentations of evidence does not mean that we are saying that public health or clinical recommendations or actions cannot proceed without perfect evidence. In many cases it is appropriate to proceed with clinical or public heath actions or recommendations even in the absence of strong evidence showing that something will work – but in doing so, we should still fulfill our duty to truthfulness and not misrepresent the evidence.
What can we do to limit the spread of these and other unsubstantiated beliefs in the obesity domain? One important factor for our field may be a reinvigoration of one aspect of our culture and the downplaying of another. In recent years, with the rise of the obesity epidemic, numerous papers have used the rhetoric of war in describing our efforts to abate this epidemic 468-475. It has been said that the first casualty of war is truth (Aeschylus). Indeed, it is well documented that under conditions of war or extreme emotional situations, propaganda is used and complex messages are distorted into simplistic slogans regardless of their truthfulness. As scientists, we should resist this sloganeering and perhaps downplay the emotional rhetoric of war, which may lead to a willingness to distort information and gloss over complexities. We can instead draw on our culture as scientists by placing the greatest premium on truthfulness and by allowing an unwavering commitment to exactitude and rationality to be the principle around which we rally.
In addition, we can take very practical steps to address the presumptions (as opposed to the myths) whenever possible by conducting the definitive studies that would put the question to rest. For some questions, such as is food addictive, exactly what the definitive study would be is not clear. In such situations, one must ask whether one is really dealing with a scientifically falsifiable hypothesis 476. In other situations (e.g. what is the effect of a particular obesity treatment on lifespan in humans), the definitive study might be easily specified but not easily doable in practice. Even so, for many questions, the definitive study is unquestionably doable. For example, in our discussion of the effects of breakfast consumption vs. breakfast skipping, the definitive study would clearly be a sufficiently large, sufficiently long, randomized experiment in humans with weight or body fat as an outcome. Indeed, doing such a study would, relative to the amount we invest in obesity research overall would, take a relatively small amount of funding and be relatively easy to do. With this in mind, several of us have conducted such a trial and as of this writing are analyzing the data. We hope that other investigators will pick up some of the ideas presented here and discontinue the repeated attempts at observational studies, anecdotal reports, descriptions of rationales, and endless debates that do not advance progress and simply do the definitive trials.
Acknowledgments
In memory of E. Michael Foster.
Funding: This work was funded in part by NIH grant P30DK056336.
References
- 1.Sawbridge DT, Fitzgerald R. ‘Lazy, slothful and indolent’: medical and social perceptions of obesity in Europe to the eighteenth century. Vesalius. 2009;15:59. [PubMed] [Google Scholar]
- 2.Bonafini BA, Pozzilli P. Body weight and beauty: the changing face of the ideal female body weight. Obes Rev. 2011;12:62. doi: 10.1111/j.1467-789X.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 3.Danielsdottir S, O'Brien KS, Ciao A. Anti-fat prejudice reduction: a review of published studies. Obes Facts. 2010;3:47. doi: 10.1159/000277067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeJong W. The stigma of obesity: the consequences of naive assumptions concerning the causes of physical deviance. J Health Soc Behav. 1980;21:75. [PubMed] [Google Scholar]
- 5.Gard M, Wright J. The Obesity Epidemic. Routledge; 2005. [Google Scholar]
- 6.Jutel A. Weighing Health: The Moral Burden of Obesity. Social Semiotics. 2005;15:2. [Google Scholar]
- 7.Egger G. Personal carbon trading: a potential “stealth intervention” for obesity reduction? Med J Aust. 2007;187:185. doi: 10.5694/j.1326-5377.2007.tb01183.x. [DOI] [PubMed] [Google Scholar]
- 8.Evans B. ‘Gluttony or sloth’: critical geographies of bodies and morality in (anti)obesity policy. Area. 2006;38 [Google Scholar]
- 9.Campos P, Saguy A, Ernsberger P, Oliver E, Gaesser G. The epidemiology of overweight and obesity: public health crisis or moral panic? Int J Epidemiol. 2006;35:55. doi: 10.1093/ije/dyi254. [DOI] [PubMed] [Google Scholar]
- 10.Saguy AC, Riley KW. Weighing both sides: morality, mortality, and framing contests over obesity. J Health Polit Policy Law. 2005;30:869. doi: 10.1215/03616878-30-5-869. [DOI] [PubMed] [Google Scholar]
- 11.Whitney E, Rolfes S. Understanding Nutrition. Brooks and Cole; 2011. [Google Scholar]
- 12.American Heart Association. 5 Goals to Losing Weight. American Heart Association; Jan 10, 2013. [Google Scholar]
- 13.Cooper K. The Very Best Way to Lose Weight and Keep it Off. Cleveland Clinic; 2013. [Google Scholar]
- 14.Astrup A, Rossner S. Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes Rev. 2000;1:17. doi: 10.1046/j.1467-789x.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 15.Weiss EC, Galuska DA, Kettel KL, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999-2002. Am J Prev Med. 2007;33:34. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 16.Jeffery RW, Wing RR, Mayer RR. Are smaller weight losses or more achievable weight loss goals better in the long term for obese patients? J Consult Clin Psychol. 1998;66:641. doi: 10.1037//0022-006x.66.4.641. [DOI] [PubMed] [Google Scholar]
- 17.Bjorvell H, Rossner S. A ten-year follow-up of weight change in severely obese subjects treated in a combined behavioural modification programme. Int J Obes Relat Metab Disord. 1992;16:623. [PubMed] [Google Scholar]
- 18.Toubro S, Astrup A. Randomised comparison of diets for maintaining obese subjects' weight after major weight loss: ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ. 1997;314:29. doi: 10.1136/bmj.314.7073.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire MT, Wing RR, Hill JO. The prevalence of weight loss maintenance among American adults. Int J Obes Relat Metab Disord. 1999;23:1314. doi: 10.1038/sj.ijo.0801075. [DOI] [PubMed] [Google Scholar]
- 20.Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis. 2011;70:1798. doi: 10.1136/ard.2010.142018. [DOI] [PubMed] [Google Scholar]
- 21.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. FDA approves Belviq to treat some overweight or obese adults. 2012 Jun 27; 7-17-2013. [PubMed] [Google Scholar]
- 23.Handjieva-Darlenska T, Handjiev S, Larsen TM, van Baak MA, Jebb S, Papadaki A, Pfeiffer AF, Martinez JA, Kunesova M, Holst C, Saris WH, Astrup A. Initial weight loss on an 800-kcal diet as a predictor of weight loss success after 8 weeks: the Diogenes study. Eur J Clin Nutr. 2010;64:994. doi: 10.1038/ejcn.2010.110. [DOI] [PubMed] [Google Scholar]
- 24.Fairburn CG, Stice E, Cooper Z, Doll HA, Norman PA, O'Connor ME. Understanding persistence in bulimia nervosa: a 5-year naturalistic study. J Consult Clin Psychol. 2003;71:103. [PubMed] [Google Scholar]
- 25.Cooper Z, Fairburn CG. Cognitive Behavioral Treatment in Obesity. New York: The Guildford Press; 2002. [Google Scholar]
- 26.Cervone D, Jiwani N, Wood R. Goal setting and the differential influence of self-regulatory processes on complex decision-making performance. J Pers Soc Psychol. 1991;61:257. doi: 10.1037//0022-3514.61.2.257. [DOI] [PubMed] [Google Scholar]
- 27.Cooper Z, Fairburn CG. A new cognitive behavioural approach to the treatment of obesity. Behav Res Ther. 2001;39:499. doi: 10.1016/s0005-7967(00)00065-6. [DOI] [PubMed] [Google Scholar]
- 28.Academy of Nutrition and Dietetics. Adult Weight Management Evidence Based Nutrition Practice Guidelines Executive Summary of Recommendations. 2012 http://www.adaevidencelibrary.com/topic.cfm?cat=3014&auth=1.
- 29.American Dietetic Association (ADA) Setting Realistic Weight Loss Goals. 2012 http://www.diabetes.org/food-and-fitness/fitness/weight-loss/setting-realistic-weight-loss.html. 5-9-0012.
- 30.Centers for Disease Control. Getting Started. 2012 http://www.cdc.gov/healthyweight/losing_weight/getting_started.html. 5-9-0012.
- 31.United States Department of Agriculture. USDA Home and Garden Bulletin. 4th. Washinton DC: 1995. Nutrition and Your Health: Dietary Guidelines for Americans. [Google Scholar]
- 32.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657. [PubMed] [Google Scholar]
- 33.National Heart LBI. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults: The Evidence Report. 1998 [Google Scholar]
- 34.Stern JS, Hirsch J, Blair SN, Foreyt JP, Frank A, Kumanyika SK, Madans JH, Marlatt GA, St Jeor ST, Stunkard AJ. Weighing the options: criteria for evaluating weight-management programs. The Committee to Develop Criteria for Evaluating the Outcomes of Approaches to Prevent and Treat Obesity. Obes Res. 1995;3:591. [PubMed] [Google Scholar]
- 35.Cunningham E. How can I support my clients in setting realistic weight loss goals? J Acad Nutr Diet. 2014;114:176. doi: 10.1016/j.jand.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients' expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol. 1997;65:79. doi: 10.1037//0022-006x.65.1.79. [DOI] [PubMed] [Google Scholar]
- 37.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119:688. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- 38.Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol. 1994;62:165. doi: 10.1037//0022-006x.62.1.165. [DOI] [PubMed] [Google Scholar]
- 39.Dalle GR, Calugi S, Molinari E, Petroni ML, Bondi M, Compare A, Marchesini G. Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obes Res. 2005;13:1961. doi: 10.1038/oby.2005.241. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira PJ, Palmeira AL, Branco TL, Martins SS, Minderico CS, Barata JT, Silva AM, Sardinha LB. Who will lose weight? A reexamination of predictors of weight loss in women. Int J Behav Nutr Phys Act. 2004;1:12. doi: 10.1186/1479-5868-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalle GR, Calugi S, Magri F, Cuzzolaro M, Dall'Aglio E, Lucchin L, Melchionda N, Marchesini G. Weight loss expectations in obese patients seeking treatment at medical centers. Obes Res. 2004;12:2005. doi: 10.1038/oby.2004.251. [DOI] [PubMed] [Google Scholar]
- 42.Foster GD, Wadden TA, Vogt RA. Body image in obese women before, during, and after weight loss treatment. Health Psychol. 1997;16:226. doi: 10.1037//0278-6133.16.3.226. [DOI] [PubMed] [Google Scholar]
- 43.Linde JA, Jeffery RW, Finch EA, Ng DM, Rothman AJ. Are unrealistic weight loss goals associated with outcomes for overweight women? Obes Res. 2004;12:569. doi: 10.1038/oby.2004.65. [DOI] [PubMed] [Google Scholar]
- 44.Provencher V, Begin C, Gagnon-Girouard MP, Gagnon HC, Tremblay A, Boivin S, Lemieux S. Defined weight expectations in overweight women: anthropometrical, psychological and eating behavioral correlates. Int J Obes (Lond) 2007;31:1731. doi: 10.1038/sj.ijo.0803656. [DOI] [PubMed] [Google Scholar]
- 45.Foster GD, Wadden TA, Phelan S, Sarwer DB, Sanderson RS. Obese patients' perceptions of treatment outcomes and the factors that influence them. Arch Intern Med. 2001;161:2133. doi: 10.1001/archinte.161.17.2133. [DOI] [PubMed] [Google Scholar]
- 46.Fabricatore AN, Wadden TA, Womble LG, Sarwer DB, Berkowitz RI, Foster GD, Brock JR. The role of patients' expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739. doi: 10.1038/sj.ijo.0803649. [DOI] [PubMed] [Google Scholar]
- 47.Linde JA, Jeffery RW, Levy RL, Pronk NP, Boyle RG. Weight loss goals and treatment outcomes among overweight men and women enrolled in a weight loss trial. Int J Obes (Lond) 2005;29:1002. doi: 10.1038/sj.ijo.0802990. [DOI] [PubMed] [Google Scholar]
- 48.Linde JA, Jeffery RW, Levy RL, Pronk NP, Boyle RG. Weight loss goals and treatment outcomes among overweight men and women enrolled in a weight loss trial. Int J Obes (Lond) 2005;29:1002. doi: 10.1038/sj.ijo.0802990. [DOI] [PubMed] [Google Scholar]
- 49.Crawford R, Glover L. The impact of pre-treatment weight-loss expectations on weight loss, weight regain, and attrition in people who are overweight and obese: a systematic review of the literature. Br J Health Psychol. 2012;17:609. doi: 10.1111/j.2044-8287.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 50.Durant NH, Joseph RP, Affuso OH, Dutton GR, Robertson HT, Allison DB. Empirical evidence does not support an association between less ambitious pre-treatment goals and better treatment outcomes: a meta-analysis. Obes Rev. 2013;14:532. doi: 10.1111/obr.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster GD, Phelan S, Wadden TA, Gill D, Ermold J, Didie E. Promoting more modest weight losses: a pilot study. Obes Res. 2004;12:1271. doi: 10.1038/oby.2004.161. [DOI] [PubMed] [Google Scholar]
- 52.Gorin AA, Marinilli PA, Tate DF, Raynor HA, Fava JL, Wing RR. Failure to meet weight loss expectations does not impact maintenance in successful weight losers. Obesity (Silver Spring) 2007;15:3086. doi: 10.1038/oby.2007.367. [DOI] [PubMed] [Google Scholar]
- 53.Ames GE, Perri MG, Fox LD, Fallon EA, De BN, Murawski ME, Pafumi L, Hausenblas HA. Changing weight-loss expectations: a randomized pilot study. Eat Behav. 2005;6:259. doi: 10.1016/j.eatbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 55.Prochaska JO, Velicer WF, Rossi JS, Goldstein MG, Marcus BH, Rakowski W, Fiore C, Harlow LL, Redding CA, Rosenbloom D. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13:39. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 56.Wee CC, Davis RB, Phillips RS. Stage of readiness to control weight and adopt weight control behaviors in primary care. J Gen Intern Med. 2005;20:410. doi: 10.1111/j.1525-1497.2005.0074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brownell KD, Hager DL, Leermakers E. The Weight Loss Readiness Test II. American Health Publishing Company; Dallas: 2004. [Google Scholar]
- 58.Fontaine KR, Cheskin LJ, Allison DB. Predicting treatment attendance and weight loss: assessing the psychometric properties and predictive validity of the Dieting Readiness Test. J Pers Assess. 1997;68:173. doi: 10.1207/s15327752jpa6801_14. [DOI] [PubMed] [Google Scholar]
- 59.Mayo Clinic. Your weight-loss success depends in large part on your readiness to take on the challenge. 2012 http://www.mayoclinic.com/health/weight-loss.
