Abstract
Selective cytotoxicity to cancer cells without compromising their normal counterparts pose a huge challenge for traditional drug design. Here we developed a tumor antigen targeted delivery of immunonanoparticle carrying a novel non-immunosuppressive FTY720 derivative OSU-2S with potent cytotoxicity against leukemic B cells. OSU-2S induces activation of protein phosphatase 2A, phosphorylation and nuclear translocation of SHP1S591 and deregulation of multiple cellular processes in chronic lymphocytic leukemia (CLL) resulting in potent cytotoxicity. To preclude OSU-2S mediated effects on these ubiquitous phosphatases in unintended cells and avoid potential adverse effects we developed a OSU-2S targeted delivery immunonanoparticles (2A2-OSU-2S-ILP), that mediated selective cytotoxicity of CLL but not normal B cells through targeting receptor tyrosine kinase ROR1 expressed in leukemic but not normal B cells. Developing a novel spontaneous CLL mouse model expressing human ROR1 (hROR1) in all leukemic B cells, we demonstrate the therapeutic benefit of enhanced survival with 2A2-OSU-2S-ILP in-vivo. The newly developed non-immunosuppressive OSU-2S, its delivery using human CLL directed immunonanoparticles and the novel transgenic mouse model of CLL that expresses hROR1 exclusively in leukemic B cell surface are highly innovative and can be applied to CLL and other ROR1+ malignancies including mantle cell lymphoma and acute lymphoblastic leukemia.
Keywords: CLL, OSU-2S, phosphatases, ROR1, immunonanoparticle, ROR1 transgenic mice
INTRODUCTION
Chronic lymphocytic leukemia (CLL) characterized by accumulation of apoptotic resistance CD19+CD5+ B lymphocytes in peripheral blood and lymphoid organs is the most prevalent adulthood leukemia in the western hemisphere.1, 2 Current therapies for CLL are not curative rendering drug adverse effects and immunosuppression.3 Several hematological malignancies including CLL display aberrantly phosphorylated proteins that have led to development of inhibitors of kinases such as SYK, BTK, PIM, mTOR and PI3K for CLL therapy.4 While this approach is very promising, limitations associated with simultaneously targeting multiple survival kinases involved in diverse survival signaling pathways, kinase independent survival signals and selective inhibition in leukemic but not normal B cells that are critical for the normal physiology warrant alternate approaches. The discovery of predominantly inactive phosphatases in a variety of cancers5–9 and the potential for phosphatase targeted therapy as an alternative to kinase inhibitors especially in situations where the efficacy of the kinase inhibitors are compromised due to resistance mechanisms attributed to mutations and single nucleotide polymorphisms of the drug targets prompted us to evaluate potential activators of phosphatases in CLL and other B cell malignancies. We have recently reported the cytotoxic activity of FTY720, a sphingosine analogue against several B cell malignancies including CLL through modulation of protein phosphatase 2A (PP2A).10–12 Despite its promising in-vitro and in-vivo activity against a variety of solid tumors and heme-malignancies,10–15 our attempts to develop FTY720 for oncology indications was hindered by its immunosuppressive property involving T cell sequestration to lymph nodes through its action on sphingosine-1-phosphate receptor (S1PR). This prompted us to develop OSU-2S, {(S)-2-amino-2-(4-{(6-methylheptyl)-oxy} phenethyl)pentan-1-ol} a synthetic derivative of FTY720 by structure activity relationship with more potent anti tumor activity but lacking the sphingosine-1-phosphate receptor mediated immunosuppressive effects.16 The promising preclinical activity of OSU-2S in prognostically poor CLL patient cells prompted us to evaluate this molecule for further studies. Prolonged exposure of unintended normal cells to chemotherapeutic drugs contribute to major adverse effects. Novel therapy options that specifically target leukemic B cells sparing normal B cells are lacking and will be highly promising for CLL patients. To address this, we entrapped OSU-2S in lipid nanoparticles targeting the tumor antigen ROR1. ROR1 is a receptor tyrosine kinase expressed in over 95% of CLL but not normal B cells. Its unaltered surface expression related to the severity of CLL disease,17, 18 and its relatively low cell surface density and internalization property makes it an attractive target for armed rather than naked monoclonal antibodies(mAbs). Liposomal immunonanoparticles(ILPs) have provided a modality for high interactive affinities with target antigens on cell surface and are used to selectively enhance drug payload to target cells, favorably alter the drug kinetics, overcome drug off-target effects and potentially the drug efflux pumps.19 To evaluate in-vivo the proof-of-principle we have generated a custom mouse model expressing hROR1 surface protein on all leukemic B cells which further closely resembled CLL. Utilizing this in-vivo model we show the therapeutic benefit of delivering OSU-2S loaded nanoparticles directed against hROR1 expressing leukemic cells for CLL therapy.