- 60.Norcross JC, Krebs PM, Prochaska JO. Stages of change. J Clin Psychol. 2011;67:143. doi: 10.1002/jclp.20758. [DOI] [PubMed] [Google Scholar]
- 61.Seals JG. Integrating the transtheoretical model into the management of overweight and obese adults. J Am Acad Nurse Pract. 2007;19:63. doi: 10.1111/j.1745-7599.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 62.Teixeira PJ, Silva MN, Mata J, Palmeira AL, Markland D. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012;9:22. doi: 10.1186/1479-5868-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Douglas JG, Ford MJ, Munro JF. Patient motivation and predicting outcome in a hospital obesity clinic. Int J Obes. 1981;5:33. [PubMed] [Google Scholar]
- 64.Edell BH, Edington S, Herd B, O'Brien RM, Witkin G. Self-efficacy and self-motivation as predictors of weight loss. Addict Behav. 1987;12:63. doi: 10.1016/0306-4603(87)90009-8. [DOI] [PubMed] [Google Scholar]
- 65.Grossschadl F, Titze S, Burkert N, Stronegger WJ. Moderate- and vigorous-intensity exercise behaviour according to the Transtheoretical Model: associations with smoking and BMI among Austrian adults. Wien Klin Wochenschr. 2013;125:270. doi: 10.1007/s00508-013-0361-z. [DOI] [PubMed] [Google Scholar]
- 66.Martin CK, O'neil PM, Binks M. An attempt to identify predictors of treatment outcome in two comprehensive weight loss programs. Eat Behav. 2002;3:239. doi: 10.1016/s1471-0153(02)00065-x. [DOI] [PubMed] [Google Scholar]
- 67.Teixeira PJ, Going SB, Houtkooper LB, Cussler EC, Martin CJ, Metcalfe LL, Finkenthal NR, Blew RM, Sardinha LB, Lohman TG. Weight loss readiness in middle-aged women: psychosocial predictors of success for behavioral weight reduction. J Behav Med. 2002;25:499. doi: 10.1023/a:1020687832448. [DOI] [PubMed] [Google Scholar]
- 68.Teixeira PJ, Palmeira AL, Branco TL, Martins SS, Minderico CS, Barata JT, Silva AM, Sardinha LB. Who will lose weight? A reexamination of predictors of weight loss in women. Int J Behav Nutr Phys Act. 2004;1:12. doi: 10.1186/1479-5868-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toth-Capelli KM, Brawer R, Plumb J, Daskalakis C. Stage of change and other predictors of participant retention in a behavioral weight management program in primary care. Health Promot Pract. 2013;14:441. doi: 10.1177/1524839912460871. [DOI] [PubMed] [Google Scholar]
- 70.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70:115. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- 71.Fontaine KR, Wiersema L. Dieting readiness test fails to predict enrollment in a weight loss program. J Am Diet Assoc. 1999;99:664. doi: 10.1016/S0002-8223(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 72.Dixon JB, Laurie CP, Anderson ML, Hayden MJ, Dixon ME, O'Brien PE. Motivation, readiness to change, and weight loss following adjustable gastric band surgery. Obesity (Silver Spring) 2009;17:698. doi: 10.1038/oby.2008.609. [DOI] [PubMed] [Google Scholar]
- 73.Tuah NA, Amiel C, Qureshi S, Car J, Kaur B, Majeed A. Transtheoretical model for dietary and physical exercise modification in weight loss management for overweight and obese adults. Cochrane Database Syst Rev. 2011:CD008066. doi: 10.1002/14651858.CD008066.pub2. [DOI] [PubMed] [Google Scholar]
- 74.Brown P, Sutterby JA, Thorton CD. Combating Childhood Obesity With Physical Play Opportunities. PTO Today; 2010. [Google Scholar]
- 75.U.S.Department of Health and Human Services. The Surgeon General's Vision for a Healthy and Fit Nation. Office of the Surgeon General, US DHHS; Washington D.C.: 2010. [PubMed] [Google Scholar]
- 76.Institute of Medicine. Preventing Childhood Obesity: Health in the Balance. The National Academies Press; Washington, D.C.: 2005. [PubMed] [Google Scholar]
- 77.Institute of Medicine. Educating the Student Body: Taking Physical Activity and Physical Education to School. The National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- 78.Fisher G, Hunter GR, Allison DB. Commentary: physical activity does influence obesity risk when it actually occurs in sufficient amount. Int J Epidemiol. 2013;42:1845. doi: 10.1093/ije/dyt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.U.S.Department of Health and Human Services. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: USDHHS; 2008. [Google Scholar]
- 80.Sallis JF, McKenzie TL. Physical education's role in public health. Res Q Exerc Sport. 1991;62:124. doi: 10.1080/02701367.1991.10608701. [DOI] [PubMed] [Google Scholar]
- 81.Sallis JF, McKenzie TL, Alcaraz JE, Kolody B, Faucette N, Hovell MF. The effects of a 2-year physical education program (SPARK) on physical activity and fitness in elementary school students. Am J Public Health. 1997;87:1328. doi: 10.2105/ajph.87.8.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McKenzie TL, Sallis JF, Prochaska JJ, Conway TL, Marshall SJ, Rosengard P. Evaluation of a two-year middle-school physical education intervention: M-SPAN. Med Sci Sports Exerc. 2004;36:1382. doi: 10.1249/01.mss.0000135792.20358.4d. [DOI] [PubMed] [Google Scholar]
- 83.Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, Webber LS, Elder JP, Feldman HA, Johnson CC. Outcomes of a field trial to improve children's dietary patterns and physical activity. The Child and Adolescent Trial for Cardiovascular Health. JAMA. 1996;275:768. doi: 10.1001/jama.1996.03530340032026. [DOI] [PubMed] [Google Scholar]
- 84.U.S.Department of Health and Human Services. Healthy People 2010. 2nd. U.S. Government Printing Office; Washington, D.C.: 2000. [Google Scholar]
- 85.Centers for Disease Control and Prevention. Guidelines for school and community programs to promote lifelong physical activity among young people. National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. J Sch Health. 1997;67:202. doi: 10.1111/j.1746-1561.1997.tb06307.x. [DOI] [PubMed] [Google Scholar]
- 86.National Association for Sport and Physical Education, American Heart Association. 2010 Shape of the Nation Report: Status of Physical Education in the USA. National Association for Sport and Physical Education; Reston, VA: 2010. [Google Scholar]
- 87.Pate RR, Davis MG, Robinson TN, Stone EJ, McKenzie TL, Young JC. Promoting physical activity in children and youth: A leadership role for schools: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism (Physical Activity Committee) in collaboration with the Councils on Cardiovascular Disease in the Young and Cardiovascular Nursing. Circulation. 2006;114:1214. doi: 10.1161/CIRCULATIONAHA.106.177052. [DOI] [PubMed] [Google Scholar]
- 88.Riis J, Grayson H, Strobino D, Ahmed S, Minkovitz C. State School Policies and Youth Obesity. Maternal and Child Health. 2012;16:S111–S118. doi: 10.1007/s10995-012-1000-4. [DOI] [PubMed] [Google Scholar]
- 89.O'Malley PM, Johnston LD, Delva J, Terry-McElrath YM. School physical activity environment related to student obesity and activity: a national study of schools and students. J Adolesc Health. 2009;45:S71–S81. doi: 10.1016/j.jadohealth.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 90.Fernandes M, Sturm R. The Role of School Physical Activity Programs in Child Body Mass Trajectory. Journal of Physical Activity and Health. 2011;8:174. doi: 10.1123/jpah.8.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim J. Are Physical Education-Related State Policies and Schools' Physical Education Requirement Related to Children's Physical Activity and Obesity. Journal of School Health. 2012;82:268. doi: 10.1111/j.1746-1561.2012.00697.x. [DOI] [PubMed] [Google Scholar]
- 92.Almas A, Islam M, Jafar TH. School-based physical activity programme in preadolescent girls (9-11 years): a feasibility trial in Karachi, Pakistan. Arch Dis Child. 2013;98:515. doi: 10.1136/archdischild-2012-303242. [DOI] [PubMed] [Google Scholar]
- 93.Bellows LL, Davies PL, Anderson J, Kennedy C. Effectiveness of a physical activity intervention for Head Start preschoolers: a randomized intervention study. Am J Occup Ther. 2013;67:28. doi: 10.5014/ajot.2013.005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brandstetter S, Klenk J, Berg S, Galm C, Fritz M, Peter R, Prokopchuk D, Steiner RP, Wartha O, Steinacker J, Wabitsch M. Overweight prevention implemented by primary school teachers: a randomised controlled trial. Obes Facts. 2012;5:1. doi: 10.1159/000336255. [DOI] [PubMed] [Google Scholar]
- 95.Story M, Hannan PJ, Fulkerson JA, Rock BH, Smyth M, Arcan C, Himes JH. Bright Start: Description and main outcomes from a group-randomized obesity prevention trial in American Indian children. Obesity (Silver Spring) 2012;20:2241. doi: 10.1038/oby.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walther C, Mende M, Gaede L, Muller U, Machalica K, Schuler G. Effects of daily physical exercise at school on cardiovascular risk--results of a 2-year cluster-randomized study. Dtsch Med Wochenschr. 2011;136:2348. doi: 10.1055/s-0031-1292049. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez-Suarez C, Worley A, Grimmer-Somers K, Dones V. School-based interventions on childhood obesity: a meta-analysis. Am J Prev Med. 2009;37:418. doi: 10.1016/j.amepre.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 98.Datar A, Sturm R. Physical Education in Elementary School and Body Mass Index: Evidence from the Early Childhood Longitudinal Study. American Journal of Public Health. 2004;94:1501. doi: 10.2105/ajph.94.9.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cawley J, Frisvold D, Meyerhoefer C. The impact of physical education on obesity among elementary school children. J Health Econ. 2013;32:743. doi: 10.1016/j.jhealeco.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 100.Lloyd RS, Oliver JL, Hughes MG, Williams CA. The effects of 4-weeks of plyometric training on reactive strength index and leg stiffness in male youths. J Strength Cond Res. 2012;26:2812. doi: 10.1519/JSC.0b013e318242d2ec. [DOI] [PubMed] [Google Scholar]
- 101.Williamson DA, Champagne CM, Harsha DW, Han H, Martin CK, Newton RL, Jr, Sothern MS, Stewart TM, Webber LS, Ryan DH. Effect of an environmental school-based obesity prevention program on changes in body fat and body weight: a randomized trial. Obesity (Silver Spring) 2012;20:1653. doi: 10.1038/oby.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kain J, Uauy R, Albala C, Vio F, Cerda R, Leyton B. School-based obesity prevention in Chilean primary school children: methodology and evaluation of a controlled study. International Journal of Obesity. 2004;28:483. doi: 10.1038/sj.ijo.0802611. [DOI] [PubMed] [Google Scholar]
- 103.Sollerhed AC, Ejlertsson G. Physical benefits of expanded physical education in primary school: findings from a 3-year intervention study in Sweden. The Journal of Sports Medicine and Physical Fitness. 2008;18:102. doi: 10.1111/j.1600-0838.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- 104.Kriemler S, Zahner L, Schindler C, Meyer U, Hartmann T, Hebestreit H, Brunner-La Rocca HP, van Mechelen W, Pruder JJ. Effect of school based physical activity programme (KISS) on fitness and adiposity in primary schoolchildren: cluster randomised controlled trial. British Medical Journal. 2010;340:c785. doi: 10.1136/bmj.c785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sallis JF, McKenzie TL, Alcarez JE, Kolody B, Hovell MF, Nader PR. Project SPARK. Effects of physical education on adiposity on children. Annals of the. 1993 doi: 10.1111/j.1749-6632.1993.tb18844.x. [DOI] [PubMed] [Google Scholar]
- 106.Manios Y, Moschandreas J, Hatzis C, Kafatos A. Evaluation of a health and nutrition education program in primary school children of Crete over a three-year period. Prev Med. 1999;28:149. doi: 10.1006/pmed.1998.0388. [DOI] [PubMed] [Google Scholar]
- 107.Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, Webber LA, Elder JP, Feldman HA, Johnson CC, Kelder SH, Wu M. for the CATCH Collaborative Group, Outcomes of a Field Trial to Improve Children's Dietary Patterns and Physical Activity. JAMA: The Journal of the American Medical Association. 1996;275:768. doi: 10.1001/jama.1996.03530340032026. [DOI] [PubMed] [Google Scholar]
- 108.Fardy PS, White RE, Haltiwanger-Schmitz K, et al. Coronary disease risk factor reduction and behavior modification in minority adolescents: The PATH program. J Adolesc Health. 1996;18:247. doi: 10.1016/1054-139X(95)00283-X. [DOI] [PubMed] [Google Scholar]
- 109.Bayne-Smith M, Fardy PS, Azzollini A, Magel J, Schmitz KH, Agin D. Improvements in Heart Health Behaviors and Reduction in Coronary Artery Disease Risk Factors in Urban Teenaged Girls Through a School-Based Intervention: The PATH Program. American Journal of Public Health. 2004;94:1538. doi: 10.2105/ajph.94.9.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dwyer T, Coonan WE, Leitch DR, Hetzel BS, Baghurst RA. An investigation of the effects of daily physical activity on the health of primary school students in South Australia. Int J Epidemiol. 1983;12:308. doi: 10.1093/ije/12.3.308. [DOI] [PubMed] [Google Scholar]
- 111.Ewart CK, Young DR, Hagberg JM. Effects of school-based aerobic exercise on blood pressure in adolescent girls at risk for hypertension. Am J Public Health. 1998;88:949. doi: 10.2105/ajph.88.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrell JS, McMurray RG, Bangdiwala SI, Frauman AC, Gansky SA, Bradley CB. Effects of a school-based intervention to reduce cardiovascular disease risk factors in elementary-school children: The Cardiovascular Health in Children (CHIC) study. J Pediatr. 1996;128:797. doi: 10.1016/s0022-3476(96)70332-3. [DOI] [PubMed] [Google Scholar]
- 113.Neumark-Sztainer D, Story M, Hannan PJ, Rex J. New Moves: a school-based obesity prevention program for adolescent girls. Preventive Medicine. 2003;37:41. doi: 10.1016/s0091-7435(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 114.Harris KC, Kuramoto LK, Schulzer M, Retallack JE. Effect of School-based physical activity interventions on body mass index in children: a meta-analysis. Canadian Medical Association Journal. 2009;180:719. doi: 10.1503/cmaj.080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, et al. The effectiveness of interventions to increase physical activity: A systematic review. Am J Prev Med. 2002;22:73. doi: 10.1016/s0749-3797(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 116.Stice E, Shaw H, Marti CN. A Meta-Analytic Review of Obesity Prevention Programs for Children and Adolescents: The Skinny on Interventions that Work. Psychology Bulletin. 2006;132:667. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levenstein H. Revolution at the Table: The Transformation of the American Diet. Oxford University Press; New York: 1988. p. 122. [Google Scholar]
- 118.Saadeh MR. A new global strategy for infant and young child feeding. Forum Nutr. 2003;56:236. [PubMed] [Google Scholar]
- 119.Obesity Prevention Strategic Plan-New York. 2013. 10-1-2013. [Google Scholar]
- 120.Obesity Prevention Strategic Plan Indiana. 2013 http://www.cdc.gov/obesity/stateprograms/fundedstates/pdf/Indiana-State-Profile.pdf.
- 121.Obesity Prevention Strategic Plan California. 2013 http://www.cdph.ca.gov/programs/COPP/Documents/COPP-ObesityPreventionPlan-2010.pdf.pdf. 10-1-2013.
- 122.Obesity Prevention Strategic Plan Texas. 2013 http://www.dshs.state.tx.us/obesity/pdf/StrategicPlanUpdate.pdf.
- 123.McGuire S U.S. Dept. of Health and Human Services. Adv Nutr. Vol. 2. U.S. Dept of Health and Human Services, Office of the Surgeon General; 2011. The Surgeon General's Call to Action to Support Breastfeeding; p. 523. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, Eidelman AI. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 125.James DC, Dobson B. Position of the American Dietetic Association: Promoting and supporting breastfeeding. J Am Diet Assoc. 2005;105:810. doi: 10.1016/j.jada.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 126.California WIC. Breastfeeding is the first defense against Obesity. 2012 http://www.calwic.org/storage/documents/wellness/bf_paper1.pdf.
- 127.Dewey KG. Is breastfeeding protective against child obesity? J Hum Lact. 2003;19:9. doi: 10.1177/0890334402239730. [DOI] [PubMed] [Google Scholar]
- 128.Haschke F, van't Hof MA. Euro-Growth references for breast-fed boys and girls: influence of breast-feeding and solids on growth until 36 months of age. Euro-Growth Study Group. J Pediatr Gastroenterol Nutr. 2000;31(Suppl 1):S60–S71. doi: 10.1097/00005176-200007001-00006. [DOI] [PubMed] [Google Scholar]
- 129.Hediger ML, Overpeck MD, Kuczmarski RJ, Ruan WJ. Association between infant breastfeeding and overweight in young children. JAMA. 2001;285:2453. doi: 10.1001/jama.285.19.2453. [DOI] [PubMed] [Google Scholar]
- 130.Nielsen GA, Thomsen BL, Michaelsen KF. Influence of breastfeeding and complementary food on growth between 5 and 10 months. Acta Paediatr. 1998;87:911. doi: 10.1080/080352598750031536. [DOI] [PubMed] [Google Scholar]
- 131.Araujo CL, Victora CG, Hallal PC, Gigante DP. Breastfeeding and overweight in childhood: evidence from the Pelotas 1993 birth cohort study. Int J Obes (Lond) 2006;30:500. doi: 10.1038/sj.ijo.0803160. [DOI] [PubMed] [Google Scholar]
- 132.Beyerlein A, Toschke AM, von KR. Breastfeeding and childhood obesity: shift of the entire BMI distribution or only the upper parts? Obesity (Silver Spring) 2008;16:2730. doi: 10.1038/oby.2008.432. [DOI] [PubMed] [Google Scholar]
- 133.Burdette HL, Whitaker RC. Differences by race and ethnicity in the relationship between breastfeeding and obesity in preschool children. Ethn Dis. 2007;17:467. [PubMed] [Google Scholar]
- 134.Davis JN, Weigensberg MJ, Shaibi GQ, Crespo NC, Kelly LA, Lane CJ, Goran MI. Influence of breastfeeding on obesity and type 2 diabetes risk factors in Latino youth with a family history of type 2 diabetes. Diabetes Care. 2007;30:784. doi: 10.2337/dc06-2008. [DOI] [PubMed] [Google Scholar]
- 135.Kramer MS, Kakuma R. The optimal duration of exclusive breastfeeding: a systematic review. Adv Exp Med Biol. 2004;554:63. doi: 10.1007/978-1-4757-4242-8_7. [DOI] [PubMed] [Google Scholar]
- 136.Kramer MS, Fombonne E, Igumnov S, Vanilovich I, Matush L, Mironova E, Bogdanovich N, Tremblay RE, Chalmers B, Zhang X, Platt RW. Effects of prolonged and exclusive breastfeeding on child behavior and maternal adjustment: evidence from a large, randomized trial. Pediatrics. 2008;121:e435–e440. doi: 10.1542/peds.2007-1248. [DOI] [PubMed] [Google Scholar]
- 137.Kramer MS, Fombonne E, Matush L, Bogdanovich N, Dahhou M, Platt RW. Long-term behavioural consequences of infant feeding: the limits of observational studies. Paediatr Perinat Epidemiol. 2011;25:500. doi: 10.1111/j.1365-3016.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martin RM, Patel R, Kramer MS, Guthrie L, Vilchuck K, Bogdanovich N, S N, G N, Foo Y, Palmer T, Rifas-Shiman SL, G MW, Smith GD, Oken E. Effects of Promoting Longer-term and Exclusive Breastfeeding on Adiposity and Insulin-like Growth Factor-I at Age 11.5 Years: A Randomized Trial. JAMA. 309:2013. doi: 10.1001/jama.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O'Tierney PF, Barker DJ, Osmond C, Kajantie E, Eriksson JG. Duration of breast-feeding and adiposity in adult life. J Nutr. 2009;139:422S. doi: 10.3945/jn.108.097089. [DOI] [PubMed] [Google Scholar]
- 140.Gillman MW, Rifas-Shiman SL, Camargo CA, Jr, Berkey CS, Frazier AL, Rockett HR, Field AE, Colditz GA. Risk of overweight among adolescents who were breastfed as infants. JAMA. 2001;285:2461. doi: 10.1001/jama.285.19.2461. [DOI] [PubMed] [Google Scholar]
- 141.Gillman MW. Commentary: breastfeeding and obesity--the 2011 Scorecard. Int J Epidemiol. 2011;40:681. doi: 10.1093/ije/dyr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arenz S, Ruckerl R, Koletzko B, von KR. Breast-feeding and childhood obesity--a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- 143.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162:397. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 144.Owen CG, Martin RM, Whincup PH, vey-Smith G, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82:1298. doi: 10.1093/ajcn/82.6.1298. [DOI] [PubMed] [Google Scholar]
- 145.Horta BL, Bahl R, Martines JC, Victora CG. Systematic Reviews and Meta-analyses. World Health Organization; 2007. Evidence of the long-term effects of breastfeeding. [Google Scholar]
- 146.Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007;1 [PMC free article] [PubMed] [Google Scholar]
- 147.Casazza K, Fernandez JR, Allsion DB. Modest Protective Effects of Breast-feeding on Obesity: Is the Evidence Truly Supportive? Nutrition Today. 2012;47(1):33–38. [Google Scholar]
- 148.Cope MB, Allison DB. Critical review of the World Health Organization's (WHO) 2007 report on ‘evidence of the long-term effects of breastfeeding: systematic reviews and meta-analysis’ with respect to obesity. Obes Rev. 2008;9:594. doi: 10.1111/j.1467-789X.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 149.Saadeh RJ. The Baby-Friendly Hospital Initiative 20 years on: facts, progress, and the way forward. J Hum Lact. 2012;28:272. doi: 10.1177/0890334412446690. [DOI] [PubMed] [Google Scholar]
- 150.Protecting, promoting and supporting breast feeding: the special role of maternity services. Nurs J India. 1993;84:3. [PubMed] [Google Scholar]
- 151.Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet JP, Vanilovich I, Mezen I, Ducruet T, Shishko G, Zubovich V, Mknuik D, Gluchanina E, Dombrovskiy V, Ustinovitch A, Kot T, Bogdanovich N, Ovchinikova L, Helsing E. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 152.Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet JP, Martin RM, Davey SG, Gillman MW, Chalmers B, Hodnett E, Shapiro S. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86:1717. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 153.Metzger MW, McDade TW. Breastfeeding as obesity prevention in the United States: A sibling difference model. Am J Hum Biol. 2009 doi: 10.1002/ajhb.20982. [DOI] [PubMed] [Google Scholar]
- 154.Nelson MC, Gordon-Larsen P, Adair LS. Are adolescents who were breast-fed less likely to be overweight? Analyses of sibling pairs to reduce confounding. Epidemiology. 2005;16:247. doi: 10.1097/01.ede.0000152900.81355.00. [DOI] [PubMed] [Google Scholar]
- 155.Evenhouse E, Reilly S. Improved estimates of the benefits of breastfeeding using sibling comparisons to reduce selection bias. Health Serv Res. 2005;40:1781. doi: 10.1111/j.1475-6773.2004.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang M, Foster EM. Duration of Breastfeeding and Childhood Obesity: A Generalized Propensity Score Approach. Health Services Research. 2012 doi: 10.1111/j.1475-6773.2012.01456.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee A. Do Not Weigh Yourself Everyday When Losing Weight. 2013 http://www.selfgrowth.com/articles/Do_Not_Weigh_Yourself_Everyday_When_Losing_Weight.html.