MATERIALS AND METHODS
Cells
Peripheral blood was obtained from CLL patients after informed consent under protocol approved by the institution’s internal review board. All primary CLL cells used are immunophenotypically defined as outlined by the modified 1996 National Cancer Institute criteria.20 CLL cells were isolated from fresh patient blood using “Rosette-Sep” kit (Stem Cell Technologies; Vancouver, BC, Canada) and ficoll density gradient centrifugation (Ficoll-Paque Plus; Amershan Biosciences, Piscataway, NJ) according to the manufacturer’s instructions. Normal B cells were purified from leukopaks from American Red Cross, Central Ohio (Columbus, OH). Isolated mononuclear cells were cultured in RPMI 1640 media (Gibco, Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, St Louis, MO); 2 mM L-glutamine and penicillin (100 U/mL); streptomycin (100 µg/mL) (Gibco) at 37°C with 5% CO2. Human Burkitt lymphoma cell lines Raji and Ramos were obtained from American Type Culture Collection (ATCC, Manassas, VA) and MEC-1 and MEC-2 cells were obtained from DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Mouse spleenocytes were cultured as previously described21.
Cell viability and apoptosis
Cell death was assessed by FACS following annexin-V-FITC and propidium iodide (PI) (BD Bioscience, San Jose, CA) staining. Briefly one million cells in 200µl of annexin binding buffer (BD Bioscience) were stained with annexin-V-FITC and PI for 15 minutes in dark and read in Beckman-Coulter FC-500 cytometer. Proliferation of the cell lines was measured by CellTiter96® MTS {3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium} proliferation assay (Promega, Madison, WI) according to manufacturer’s instruction.
Immunoblotting
Immunoblotting was done using standard methods as described previously.10 Details in supplemental information.
Phosphatase assays
Non-radioactive PP2A assay was done as previously described10 and detailed in supplemental information.
Chemicals and reagents
OSU-2S was synthesized at The Ohio State University (OSU) Medicinal Chemistry Shared Resource as described previously.16 Nuclear magnetic resonance and mass spectrometry were used to confirm the purity and identity of the compound. FTY720 was purchased from ChemieTek (Indianapolis, IN). DMSO (Gibco) was used to dissolve FTY720/OSU-2S for in-vitro studies. 25% (2-Hydroxypropyl)-β-cyclodextrin (HPβCD) (Sigma-Aldrich) was used to dissolve OSU-2S for in-vivo studies. Phorbol-12-myristate-13-acetate (PMA) (Calbiochem, Millipore, Billerica, MA); Bisindolylmaleimide (Cell Signaling Technology, Danvers, MA); Egg phosphatidylcholine (Egg PC), methoxy-polyethylene glycol (MW~2,000Da)-distearoyl phosphatidyl- ethanolamine (PEG-DSPE) were purchased from Lipoid (Newark, NJ). 2-Iminothiolane (Traut’s reagent), cholesterol (Chol) and other chemicals were purchased from Sigma (St. Louis, MO). DSPE-PEG-maleimide (DSPE-PEG-mal) was obtained from Avanti Polar Lipids (Alabaster, AL). PKC activity was measured using PepTag assay kit, Promega (Madison, WI).