- 158.Trotter K. Why You Shouldn't Weigh Yourself Daily. Huffington Post; Dec 5, 2012. [Google Scholar]
- 159.Stuart R. Behavioral control of overeating. Behavior Research & Therapy. 1967;5:357. doi: 10.1002/j.1550-8528.1996.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 160.Fujimoto K, Sakata T, Etou H, Fukagawa K, Ookuma K, Terada K, Kurata K. Charting of daily weight pattern reinforces maintenance of weight reduction in moderately obese patients. Am J Med Sci. 1992;303:145. doi: 10.1097/00000441-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 161.Heckerman CL, Brownell KD, Westlake RJ. Self and external monitoring of weight. Psychol Rep. 1978;43:375. doi: 10.2466/pr0.1978.43.2.375. [DOI] [PubMed] [Google Scholar]
- 162.Quayle CM, Powers RB. The Self-recording of Weights and Bites in the Treatment of Obesity. The Psycological Record. 1979;29:517. [Google Scholar]
- 163.Romanczyk RG. Self-monitoring in the treatment of obesity: Parameters of reactivity. Behavior Therapy. 1974;5:531. [Google Scholar]
- 164.Mahoney MJ, Moura NG, Wade TC. Relative efficacy of self-reward, self-punishment, and self-monitoring techniques for weight loss. J Consult Clin Psychol. 1973;40:404. doi: 10.1037/h0034565. [DOI] [PubMed] [Google Scholar]
- 165.Oshima Y, Matsuoka Y, Sakane N. Effect of weight-loss program using self-weighing twice a day and feedback in overweight and obese subjects:A randomized control trial. Obesity Research & Clinical Practice. 2012;7:e361–e366. doi: 10.1016/j.orcp.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 166.Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity (Silver Spring) 2013;21:1789. doi: 10.1002/oby.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pacanowski CR. Effects of self-weighing and visual feedback on weight control in adults. 2013 [Google Scholar]
- 168.Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring) 2007;15:3091. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 169.Gokee-LaRose J, Gorin AA, Wing RR. Behavioral self-regulation for weight loss in young adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2009;6:10. doi: 10.1186/1479-5868-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Kurth CL, Johnson SL. Correlates of weight loss and its maintenance over two years of follow-up among middle-aged men. Prev Med. 1984;13:155. doi: 10.1016/0091-7435(84)90048-3. [DOI] [PubMed] [Google Scholar]
- 171.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66:239. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 172.Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med. 2005;30:210. doi: 10.1207/s15324796abm3003_5. [DOI] [PubMed] [Google Scholar]
- 173.VanWormer JJ, Linde JA, Harnack LJ, Stovitz SD, Jeffery RW. Self-weighing frequency is associated with weight gain prevention over 2 years among working adults. Int J Behav Med. 2012;19:351. doi: 10.1007/s12529-011-9178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 175.Levitsky DA, Garay J, Nausbaum M, Neighbors L, Dellavalle DM. Monitoring weight daily blocks the freshman weight gain: a model for combating the epidemic of obesity. Int J Obes (Lond) 2006;30:1003. doi: 10.1038/sj.ijo.0803221. [DOI] [PubMed] [Google Scholar]
- 176.Gow RW, Trace SE, Mazzeo SE. Preventing weight gain in first year college students: an online intervention to prevent the “freshman fifteen”. Eat Behav. 2010;11:33. doi: 10.1016/j.eatbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Strimas R, Dionne MM. Differential effects of self-weighing in restrained and unrestrained eaters. Personality and Individual Differences. 2010;49:1011. [Google Scholar]
- 178.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 179.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States--no statistically significant chance since 2003-2004. NCHS Data Brief. 2007;1 [PubMed] [Google Scholar]
- 180.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90:1453. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 181.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6:e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, Wang C, Aronne LJ, Wright SM, Baskin M, Dhurandhar NV, Lijoi MC, Grilo CM, DeLuca M, Westfall AO, Allison DB. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 183.Hebebrand J, Wulftange H, Goerg T, Ziegler A, Hinney A, Barth N, Mayer H, Remschmidt H. Epidemic obesity: are genetic factors involved via increased rates of assortative mating? Int J Obes Relat Metab Disord. 2000;24:345. doi: 10.1038/sj.ijo.0801135. [DOI] [PubMed] [Google Scholar]
- 184.Gossman M. Program Report: Health Economics. NBER Reporter Online. 2009 7-24-2013. [Google Scholar]
- 185.Ajslev TA, Angquist L, Silventoinen K, Gamborg M, Allison DB, Baker JL, Sorensen TI. Assortative marriages by body mass index have increased simultaneously with the obesity epidemic. Front Genet. 2012;3:125. doi: 10.3389/fgene.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bastian LA, West NA, Corcoran C, Munger RG. Number of children and the risk of obesity in older women. Prev Med. 2005;40:99. doi: 10.1016/j.ypmed.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 187.Dawson JA, Dhurandhar EJ, Vazquez AI, Peng B, Allison DB. Propagation of Obesity across Generations: The Roles of Differential Realized Fertility and Assortative Mating by Body Mass Index. Hum Hered. 2013;75:204. doi: 10.1159/000352007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Garn SM, Sullivan TV, Hawthorne VM. Educational level, fatness, and fatness differences between husbands and wives. Am J Clin Nutr. 1989;50:740. doi: 10.1093/ajcn/50.4.740. [DOI] [PubMed] [Google Scholar]
- 189.Hur YM. Assortive mating for personaltiy traits, educational level, religious affiliation, height, weight, adn body mass index in parents of Korean twin sample. Twin Res. 2003;6:467. doi: 10.1375/136905203322686446. [DOI] [PubMed] [Google Scholar]
- 190.Jacobson P, Torgerson JS, Sjostrom L, Bouchard C. Spouse resemblance in body mass index: effects on adult obesity prevalence in the offspring generation. Am J Epidemiol. 2007;165:101. doi: 10.1093/aje/kwj342. [DOI] [PubMed] [Google Scholar]
- 191.Rosenberg L, Palmer JR, Wise LA, Horton NJ, Kumanyika SK, Adams-Campbell LL. A prospective study of the effect of childbearing on weight gain in African-American women. Obes Res. 2003;11:1526. doi: 10.1038/oby.2003.204. [DOI] [PubMed] [Google Scholar]
- 192.Speakman JR, Djafarian K, Stewart J, Jackson DM. Assortative mating for obesity. Am J Clin Nutr. 2007;86:316. doi: 10.1093/ajcn/86.2.316. [DOI] [PubMed] [Google Scholar]
- 193.Weng HH, Bastian LA, Taylor DH, Jr, Moser BK, Ostbye T. Number of children associated with obesity in middle-aged women and men: results from the health and retirement study. J Womens Health (Larchmt) 2004;13:85. doi: 10.1089/154099904322836492. [DOI] [PubMed] [Google Scholar]
- 194.Symonds ME, Sebert S, Budge H. The obesity epidemic: from the environment to epigenetics - not simply a response to dietary manipulation in a thermoneutral environment. Front Genet. 2011;2:24. doi: 10.3389/fgene.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Douglas KA, Collins JL, Warren C, Kann L, Gold R, Clayton S, Ross JG, Kolbe LJ. Results from the 1995 National College Health Risk Behavior Survey. J Am Coll Health. 1997;46:55. doi: 10.1080/07448489709595589. [DOI] [PubMed] [Google Scholar]
- 196.Lowry R, Galuska DA, Fulton JE, Wechsler H, Kann L, Collins JL. Physical activity, food choice, and weight management goals and practices among US college students. Am J Prev Med. 2000;18:18. doi: 10.1016/s0749-3797(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 197.Haberman S, Luffey D. Weighing in college students' diet and exercise behaviors. J Am Coll Health. 1998;46:189. doi: 10.1080/07448489809595610. [DOI] [PubMed] [Google Scholar]
- 198.Pinto BM, Marcus BH. A stages of change approach to understanding college students' physical activity. J Am Coll Health. 1995;44:27. doi: 10.1080/07448481.1995.9937506. [DOI] [PubMed] [Google Scholar]
- 199.Racette SB, Deusinger SS, Strube MJ, Highstein GR, Deusinger RH. Changes in weight and health behaviors from freshman through senior year of college. J Nutr Educ Behav. 2008;40:39. doi: 10.1016/j.jneb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 200.Meilman PW, Presley CA, Cashin JR. Average weekly alcohol consumption: drinking percentiles for American college students. J Am Coll Health. 1997;45:201. doi: 10.1080/07448481.1997.9936885. [DOI] [PubMed] [Google Scholar]
- 201.Wechsler H, Molnar BE, Davenport AE, Baer JS. College alcohol use: a full or empty glass? J Am Coll Health. 1999;47:247. doi: 10.1080/07448489909595655. [DOI] [PubMed] [Google Scholar]
- 202.Brevard PB, Ricketts CD. Residence of college students affects dietary intake, physical activity, and serum lipid levels. J Am Diet Assoc. 1996;96:35. doi: 10.1016/S0002-8223(96)00011-9. [DOI] [PubMed] [Google Scholar]
- 203.Kuo M, Adlaf EM, Lee H, Gliksman L, Demers A, Wechsler H. More Canadian students drink but American students drink more: comparing college alcohol use in two countries. Addiction. 2002;97:1583. doi: 10.1046/j.1360-0443.2002.00240.x. [DOI] [PubMed] [Google Scholar]
- 204.Crombie AP, Ilich JZ, Dutton GR, Panton LB, Abood DA. The freshman weight gain phenomenon revisited. Nutr Rev. 2009;67:83. doi: 10.1111/j.1753-4887.2008.00143.x. [DOI] [PubMed] [Google Scholar]
- 205.Graham MA, Jones AL. Freshman 15: valid theory or harmful myth? J Am Coll Health. 2002;50:171. doi: 10.1080/07448480209596023. [DOI] [PubMed] [Google Scholar]
- 206.Gropper SS, Simmons KP, Gaines A, Drawdy K, Saunders D, Ulrich P, Connell LJ. The freshman 15-a closer look. J Am Coll Health. 2009;58:223. doi: 10.1080/07448480903295334. [DOI] [PubMed] [Google Scholar]
- 207.Mihalopoulos NL, Auinger P, Klein JD. The Freshman 15: is it real? J Am Coll Health. 2008;56:531. doi: 10.3200/JACH.56.5.531-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morrow ML, Heesch KC, Dinger MK, Hull HR, Kneehans AW, Fields DA. Freshman 15: fact or fiction? Obesity (Silver Spring) 2006;14:1438. doi: 10.1038/oby.2006.163. [DOI] [PubMed] [Google Scholar]
- 209.Andrade FC, Vazquez-Vidal I, Flood T, Aradillas-Garcia C, Vargas-Morales JM, Medina-Cerda E, Teran-Garcia M. One-year follow-up changes in weight are associated with changes in blood pressure in young Mexican adults. Public Health. 2012;126:535. doi: 10.1016/j.puhe.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 210.Matvienko O, Lewis DS, Schafer E. A college nutrition science course as an intervention to prevent weight gain in female college freshmen. J Nutr Educ. 2001;33:95. doi: 10.1016/s1499-4046(06)60172-3. [DOI] [PubMed] [Google Scholar]
- 211.Anderson DA, Shapiro JR, Lundgren JD. The freshman year of college as a critical period for weight gain: an initial evaluation. Eat Behav. 2003;4:363. doi: 10.1016/S1471-0153(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 212.Edmonds MJ, Ferreira KJ, Nikiforuk EA, Finnie AK, Leavey SH, Duncan AM, Randall Simpson JA. Body weight and percent body fat increase during the transition from high school to university in females. J Am Diet Assoc. 2008;108:1033. doi: 10.1016/j.jada.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 213.Hivert MF, Langlois MF, Berard P, Cuerrier JP, Carpentier AC. Prevention of weight gain in young adults through a seminar-based intervention program. Int J Obes (Lond) 2007;31:1262. doi: 10.1038/sj.ijo.0803572. [DOI] [PubMed] [Google Scholar]
- 214.Holm-Denoma JM, Joiner TE, Vohs KD, Heatherton TF. The “freshman fifteen” (the “freshman five” actually): predictors and possible explanations. Health Psychol. 2008;27:S3–S9. doi: 10.1037/0278-6133.27.1.S3. [DOI] [PubMed] [Google Scholar]
- 215.Lloyd-Richardson EE, Bailey S, Fava JL, Wing R. A prospective study of weight gain during the college freshman and sophomore years. Prev Med. 2009;48:256. doi: 10.1016/j.ypmed.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lowe MR, Annunziato RA, Markowitz JT, Didie E, Bellace DL, Riddell L, Maille C, McKinney S, Stice E. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite. 2006;47:83. doi: 10.1016/j.appet.2006.03.160. [DOI] [PubMed] [Google Scholar]
- 217.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System 2011 Survey Data and Documentation. CDC; 2012. Centers for Disease Control and Prevention. [Google Scholar]
- 218.Levitsky DA, Pacanowski CR. Free will and the obesity epidemic. Public Health Nutr. 2012;15:126. doi: 10.1017/S1368980011002187. [DOI] [PubMed] [Google Scholar]
- 219.Kolata G. Studies Question the Pairing of Food Deserts and Obesity. New York Times Online; Apr 1, 2012. Ref Type: Online Source. [Google Scholar]
- 220.United States Department of Agriculture. The Food Access Research Atlas. USDA Econmic Research Service; May 8, 2013. [Google Scholar]
- 221.Jiao J, Moudon AV, Ulmer J, Hurvitz PM, Drewnowski A. How to identify food deserts: measuring physical and economic access to supermarkets in King County, Washington. Am J Public Health. 2012;102:e32–e39. doi: 10.2105/AJPH.2012.300675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.GrantWatch. Foundations Aim to Foster Nourishment and Banish ‘Food Deserts’. Health Affairs. 2013;31:1119. [Google Scholar]
- 223.Alwitt LF, Donley TD. Retail Stores in Poor Urban Neighborhoods. J Consumer Affairs. 2012;31:1119. [Google Scholar]
- 224.Beaulac J, Kristjansson E, Cummins S. A systematic review of food deserts, 1966-2007. Prev Chronic Dis. 2009;6:A105. [PMC free article] [PubMed] [Google Scholar]
- 225.Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. Am J Public Health. 2006;96:325. doi: 10.2105/AJPH.2004.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Morland K, Wing S, Diez RA, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med. 2002;22:23. doi: 10.1016/s0749-3797(01)00403-2. [DOI] [PubMed] [Google Scholar]
- 227.Powell LM, Slater S, Mirtcheva D, Bao Y, Chaloupka FJ. Food store availability and neighborhood characteristics in the United States. Prev Med. 2007;44:189. doi: 10.1016/j.ypmed.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 228.Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. Neighborhood racial composition, neighborhood poverty, and the spatial accessibility of supermarkets in metropolitan Detroit. Am J Public Health. 2005;95:660. doi: 10.2105/AJPH.2004.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.ver Ploeg M, Breneman V, Farrigan T, Hopkins D, Biing-Hwan L, Nord M, Smith TA, Williams R, Kinnison K, Olander C, Singh A, Tuckermanty E. Access to Affordable and Nutrition Food-Measuring and Understanding Food Desers and their Consequences: Report to Congress. United States Department of Agriculture; 2012. [Google Scholar]
- 230.Lee H. The role of local food availability in explaining obesity risk among young school-aged children. Soc Sci Med. 2012;74:1193. doi: 10.1016/j.socscimed.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 231.An R, Sturm R. School and residential neighborhood food environment and diet among California youth. Am J Prev Med. 2012;42:129. doi: 10.1016/j.amepre.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Boone-Heinonen J, Gordon-Larsen P, Kiefe CI, Shikany JM, Lewis CE, Popkin BM. Fast food restaurants and food stores: longitudinal associations with diet in young to middle-aged adults: the CARDIA study. Arch Intern Med. 2011;171:1162. doi: 10.1001/archinternmed.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Cummins S, Petticrew M, Higgins C, Findlay A, Sparks L. Large scale food retailing as an intervention for diet and health: quasi-experimental evaluation of a natural experiment. J Epidemiol Community Health. 2005;59:1035. doi: 10.1136/jech.2004.029843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wrigley N, Warm D, Margetts B. Deprivation, diet, and food-retail access: findings from the Leeds ‘food deserts’ study. Environment and Planning A. 2003;35:151. [Google Scholar]
- 235.RANDHealth. PHRESH: Pittsburgh Hill/Homewood Research on Eating, Shopping, and Health. 2013 [Google Scholar]
- 236.Elbel B, Taksler GB, Mijanovich T, Abrams CB, Dixon LB. Promotion of healthy eating through public policy: a controlled experiment. Am J Prev Med. 2013;45:49. doi: 10.1016/j.amepre.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lerche DJ. Lose Weight: Eat Breakfast. WebMD; 2012. [Google Scholar]
- 238.Academy of Nutrition and Dietetics. A lickety split breakfast. 2012 Eatright.org. 5-10-2012.
- 239.Salge Blake J. Want to trim your waist? Try eating breakfast. 2010 Eatright.org. 5-10-2012.
- 240.Zeratsky K. Why does eating a healthy breakfast help control weight? Mayo Clinic; 2011. 5-10-2012. [Google Scholar]
- 241.McAllister C. What is the effect of skipping breakfast? Livestrong Foundation; 2011. 5-10-2012. [Google Scholar]
- 242.Helm J. Debating the Merits of Breakfast. WebMD; Sep 12, 2013. 1-10-2014. [Google Scholar]
- 243.Brown AW, Bohan Brown MM, Allison DB. Belief beyond the evidence: using the proposed effect of breakfast on obesity to show 2 practices that distort scientific evidence. Am J Clin Nutr. 2013;98:1298. doi: 10.3945/ajcn.113.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Astbury NM, Taylor MA, Macdonald IA. Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J Nutr. 2011;141:1381. doi: 10.3945/jn.110.128645. [DOI] [PubMed] [Google Scholar]
- 245.Leidy HJ, Racki EM. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in ‘breakfast-skipping’ adolescents. Int J Obes (Lond) 2010;34:1125. doi: 10.1038/ijo.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jakubowicz D, Froy O, Wainstein J, Boaz M. Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids. 2012;77:323. doi: 10.1016/j.steroids.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 247.Jakubowicz D, Barnea M, Wainstein J, Froy O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 248.Carels RA, Young KM, Coit C, Clayton AM, Spencer A, Wagner M. Skipping meals and alcohol consumption. The regulation of energy intake and expenditure among weight loss participants. Appetite. 2008;51:538. doi: 10.1016/j.appet.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 249.Wyatt HR, Grunwald GK, Mosca CL, Klem ML, Wing RR, Hill JO. Long-term weight loss and breakfast in subjects in the National Weight Control Registry. Obes Res. 2002;10:78. doi: 10.1038/oby.2002.13. [DOI] [PubMed] [Google Scholar]
- 250.Kral TV, Whiteford LM, Heo M, Faith MS. Effects of eating breakfast compared with skipping breakfast on ratings of appetite and intake at subsequent meals in 8- to 10-y-old children. Am J Clin Nutr. 2011;93:284. doi: 10.3945/ajcn.110.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Levitsky DA, Pacanowski C. Skipping breakfast results in reduced daily energy intake: Experimental evidence. 2012 [Google Scholar]
- 252.Schusdziarra V, Hausmann M, Wittke C, Mittermeier J, Kellner M, Naumann A, Wagenpfeil S, Erdmann J. Impact of breakfast on daily energy intake--an analysis of absolute versus relative breakfast calories. Nutr J. 2011;10:5. doi: 10.1186/1475-2891-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Powell CA, Walker SP, Chang SM, Grantham-McGregor SM. Nutrition and education: a randomized trial of the effects of breakfast in rural primary school children. Am J Clin Nutr. 1998;68:873. doi: 10.1093/ajcn/68.4.873. [DOI] [PubMed] [Google Scholar]
- 254.Schlundt DG, Hill JO, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr. 1992;55:645. doi: 10.1093/ajcn/55.3.645. [DOI] [PubMed] [Google Scholar]
- 255.Halberg F, Erhard H, Cornelissen G. Not eating enough: overcoming underconsumption of military operational rations. Washington DC: National Academy Press; 1995. From biological rhythms to chronomes relevant for nutrition; p. 361. [PubMed] [Google Scholar]
- 256.Rosado JL, del RA, Montemayor K, Garcia OP, Caamano MC. An increase of cereal intake as an approach to weight reduction in children is effective only when accompanied by nutrition education: a randomized controlled trial. Nutr J. 2008;7:28. doi: 10.1186/1475-2891-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Davis A. The Quotations Page. 2013 Jul 7; [Google Scholar]
- 258.DietAdvices.com. Not eating after 6pm Actually Helps Your Diet. 2010 Jul 4; DietAdvices.com. 7-22-2013.
- 259.Martin RM. How to Lose weight by not eating after 6pm. 9-2-0010. [Google Scholar]
- 260.Team WF. Eating late and fast: contributing factors in obesity. Women's Fitness; 2013. [Google Scholar]
- 261.Michaels J, van Alst M. Master Your Metabolism, by Jillian Michaels - Excerpt. 2009 7-15-2013. [Google Scholar]
- 262.Taft W. Eat At Least 3 Hours Before Going to Sleep. 2013 WillTaft.com.
- 263.Men's Health. The 8-hour Cheat Sheet. Men's Health; Dec 14, 2012. [Google Scholar]
- 264.Ignarro L, Myers A. Eating after 8PM may increase risk of obesity. HealthIsWealth; 5-9-0011. [Google Scholar]
- 265.Geliebter A, Gluck ME, Tanowitz M, Aronoff NJ, Zammit GK. Work-shift period and weight change. Nutrition. 2000;16:27. doi: 10.1016/s0899-9007(99)00228-2. [DOI] [PubMed] [Google Scholar]
- 266.Allison DB, Heshka S. Toward an empirically derived typology of obese persons: derivation in a nonclinical sample. Int J Eat Disord. 1993;13:93. doi: 10.1002/1098-108x(199301)13:1<93::aid-eat2260130112>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 267.Vander Wal JS. Night eating syndrome: a critical review of the literature. Clin Psychol Rev. 2012;32:49. doi: 10.1016/j.cpr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 268.Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2013 doi: 10.1111/jhn.12141. [DOI] [PubMed] [Google Scholar]
- 269.Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Gonzalez R, Kueht M, McElfresh TA, Brewer RA, Chandler MP, Bray MS, Young ME. Influence of dark phase restricted high fat feeding on myocardial adaptation in mice. J Mol Cell Cardiol. 2013;55:147. doi: 10.1016/j.yjmcc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kong A, Beresford SA, Alfano CM, Foster-Schubert KE, Neuhouser ML, Johnson DB, Duggan C, Wang CY, Xiao L, Bain CE, McTiernan A. Associations between snacking and weight loss and nutrient intake among postmenopausal overweight to obese women in a dietary weight-loss intervention. J Am Diet Assoc. 2011;111:1898. doi: 10.1016/j.jada.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Waller SM, Vander Wal JS, Klurfeld DM, McBurney MI, Cho S, Bijlani S, Dhurandhar NV. Evening ready-to-eat cereal consumption contributes to weight management. J Am Coll Nutr. 2004;23:316. doi: 10.1080/07315724.2004.10719374. [DOI] [PubMed] [Google Scholar]
- 272.Hibi M, Masumoto A, Naito Y, Kiuchi K, Yoshimoto Y, Matsumoto M, Katashima M, Oka J, Ikemoto S. Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am J Physiol Regul Integr Comp Physiol. 2013;304:R94–R101. doi: 10.1152/ajpregu.00115.2012. [DOI] [PubMed] [Google Scholar]
- 273.Keim NL, Van Loan MD, Horn WF, Barbieri TF, Mayclin PL. Weight loss is greater with consumption of large morning meals and fat-free mass is preserved with large evening meals in women on a controlled weight reduction regimen. J Nutr. 1997;127:75. doi: 10.1093/jn/127.1.75. [DOI] [PubMed] [Google Scholar]
- 274.Schuna C. Why not to eat after 7 P M. Livestrong; 2011. [Google Scholar]
- 275.Snyderman N. You Can Eat After 8 P M. 2009 May 6; [Google Scholar]
- 276.Bray MS, Young ME. Chronobiological Effects on Obesity. Curr Obes Rep. 2012;1:9. doi: 10.1007/s13679-011-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.International Fruit and Vegetable Alliance. F&V Intake and Unhealthy Snacks. 2011 http://www.pbhfoundation.org/pdfs/about/donor_updates/jun24_2011/IfavaNewsJun11.pdf.
- 278.World Health Organization. Promoting fruit and vegetable consumption around the world. 2002 http://www.who.int/dietphysicalactivity/fruit/en/index2.html.
- 279.Mizrahi A, Knekt P, Montonen J, Laaksonen MA, Heliovaara M, Jarvinen R. Plant foods and the risk of cerebrovascular diseases: a potential protection of fruit consumption. Br J Nutr. 2009;102:1075. doi: 10.1017/S0007114509359097. [DOI] [PubMed] [Google Scholar]
- 280.More than Medication. Eat vegetables and lose weight. 2012 http://www.morethanmedication.com.au/article/article.aspx?idcontent=80.
- 281.Lin BH, Wendt M, Guthrie JF. Impact on energy, sodium and dietary fibre intakes of vegetables prepared at home and away from home in the USA. Public Health Nutr. 2013;16:1937. doi: 10.1017/S1368980013001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rolls BJ, Ello-Martin JA, Tohill BC. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr Rev. 2004;62:1. doi: 10.1111/j.1753-4887.2004.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 283.Rolls BJ, Roe LS, Meengs JS. Portion size can be used strategically to increase vegetable consumption in adults. Am J Clin Nutr. 2010;91:913. doi: 10.3945/ajcn.2009.28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Perez-Escamilla R, Obbagy JE, Altman JM, Essery EV, McGrane MM, Wong YP, Spahn JM, Williams CL. Dietary energy density and body weight in adults and children: a systematic review. J Acad Nutr Diet. 2012;112:671. doi: 10.1016/j.jand.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 285.Tohill BC, Seymour J, Serdula M, Kettel-Khan L, Rolls BJ. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62:365. doi: 10.1111/j.1753-4887.2004.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 286.Tapsell LC, Dunning A, Warensjo E, Lyons-Wall P, Dehlsen K. Effects of vegetable consumption on weight loss: a review of the evidence with implications for design of randomized controlled trials. Crit Rev Food Sci Nutr. 2014;54:1529. doi: 10.1080/10408398.2011.642029. [DOI] [PubMed] [Google Scholar]
- 287.Heo M, Kim RS, Wylie-Rosett J, Allison DB, Heymsfield SB, Faith MS. Inverse association between fruit and vegetable intake and BMI even after controlling for demographic, socioeconomic and lifestyle factors. Obes Facts. 2011;4:449. doi: 10.1159/000335279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Buijsse B, Feskens EJ, Schulze MB, Forouhi NG, Wareham NJ, Sharp S, Palli D, Tognon G, Halkjaer J, Tjonneland A, Jakobsen MU, Overvad K, van der AD, Du H, Sorensen TI, Boeing H. Fruit and vegetable intakes and subsequent changes in body weight in European populations: results from the project on Diet, Obesity, and Genes (DiOGenes) Am J Clin Nutr. 2009;90:202. doi: 10.3945/ajcn.2008.27394. [DOI] [PubMed] [Google Scholar]
- 289.Vergnaud AC, Norat T, Romaguera D, Mouw T, May AM, Romieu I, Freisling H, Slimani N, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Teucher B, Boeing H, Buijsse B, Tjonneland A, Halkjaer J, Overvad K, Jakobsen MU, Rodriguez L, Agudo A, Sanchez MJ, Amiano P, Huerta JM, Gurrea AB, Wareham N, Khaw KT, Crowe F, Orfanos P, Naska A, Trichopoulou A, Masala G, Pala V, Tumino R, Sacerdote C, Mattiello A, Bueno-de-Mesquita HB, van Duijnhoven FJ, Drake I, Wirfalt E, Johansson I, Hallmans G, Engeset D, Braaten T, Parr CL, Odysseos A, Riboli E, Peeters PH. Fruit and vegetable consumption and prospective weight change in participants of the European Prospective Investigation into Cancer and Nutrition-Physical Activity, Nutrition, Alcohol, Cessation of Smoking, Eating Out of Home, and Obesity study. Am J Clin Nutr. 2012;95:184. doi: 10.3945/ajcn.111.019968. [DOI] [PubMed] [Google Scholar]
- 290.Svendsen M, Blomhoff R, Holme I, Tonstad S. The effect of an increased intake of vegetables and fruit on weight loss, blood pressure and antioxidant defense in subjects with sleep related breathing disorders. Eur J Clin Nutr. 2007;61:1301. doi: 10.1038/sj.ejcn.1602652. [DOI] [PubMed] [Google Scholar]
- 291.Houchins JA, Burgess JR, Campbell WW, Daniel JR, Ferruzzi MG, McCabe GP, Mattes RD. Beverage vs. solid fruits and vegetables: effects on energy intake and body weight. Obesity (Silver Spring) 2012;20:1844. doi: 10.1038/oby.2011.192. [DOI] [PubMed] [Google Scholar]
- 292.Whybrow S, Harrison CL, Mayer C, James SR. Effects of added fruits and vegetables on dietary intakes and body weight in Scottish adults. Br J Nutr. 2006;95:496. doi: 10.1079/bjn20051489. [DOI] [PubMed] [Google Scholar]
- 293.Tapsell LC, Batterham MJ, Thorne RL, O'Shea JE, Grafenauer SJ, Probst YC. Weight loss effects from vegetable intake: a 12-month randomised controlled trial. Eur J Clin Nutr. 2014 doi: 10.1038/ejcn.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Tanumihardjo SA, Valentine AR, Zhang Z, Whigham LD, Lai HJ, Atkinson RL. Strategies to increase vegetable or reduce energy and fat intake induce weight loss in adults. Exp Biol Med (Maywood) 2009;234:542. doi: 10.3181/0810-RM-293. [DOI] [PubMed] [Google Scholar]
- 295.Lapointe A, Weisnagel SJ, Provencher V, Begin C, Dufour-Bouchard AA, Trudeau C, Lemieux S. Comparison of a dietary intervention promoting high intakes of fruits and vegetables with a low-fat approach: long-term effects on dietary intakes, eating behaviours and body weight in postmenopausal women. Br J Nutr. 2010;104:1080. doi: 10.1017/S0007114510001716. [DOI] [PubMed] [Google Scholar]
- 296.Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85:1465. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ledikwe JH, Rolls BJ, Smiciklas-Wright H, Mitchell DC, Ard JD, Champagne C, Karanja N, Lin PH, Stevens VJ, Appel LJ. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr. 2007;85:1212. doi: 10.1093/ajcn/85.5.1212. [DOI] [PubMed] [Google Scholar]
- 298.Mason I. Yo-Yo Dieting - Dangerous Weight Loss? Ezine@rticles; 2012. [Google Scholar]
- 299.FDA. Federal Register Final Rule - FR69 6787 Final Rule Declaring Dietary Supplements Containing Ephedrine Alkaloids Adulterated Because They Present an Unreasonable Risk February 11, 2004. US Department of Health & Human Services; 2004. [PubMed] [Google Scholar]
- 300.National Institute of Diabetes and Digestive and Kidney Disease. Weight Cycling. 2006 http://www.weightwatchers.com/images/1033/dynamic/GCMSImages/wtcycling2bw_LIBRARY_SEPT.pdf.