Antibodies against phospho S591 SHP1 (ECM Biosciences); GAPDH, SHP1, PP2Ac (Millipore); Brg1, (Santa Cruz Biotechnology, Dallas, TX); TCL1A (MBL International). Goat F(ab’)2 against human IgA+IgG+IgM (H+L) (Jackson ImmunoResearch Laboratories, West Grove, PA) were commercially obtained. Anti-human CD19-FITC/PE, CD20-PE, CD3-FITC/PE, CD37-FITC, CD86-FITC and mouse B220-FITC/PE/APC-Cy7, CD19-PE, CD3-FITC/PE, CD3-PE-Cy7, CD5-FITC/PE, CD45-APC, Streptavidin-PE (BD Bioscience); hROR1 Biotinylated/ Alexa Fluor 488 (R&D Systems) were used for flowcytometric analyses. 2A2-IgG against hROR1 was from Dr.Christoph Rader, CCR, NCI.
Quantitative RT-PCR
Cells suspended in TRizol®(Ambion, Life Technologies) were kept at −70°C until RNA was extracted following manufacturer’s instruction. cDNA was made using random primers (Invitrogen, Life Technologies). Real-time PCR was performed using TaqMan (Life Technologies) gene expression assay probe-primer sets for Tcl1A (ID:Hs00951350_m1) and 18S (ID:HS03003631_g1) and ViiA™ 7 Real-Time PCR System (Applied Biosystems). The expression of target genes relative to internal control gene was calculated using the threshold cycle number (Ct). The relative target gene expression for each condition was normalized to vehicle control and fold change determined using the comparative method (2ΔΔCt).
Gene expression-microarray analysis
RNA extracted from primary CLL cells that responded to OSU-2S was used for gene expression profiling (GEP) performed in Microarray Shared Resource (MASR) in the OSU Comprehensive Cancer Center. RNA Nano bioanalyzer was used to assess the integrity of the RNA samples. The labeled, fragmented cRNA samples were hybridized to GeneChip Human Genome U133 plus 2.0 (Affymetrix, GPL570).22 Detailed data analysis is described in statistics section. To identify possible molecular and cellular functions affected by OSU-2S, genes changed by >2 fold (p<0.0005) were selected for function and network analyses using Ingenuity pathway analysis (IPA) (http://www.ingenuity.com).
Preparation of immunonanoparticle 2A2-ILPs
Liposomes were synthesized with Cholestrol: Egg-PC: PEG-DSPE (molar ratio = 33.5: 65: 1.5) by rapidly injecting lipids dissolved in absolute ethanol into 10 times of phosphate buffered saline (PBS, pH7.4). 2A2-IgG was reacted with 10x Traut’s reagent at room temperature for 1hr at pH 8.0 to yield 2A2-IgG-SH, thus it further reacted with DSPE-PEG-mal at a molar ratio of 1:10 to form micelles at pH 6.8 for 1hr, followed by further incubation with liposomes at the lipid to 2A2-IgG ratio of 2000:1 at 37°C for 1hr. Fluorescent-labeled liposomes (LP-calcein or LP-FAM ODN) were prepared as previously described.23
Internalization study
The internalization rate of antibodies was determined by measuring and comparing the mean fluorescent intensity of fluorescently-labeled antibodies as described previously24 and outlined in the supplementary information.
Design and preparation of liposomal OSU-2S
The lipids chosen for our study (Cholestrol: Egg-PC: PEG-DSPE; molar ratio = 33.5: 65: 1.5) are widely used to encapsulate small molecule drugs. Characteristics of empty liposome, OSU-2S-LP and 2A2-OSU-2S-ILP are shown in supplementary table 1. The mean diameter by volume of nanoparticles did not change following addition of OSU-2S into the composition. OSU-2S altered the zeta potential of nanoparticles from - 4.10±0.34 to 5.41±0.12 mV. By measuring and calculating the amount of OSU-2S before and after dialysis against PBS with LC-MS/MS, the drug entrapment efficiency was 90.09 ± 2.69% OSU-2S. The step to immobilize 2A2-IgG-PEG-DSPE onto the surface of OSU-2S-LP did not alter the entrapment efficiency of OSU-2S, and still retained 88.25 ± 4.81% of OSU-2S in the liposomal form.
Eµ-ROR1 transgenic mice generation
The Eµ-