- 301.Voss G. Break the dangerous cycle of yo-yo dieting. MSNBC Today Health; 2012. [Google Scholar]
- 302.Kirkova D. A woman's weight fluctuates by 1.6 stone during her lifetime… and we're 7.4lbs lighter in the summer. Mail Online; 2013. [Google Scholar]
- 303.Mashta O. Mail Online. 2013 7-15-2013. [Google Scholar]
- 304.NIDDK. Weight Cycling. Weight-control Information Network. 2013 7-15-2013. [Google Scholar]
- 305.Osborn RL, Forys KL, Psota TL, Sbrocco T. Yo-yo dieting in African American women: weight cycling and health. Ethn Dis. 2011;21:274. [PMC free article] [PubMed] [Google Scholar]
- 306.Jeffery RW. Does weight cycling present a health risk? Am J Clin Nutr. 1996;63:452S. doi: 10.1093/ajcn/63.3.452. [DOI] [PubMed] [Google Scholar]
- 307.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 308.Hollar D. Cross-sectional changes in patterns of allostatic load among persons with varying disabilities, NHANES: 2001-2010. Disabil Health J. 2013;6:177. doi: 10.1016/j.dhjo.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 309.Tremblay A, Chaput JP. Obesity: the allostatic load of weight loss dieting. Physiol Behav. 2012;106:16. doi: 10.1016/j.physbeh.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 310.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 311.Lee IM, Paffenbarger RS., Jr Change in body weight and longevity. JAMA. 1992;268:2045. [PubMed] [Google Scholar]
- 312.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169:881. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Stevens VL, Jacobs EJ, Sun J, Patel AV, McCullough ML, Teras LR, Gapstur SM. Weight cycling and mortality in a large prospective US study. Am J Epidemiol. 2012;175:785. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 314.Dyer AR, Stamler J, Greenland P. Associations of weight change and weight variability with cardiovascular and all-cause mortality in the Chicago Western Electric Company Study. Am J Epidemiol. 2000;152:324. doi: 10.1093/aje/152.4.324. [DOI] [PubMed] [Google Scholar]
- 315.Rzehak P, Meisinger C, Woelke G, Brasche S, Strube G, Heinrich J. Weight change, weight cycling and mortality in the ERFORT Male Cohort Study. Eur J Epidemiol. 2007;22:665. doi: 10.1007/s10654-007-9167-5. [DOI] [PubMed] [Google Scholar]
- 316.Coffey CS, Gadbury GL, Fontaine KR, Wang C, Weindruch R, Allison DB. The effects of intentional weight loss as a latent variable problem. Stat Med. 2005;24:941. doi: 10.1002/sim.1964. [DOI] [PubMed] [Google Scholar]
- 317.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169:881. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Stevens VL, Jacobs EJ, Sun J, Patel AV, McCullough ML, Teras LR, Gapstur SM. Weight cycling and mortality in a large prospective US study. Am J Epidemiol. 2012;175:785. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 319.Taing KY, Ardern CI, Kuk JL. Effect of the timing of weight cycling during adulthood on mortality risk in overweight and obese postmenopausal women. Obesity (Silver Spring) 2012;20:407. doi: 10.1038/oby.2011.207. [DOI] [PubMed] [Google Scholar]
- 320.List EO, Berryman DE, Wright-Piekarski J, Jara A, Funk K, Kopchick JJ. The effects of weight cycling on lifespan in male C57BL/6J mice. Int J Obes (Lond) 2013;37:1088. doi: 10.1038/ijo.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Anson RM, Jones B, de CR. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. Age (Dordr) 2005;27:17. doi: 10.1007/s11357-005-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J Gerontol. 1983;38:36. doi: 10.1093/geronj/38.1.36. [DOI] [PubMed] [Google Scholar]
- 323.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 324.WebMD. Snacking Into Obesity. WebMD; 2012. [Google Scholar]
- 325.Livestrong.com. Obesity & Snacks at School. 2010 Jan 19; Livestrong.com.
- 326.Harding A. Snacking, not portion size, largely driving U S overeating. Yahoo Health; Jun 29, 2011. [Google Scholar]
- 327.Mayo Clinic Staff. Snacks: How they fit into your weight-loss plan. MayoClinic; May 24, 2012. [Google Scholar]
- 328.US DHHS. Snacks-100 Calories or Less. WeCan; 2012. [Google Scholar]
- 329.Marino DD, King JC. Nutritional concerns during adolescence. Pediatr Clin North Am. 1980;27:125. doi: 10.1016/s0031-3955(16)33824-x. [DOI] [PubMed] [Google Scholar]
- 330.Berteus FH, Lindroos AK, Sjostrom L, Lissner L. Meal patterns and obesity in Swedish women-a simple instrument describing usual meal types, frequency and temporal distribution. Eur J Clin Nutr. 2002;56:740. doi: 10.1038/sj.ejcn.1601387. [DOI] [PubMed] [Google Scholar]
- 331.Piernas C, Popkin BM. Snacking increased among U.S. adults between 1977 and 2006. J Nutr. 2010;140:325. doi: 10.3945/jn.109.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Duffey KJ, Popkin BM. Energy density, portion size, and eating occasions: contributions to increased energy intake in the United States, 1977-2006. PLoS Med. 2011;8:e1001050. doi: 10.1371/journal.pmed.1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zizza C, Siega-Riz AM, Popkin BM. Significant increase in young adults' snacking between 1977-1978 and 1994-1996 represents a cause for concern! Prev Med. 2001;32:303. doi: 10.1006/pmed.2000.0817. [DOI] [PubMed] [Google Scholar]
- 334.McCrory MA, Campbell WW. Effects of eating frequency, snacking, and breakfast skipping on energy regulation: symposium overview. J Nutr. 2011;141:144. doi: 10.3945/jn.109.114918. [DOI] [PubMed] [Google Scholar]
- 335.Palmer MA, Capra S, Baines SK. Association between eating frequency, weight, and health. Nutr Rev. 2009;67:379. doi: 10.1111/j.1753-4887.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 336.Mesas AE, Munoz-Pareja M, Lopez-Garcia E, Rodriguez-Artalejo F. Selected eating behaviours and excess body weight: a systematic review. Obes Rev. 2012;13:106. doi: 10.1111/j.1467-789X.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 337.Rodriguez G, Moreno LA. Is dietary intake able to explain differences in body fatness in children and adolescents? Nutr Metab Cardiovasc Dis. 2006;16:294. doi: 10.1016/j.numecd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 338.Newby PK. Are dietary intakes and eating behaviors related to childhood obesity? A comprehensive review of the evidence. J Law Med Ethics. 2007;35:35. doi: 10.1111/j.1748-720X.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 339.Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nunez-Cordoba JM, Toledo E, Serrano-Martinez M. Prospective study of self-reported usual snacking and weight gain in a Mediterranean cohort: the SUN project. Clin Nutr. 2010;29:323. doi: 10.1016/j.clnu.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 340.Keski-Rahkonen A, Bulik CM, Pietilainen KH, Rose RJ, Kaprio J, Rissanen A. Eating styles, overweight and obesity in young adult twins. Eur J Clin Nutr. 2007;61:822. doi: 10.1038/sj.ejcn.1602601. [DOI] [PubMed] [Google Scholar]
- 341.Takahashi E, Yoshida K, Sugimori H, Miyakawa M, Izuno T, Yamagami T, Kagamimori S. Influence factors on the development of obesity in 3-year-old children based on the Toyama study. Prev Med. 1999;28:293. doi: 10.1006/pmed.1998.0428. [DOI] [PubMed] [Google Scholar]
- 342.Ritchie LD. Less frequent eating predicts greater BMI and waist circumference in female adolescents. Am J Clin Nutr. 2012;95:290. doi: 10.3945/ajcn.111.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kong A, Beresford SA, Alfano CM, Foster-Schubert KE, Neuhouser ML, Johnson DB, Duggan C, Wang CY, Xiao L, Bain CE, McTiernan A. Associations between snacking and weight loss and nutrient intake among postmenopausal overweight to obese women in a dietary weight-loss intervention. J Am Diet Assoc. 2011;111:1898. doi: 10.1016/j.jada.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Johnstone AM, Shannon E, Whybrow S, Reid CA, Stubbs RJ. Altering the temporal distribution of energy intake with isoenergetically dense foods given as snacks does not affect total daily energy intake in normal-weight men. Br J Nutr. 2000;83:7. [PubMed] [Google Scholar]
- 345.Poston WS, Haddock CK, Pinkston MM, Pace P, Karakoc ND, Reeves RS, Foreyt JP. Weight loss with meal replacement and meal replacement plus snacks: a randomized trial. Int J Obes (Lond) 2005;29:1107. doi: 10.1038/sj.ijo.0803007. [DOI] [PubMed] [Google Scholar]
- 346.Vander Wal JS, Waller SM, Klurfeld DM, McBurney MI, Cho S, Kapila M, Dhurandhar NV. Effect of a post-dinner snack and partial meal replacement program on weight loss. Int J Food Sci Nutr. 2006;57:97. doi: 10.1080/09637480600658369. [DOI] [PubMed] [Google Scholar]
- 347.Tey SL, Brown R, Gray A, Chisholm A, Delahunty C. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J Nutr Metab. 2011;2011:357350. doi: 10.1155/2011/357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Viskaal-van DM, Kok FJ, de GC. Effects of snack consumption for 8 weeks on energy intake and body weight. Int J Obes (Lond) 2010;34:319. doi: 10.1038/ijo.2009.243. [DOI] [PubMed] [Google Scholar]
- 349.Zaveri S, Drummond S. The effect of including a conventional snack (cereal bar) and a nonconventional snack (almonds) on hunger, eating frequency, dietary intake and body weight. J Hum Nutr Diet. 2009;22:461. doi: 10.1111/j.1365-277X.2009.00983.x. [DOI] [PubMed] [Google Scholar]
- 350.Sallis JF, Cervero RB, Ascher W, Henderson KA, Kraft MK, Kerr J. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 351.Community Prevention Services Task Force. Environmental and Policy Approaches to Increase Physical Activity: Point to Decision Prompts to Encourage Use of Stairs. 2005 [Google Scholar]
- 352.Koplan JP, Liverman CT, Kraak VI. Preventing childhood obesity: health in the balance: executive summary. J Am Diet Assoc. 2005;105:131. doi: 10.1016/j.jada.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 353.Associated Press. Fast-food zoning limits and more walkable neighborhoods keys to fight obesity. Daily News; 2013. [Google Scholar]
- 354.Aytur SA, Rodriguez DA, Evenson KR, Catellier DJ, Rosamond WD. The sociodemographics of land use planning: relationships to physical activity, accessibility, and equity. Health Place. 2008;14:367. doi: 10.1016/j.healthplace.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 355.Craig CL, Brownson RC, Cragg SE, Dunn AL. Exploring the effect of the environment on physical activity: a study examining walking to work. Am J Prev Med. 2002;23:36. doi: 10.1016/s0749-3797(02)00472-5. [DOI] [PubMed] [Google Scholar]
- 356.Davison KK, Werder JL, Lawson CT. Children's active commuting to school: current knowledge and future directions. Prev Chronic Dis. 2008;5:A100. [PMC free article] [PubMed] [Google Scholar]
- 357.De BI, Sallis JF, Saelens BE. Environmental correlates of physical activity in a sample of Belgian adults. Am J Health Promot. 2003;18:83. doi: 10.4278/0890-1171-18.1.83. [DOI] [PubMed] [Google Scholar]
- 358.Dollman J, Lewis NR. Active transport to school as part of a broader habit of walking and cycling among South Australian youth. Pediatr Exerc Sci. 2007;19:436. doi: 10.1123/pes.19.4.436. [DOI] [PubMed] [Google Scholar]
- 359.Smith KR, Brown BB, Yamada I, Kowaleski-Jones L, Zick CD, Fan JX. Walkability and body mass index density, design, and new diversity measures. Am J Prev Med. 2008;35:237. doi: 10.1016/j.amepre.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 360.Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:417. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- 361.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 362.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 363.Schoenborn CA, Adams PF, Barnes PM. Body weight status of adults: United States, 1997-98. Adv Data. 1:2002. [PubMed] [Google Scholar]
- 364.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 365.Kruger J, Yore MM, Kohl HW., III Leisure-time physical activity patterns by weight control status: 1999-2002 NHANES. Med Sci Sports Exerc. 2007;39:788. doi: 10.1249/mss.0b013e3180333efc. [DOI] [PubMed] [Google Scholar]
- 366.Glass TA, Rasmussen MD, Schwartz BS. Neighborhoods and obesity in older adults: the Baltimore Memory Study. Am J Prev Med. 2006;31:455. doi: 10.1016/j.amepre.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ferdinand A, Sen B, Rahurkar S, Engler S, Menachemi N. The Relationship Between Built Environments and Physical Activity: A Systematic Review. Am J Public Health. 2012 doi: 10.2105/AJPH.2012.300740. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev. 2007;29:129. doi: 10.1093/epirev/mxm009. [DOI] [PubMed] [Google Scholar]
- 369.Rahman T, Cushing RA, Jackson RJ. Contributions of built environment to childhood obesity. Mt Sinai J Med. 2011;78:49. doi: 10.1002/msj.20235. [DOI] [PubMed] [Google Scholar]
- 370.Cummins S, Fagg J. Does greener mean thinner? Associations between neighbourhood greenspace and weight status among adults in England. Int J Obes (Lond) 2012;36:1108. doi: 10.1038/ijo.2011.195. [DOI] [PubMed] [Google Scholar]
- 371.Farley TA, Meriwether RA, Baker ET, Watkins LT, Johnson CC, Webber LS. Safe play spaces to promote physical activity in inner-city children: results from a pilot study of an environmental intervention. Am J Public Health. 2007;97:1625. doi: 10.2105/AJPH.2006.092692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Gustat J, Rice J, Parker KM, Becker AB, Farley TA. Effect of changes to the neighborhood built environment on physical activity in a low-income African American neighborhood. Prev Chronic Dis. 2012;9:E57. [PMC free article] [PubMed] [Google Scholar]
- 373.American Academy of Pediatrics Committee on Communications: Children, adolescents, and television. Pediatrics. 1984;85:1119. [PubMed] [Google Scholar]
- 374.Dietz WH, Jr, Gortmaker SL. Do we fatten our children at the television set? Obesity and television viewing in children and adolescents. Pediatrics. 1985;75:807. [PubMed] [Google Scholar]
- 375.American Academy of Pediatrcis CoC. Children, adolescents and television. 1990;85:1119–1120. [PubMed] [Google Scholar]
- 376.Boone JE, Gordon-Larsen P, Adair LS, Popkin BM. Screen time and physical activity during adolescence: longitudinal effects on obesity in young adulthood. Int J Behav Nutr Phys Act. 2007;4:26. doi: 10.1186/1479-5868-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Carson V, Janssen I. The mediating effects of dietary habits on the relationship between television viewing and body mass index among youth. Pediatr Obes. 2012;7:391. doi: 10.1111/j.2047-6310.2012.00049.x. [DOI] [PubMed] [Google Scholar]
- 378.Crespo CJ, Smit E, Troiano RP, Bartlett SJ, Macera CA, Andersen RE. Television watching, energy intake, and obesity in US children: results from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2001;155:360. doi: 10.1001/archpedi.155.3.360. [DOI] [PubMed] [Google Scholar]
- 379.Anderson SE, Economos CD, Must A. Active play and screen time in US children aged 4 to 11 years in relation to sociodemographic and weight status characteristics: a nationally representative cross-sectional analysis. BMC Public Health. 2008;8:366. doi: 10.1186/1471-2458-8-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Mitchell JA, Rodriguez D, Schmitz KH, Audrain-McGovern J. Greater screen time is associated with adolescent obesity: a longitudinal study of the BMI distribution from Ages 14 to 18. Obesity (Silver Spring) 2013;21:572. doi: 10.1002/oby.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.te Velde SJ, van NF, Uijtdewilligen L, van Stralen MM, Cardon G, De CM, Manios Y, Brug J, Chinapaw MJ. Energy balance-related behaviours associated with overweight and obesity in preschool children: a systematic review of prospective studies. Obes Rev. 2012;13(Suppl 1):56. doi: 10.1111/j.1467-789X.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 382.Rideout V, Foehr U, Roberts D. Generation M2:media in the lives of 8- to 18-year-olds. The Henry J.Kaiser Family Foundation; Menlo Park, CA: 2010. [Google Scholar]
- 383.American Academy of Pediatrics Committee on Public Education. Children, adolescents and television. 2001;107:423–426. doi: 10.1542/peds.107.2.423. [DOI] [PubMed] [Google Scholar]
- 384.American Academy of Pediatrics Council on Communications and Media. Children, adolescents, obesity and the media. 2011;128:201–208. doi: 10.1542/peds.2011-1066. [DOI] [PubMed] [Google Scholar]
- 385.Hagan JF, Jr, Shaw JS, Duncan PM. Bright Futures:Guidelines for Health Supervision of Infants, Children and Adolescents. Pediatrics. 2007:S254–S288. [Google Scholar]
- 386.Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, Taveras EM. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 387.Epstein LH, Paluch RA, Consalvi A, Riordan K, Scholl T. Effects of manipulating sedentary behavior on physical activity and food intake. J Pediatr. 2002;140:334. doi: 10.1067/mpd.2002.122395. [DOI] [PubMed] [Google Scholar]
- 388.Epstein LH, Roemmich JN, Paluch RA, Raynor HA. Influence of changes in sedentary behavior on energy and macronutrient intake in youth. Am J Clin Nutr. 2005;81:361. doi: 10.1093/ajcn.81.2.361. [DOI] [PubMed] [Google Scholar]
- 389.Rey-Lopez JP, Vicente-Rodriguez G, Biosca M, Moreno LA. Sedentary behaviour and obesity development in children and adolescents. Nutr Metab Cardiovasc Dis. 2008;18:242. doi: 10.1016/j.numecd.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 390.Temple JL, Giacomelli AM, Kent KM, Roemmich JN, Epstein LH. Television watching increases motivated responding for food and energy intake in children. Am J Clin Nutr. 2007;85:355. doi: 10.1093/ajcn/85.2.355. [DOI] [PubMed] [Google Scholar]
- 391.Klesges RC, Shelton ML, Klesges LM. Effects of television on metabolic rate: potential implications for childhood obesity. Pediatrics. 1993;91:281. [PubMed] [Google Scholar]
- 392.Epstein LH, Roemmich JN, Paluch RA, Raynor HA. Physical activity as a substitute for sedentary behavior in youth. Ann Behav Med. 2005;29:200. doi: 10.1207/s15324796abm2903_6. [DOI] [PubMed] [Google Scholar]
- 393.Chahal H, Fung C, Kuhle S, Veugelers PJ. Availability and night-time use of electronic entertainment and communication devices are associated with short sleep duration and obesity among Canadian children. Pediatr Obes. 2013;8:42. doi: 10.1111/j.2047-6310.2012.00085.x. [DOI] [PubMed] [Google Scholar]
- 394.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 395.Pearson N, Biddle SJ. Sedentary behavior and dietary intake in children, adolescents, and adults. A systematic review. Am J Prev Med. 2011;41:178. doi: 10.1016/j.amepre.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 396.Bellissimo N, Pencharz PB, Thomas SG, Anderson GH. Effect of television viewing at mealtime on food intake after a glucose preload in boys. Pediatr Res. 2007;61:745. doi: 10.1203/pdr.0b013e3180536591. [DOI] [PubMed] [Google Scholar]
- 397.Francis LA, Birch LL. Does eating during television viewing affect preschool children's intake? J Am Diet Assoc. 2006;106:598. doi: 10.1016/j.jada.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Patel BP, Bellissimo N, Thomas SG, Hamilton JK, Anderson GH. Television viewing at mealtime reduces caloric compensation in peripubertal, but not postpubertal, girls. Pediatr Res. 2011;70:513. doi: 10.1203/PDR.0b013e31822d783e. [DOI] [PubMed] [Google Scholar]
- 399.Fakhouri TH, Hughes JP, Brody DJ, Kit BK, Ogden CL. Physical activity and screen-time viewing among elementary school-aged children in the United States from 2009 to 2010. JAMA Pediatr. 2013;167:223. doi: 10.1001/2013.jamapediatrics.122. [DOI] [PubMed] [Google Scholar]
- 400.Laurson KR, Eisenmann JC, Welk GJ, Wickel EE, Gentile DA, Walsh DA. Combined influence of physical activity and screen time recommendations on childhood overweight. J Pediatr. 2008;153:209. doi: 10.1016/j.jpeds.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 401.Halford JC, Boyland EJ, Hughes G, Oliveira LP, Dovey TM. Beyond-brand effect of television (TV) food advertisements/commercials on caloric intake and food choice of 5-7-year-old children. Appetite. 2007;49:263. doi: 10.1016/j.appet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 402.Halford JC, Boyland EJ, Hughes GM, Stacey L, McKean S, Dovey TM. Beyond-brand effect of television food advertisements on food choice in children: the effects of weight status. Public Health Nutr. 2008;11:897. doi: 10.1017/S1368980007001231. [DOI] [PubMed] [Google Scholar]
- 403.Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol. 2009;28:404. doi: 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wahi G, Parkin PC, Beyene J, Uleryk EM, Birken CS. Effectiveness of interventions aimed at reducing screen time in children: a systematic review and meta-analysis of randomized controlled trials. Arch Pediatr Adolesc Med. 2011;165:979. doi: 10.1001/archpediatrics.2011.122. [DOI] [PubMed] [Google Scholar]
- 405.Schmidt ME, Haines J, O'Brien A, McDonald J, Price S, Sherry B, Taveras EM. Systematic review of effective strategies for reducing screen time among young children. Obesity (Silver Spring) 2012;20:1338. doi: 10.1038/oby.2011.348. [DOI] [PubMed] [Google Scholar]
- 406.Rolls BJ, Roe LS, Meengs JS. The effect of large portion sizes on energy intake is sustained for 11 days. Obesity (Silver Spring) 2007;15:1535. doi: 10.1038/oby.2007.182. [DOI] [PubMed] [Google Scholar]
- 407.Division of Nutrition and Physical Activity. Do Increased Portion Sizes Affect How Much We Eat? Centers for Disease Control; 2006. [Google Scholar]
- 408.Hook DB. How Portion Size Adds Up to Obesity. Everyday Health; Aug 5, 2009. [Google Scholar]
- 409.Wansink B, van IK, Painter JE. Ice cream illusions bowls, spoons, and self-served portion sizes. Am J Prev Med. 2006;31:240. doi: 10.1016/j.amepre.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 410.Devitt AA, Mattes RD. Effects of food unit size and energy density on intake in humans. Appetite. 2004;42:213. doi: 10.1016/j.appet.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 411.Fisher JO, Liu Y, Birch LL, Rolls BJ. Effects of portion size and energy density on young children's intake at a meal. Am J Clin Nutr. 2007;86:174. doi: 10.1093/ajcn/86.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Geier AB, Rozin P, Doros G. Unit bias. A new heuristic that helps explain the effect of portion size on food intake. Psychol Sci. 2006;17:521. doi: 10.1111/j.1467-9280.2006.01738.x. [DOI] [PubMed] [Google Scholar]
- 413.Marchiori D, Corneille O, Klein O. Container size influences snack food intake independently of portion size. Appetite. 2012;58:814. doi: 10.1016/j.appet.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 414.Steenhuis IH, Vermeer WM. Portion size: review and framework for interventions. Int J Behav Nutr Phys Act. 2009;6:58. doi: 10.1186/1479-5868-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Diliberti N, Bordi PL, Conklin MT, Roe LS, Rolls BJ. Increased portion size leads to increased energy intake in a restaurant meal. Obes Res. 2004;12:562. doi: 10.1038/oby.2004.64. [DOI] [PubMed] [Google Scholar]
- 416.Wansink B, Payne CR, Shimizu M. The 100-calorie semi-solution: sub-packaging most reduces intake among the heaviest. Obesity (Silver Spring) 2011;19:1098. doi: 10.1038/oby.2010.306. [DOI] [PubMed] [Google Scholar]
- 417.Wansink B, van IK, Payne CR. Larger Bowl Size Increases the Amount of Cereal Children Request, Consume, and Waste. J Pediatr. 2013 doi: 10.1016/j.jpeds.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 418.Rolls BJ, Roe LS, Meengs JS. Reductions in portion size and energy density of foods are additive and lead to sustained decreases in energy intake. Am J Clin Nutr. 2006;83:11. doi: 10.1093/ajcn/83.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Zlatevska N, Dubelaar C, Holden SS. Increasing serving size increases amount consumed: Catch-22. J Marketing. 2014 In press. [Google Scholar]
- 420.Just DR, Wansink B. One man's tall is another man's small: how the framing of portion size influences food choice. Health Econ. 2013 doi: 10.1002/hec.2949. [DOI] [PubMed] [Google Scholar]
- 421.Levitsky DA, Pacanowski C. Losing weight without dieting. Use of commercial foods as meal replacements for lunch produces an extended energy deficit. Appetite. 2011;57:311. doi: 10.1016/j.appet.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 422.Benton D. Portion Size: What we know and what we need to know. Critical Reviews in Food Science and Nutrition. 2013 doi: 10.1080/10408398.2012.679980. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Kesman RL, Ebbert JO, Harris KI, Schroeder DR. Portion control for the treatment of obesity in the primary care setting. BMC Res Notes. 2011;4:346. doi: 10.1186/1756-0500-4-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.McClain AD, van den Bos W, Matheson D, Desai M, McClure SM, Robinson TN. Visual illusions and plate design: the effects of plate rim widths and rim coloring on perceived food portion size. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Rolls BJ, Roe LS, Halverson KH, Meengs JS. Using a smaller plate did not reduce energy intake at meals. Appetite. 2007;49:652. doi: 10.1016/j.appet.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Shah M, Schroeder R, Winn W, Adams-Huet B. A pilot study to investigate the effect of plate size on meal energy intake in normal weight and overweight/obese women. J Hum Nutr Diet. 2011;24:612. doi: 10.1111/j.1365-277X.2011.01210.x. [DOI] [PubMed] [Google Scholar]
- 427.Robinson TN, Matheson DM. Environmental interventions to reduce overeating in children. 2014 [Google Scholar]
- 428.Hanks AS, Kaipainen K, Wansink B. The Syracuse Plate: Reducing BMI by Introducing Smaller Plates in Households. J Nutr Educ Behav. 2013;45:41. [Google Scholar]
- 429.Reeves GM, Postolache TT, Snitker S. Childhood Obesity and Depression: Connection between these Growing Problems in Growing Children. Int J Child Health Hum Dev. 2008;1:103. [PMC free article] [PubMed] [Google Scholar]
- 430.Gillman MW, Shiman SL, Frazier AL. Family dinner and diet quality among older children and adolescents. Archives of Family Medicine. 2000;9:235. doi: 10.1001/archfami.9.3.235. [DOI] [PubMed] [Google Scholar]
- 431.Neumark-Sztainer D, Hannan PJ, Story M, Croll J, Perry C. Family meal patterns: associations with sociodemographic characteristics and improved dietary intake among adolescents. J Am Diet Assoc. 2003;103:317. doi: 10.1053/jada.2003.50048. [DOI] [PubMed] [Google Scholar]
- 432.Eisenberg ME, Olson RE, Neumark-Sztainer D, Story M, Bearinger LH. Correlations between family meals and psychosocial well-being among adolescents. Arch Pediatr Adolesc Med. 2004;158:792. doi: 10.1001/archpedi.158.8.792. [DOI] [PubMed] [Google Scholar]
- 433.Neumark-Sztainer D, Wall M, Story M, Fulkerson JA. Are family meal patterns associated with disordered eating behaviors among adolescents? J Adolesc Health. 2004;35:350. doi: 10.1016/j.jadohealth.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 434.Anderson SE, Whitaker RC. Household routines and obesity in US preschool-aged children. Pediatrics. 2010;125:420. doi: 10.1542/peds.2009-0417. [DOI] [PubMed] [Google Scholar]
- 435.Sen B. Frequency of family dinner and adolescent body weight status: evidence from the national longitudinal survey of youth, 1997. Obesity (Silver Spring) 2006;14:2266. doi: 10.1038/oby.2006.266. [DOI] [PubMed] [Google Scholar]
- 436.Sen B. The relationship between frequency of family dinner and adolescent problem behaviors after adjusting for other family characteristics. J Adolesc. 2010;33:187. doi: 10.1016/j.adolescence.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 437.Taveras EM, Rifas-Shiman SL, Berkey CS, Rockett HR, Field AE, Frazier AL, Colditz GA, Gillman MW. Family dinner and adolescent overweight. Obes Res. 2005;13:900. doi: 10.1038/oby.2005.104. [DOI] [PubMed] [Google Scholar]
- 438.Hammons AJ, Fiese BH. Is frequency of shared family meals related to the nutritional health of children and adolescents? Pediatrics. 2011;127:e1565–e1574. doi: 10.1542/peds.2010-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Taveras EM, McDonald J, O'Brien A, Haines J, Sherry B, Bottino CJ, Troncoso K, Schmidt ME, Koziol R. Healthy Habits, Happy Homes: methods and baseline data of a randomized controlled trial to improve household routines for obesity prevention. Prev Med. 2012;55:418. doi: 10.1016/j.ypmed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 440.Dennis EA, Flack KD, Davy BM. Beverage consumption and adult weight management: A review. Eat Behav. 2009;10:237. doi: 10.1016/j.eatbeh.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin B. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2012;95:555. doi: 10.3945/ajcn.111.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Sinpetru L. Drinking Water Promotes Weight Loss. Softpedia; Jul 5, 2013. [Google Scholar]
- 443.Hendrick B. Water May Be Secret Weapon in Weight Loss. WebMD; Aug 23, 2010. [Google Scholar]
- 444.Sciamanna CN, Kiernan M, Rolls BJ, Boan J, Stuckey H, Kephart D, Miller CK, Jensen G, Hartmann TJ, Loken E, Hwang KO, Williams RJ, Clark MA, Schubart JR, Nezu AM, Lehman E, Dellasega C. Practices associated with weight loss versus weight-loss maintenance results of a national survey. Am J Prev Med. 2011;41:159. doi: 10.1016/j.amepre.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 445.Robert Wood Johnson Foundation. Recommendations for Healthier Beverages. Robert Wood Johnson Foundation; Mar 1, 2013. [Google Scholar]
- 446.Handbook of Non Drug Intervention Project Team. Pre-meal water consumption for weight loss. Aust Fam Physician. 2013;42:478. [PubMed] [Google Scholar]
- 447.Rolls BJ, Wood RJ, Rolls ET, Lind H, Lind W, Ledingham JG. Thirst following water deprivation in humans. Am J Physiol. 1980;239:R476–R482. doi: 10.1152/ajpregu.1980.239.5.R476. [DOI] [PubMed] [Google Scholar]
- 448.Engell D. Interdependency of food and water intake in humans. Appetite. 1988;10:133. doi: 10.1016/0195-6663(88)90064-5. [DOI] [PubMed] [Google Scholar]
- 449.Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev. 2010;68:505. doi: 10.1111/j.1753-4887.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Lappalainen R, Mennen L, van WL, Mykkanen H. Drinking water with a meal: a simple method of coping with feelings of hunger, satiety and desire to eat. Eur J Clin Nutr. 1993;47:815. [PubMed] [Google Scholar]
- 451.Flood JF, Morley JE. Increased food intake by neuropeptide Y is due to an increased motivation to eat. Peptides. 1991;12:1329. doi: 10.1016/0196-9781(91)90215-b. [DOI] [PubMed] [Google Scholar]
- 452.Panahi S, Luhovyy BL, Liu TT, Akhavan T, El KD, Goff HD, Harvey AG. Energy and macronutrient content of familiar beverages interact with pre-meal intervals to determine later food intake, appetite and glycemic response in young adults. Appetite. 2013;60:154. doi: 10.1016/j.appet.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 453.Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol Behav. 1990;48:19. doi: 10.1016/0031-9384(90)90254-2. [DOI] [PubMed] [Google Scholar]
- 454.Maersk M, Belza A, Holst JJ, Fenger-Gron M, Pedersen SB, Astrup A, Richelsen B. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: a controlled trial. Eur J Clin Nutr. 2012;66:523. doi: 10.1038/ejcn.2011.223. [DOI] [PubMed] [Google Scholar]
- 455.Van Walleghen EL, Orr JS, Gentile CL, Davy BM. Pre-meal water consumption reduces meal energy intake in older but not younger subjects. Obesity (Silver Spring) 2007;15:93. doi: 10.1038/oby.2007.506. [DOI] [PubMed] [Google Scholar]
- 456.Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. J Am Diet Assoc. 2008;108:1236. doi: 10.1016/j.jada.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Marciani L, Hall N, Pritchard SE, Cox EF, Totman JJ, Lad M, Hoad CL, Foster TJ, Gowland PA, Spiller RC. Preventing gastric sieving by blending a solid/water meal enhances satiation in healthy humans. J Nutr. 2012;142:1253. doi: 10.3945/jn.112.159830. [DOI] [PubMed] [Google Scholar]
- 458.Rolls BJ, Bell EA, Thorwart ML. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr. 1999;70:448. doi: 10.1093/ajcn/70.4.448. [DOI] [PubMed] [Google Scholar]
- 459.Clarkston WK, Pantano MM, Morley JE, Horowitz M, Littlefield JM, Burton FR. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am J Physiol. 1997;272:R243–R248. doi: 10.1152/ajpregu.1997.272.1.R243. [DOI] [PubMed] [Google Scholar]
- 460.Muckelbauer R, Sarganas G, Gruneis A, Muller-Nordhorn J. Association between water consumption and body weight outcomes: a systematic review. Am J Clin Nutr. 2013;98:282. doi: 10.3945/ajcn.112.055061. [DOI] [PubMed] [Google Scholar]
- 461.Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008;16:2481. doi: 10.1038/oby.2008.409. [DOI] [PubMed] [Google Scholar]
- 462.Dennis EA, Dengo AL, Comber DL, Flack KD, Savla J, Davy KP, Davy BM. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity (Silver Spring) 2010;18:300. doi: 10.1038/oby.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Akers JD, Cornett RA, Savla JS, Davy KP, Davy BM. Daily self-monitoring of body weight, step count, fruit/vegetable intake, and water consumption: a feasible and effective long-term weight loss maintenance approach. J Acad Nutr Diet. 2012;112:685. doi: 10.1016/j.jand.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Brown AW, Bohan Brown MM, Allison DB. Reply to RA Mekary and E Giovannucci. Am J Clin Nutr. 2014;99:213. doi: 10.3945/ajcn.113.077354. [DOI] [PubMed] [Google Scholar]
- 465.Casazza K, Allison DB. Stagnation in the clinical, community and public health domain of obesity: the need for probative research. Clinical Obesity. 2012;2:83. doi: 10.1111/j.1758-8111.2012.00052.x. [DOI] [PubMed] [Google Scholar]
- 466.Whitney WD. The Century dictionary and cyclopedia. 1906:379. [Google Scholar]
- 467.Bornstein RF, D'Agostino PR. Stimulus recognition and the mere exposure effect. J Pers Soc Psychol. 1992;63:545. doi: 10.1037//0022-3514.63.4.545. [DOI] [PubMed] [Google Scholar]
- 468.Fraze DS. Kentucky's war on weight & type 2 diabetes in youth: the battle continues as a legislative victory emerges. Ky Nurse. 2005;53:17. [PubMed] [Google Scholar]
- 469.Friedman JM. A war on obesity, not the obese. Science. 2003;299:856. doi: 10.1126/science.1079856. [DOI] [PubMed] [Google Scholar]
- 470.Gluckman PD, Hanson M, Zimmet P, Forrester T. Losing the war against obesity: the need for a developmental perspective. Sci Transl Med. 2011;3:93cm19. doi: 10.1126/scitranslmed.3002554. [DOI] [PubMed] [Google Scholar]
- 471.Greenwood L. Child health. Paras mark D-day in war on obesity. Health Serv J. 2009;119:20. [PubMed] [Google Scholar]
- 472.Kendall L. Public health. Early learning is key weapon in obesity war. Health Serv J. 2007;117:16. [PubMed] [Google Scholar]
- 473.Klein S. The war against obesity: attacking a new front. Am J Clin Nutr. 1999;69:1061. doi: 10.1093/ajcn/69.6.1061. [DOI] [PubMed] [Google Scholar]
- 474.Mello MM. New York City's war on fat. N Engl J Med. 2009;360:2015. doi: 10.1056/NEJMhle0806121. [DOI] [PubMed] [Google Scholar]
- 475.O'Hara L, Gregg J. The war on obesity: a social determinant of health. Health Promot J Austr. 2006;17:260. doi: 10.1071/he06260. [DOI] [PubMed] [Google Scholar]
- 476.Popper KR. Science: Problems, Aims, Responsibilities. Fed Proc. 1963;22:961. [PubMed] [Google Scholar]


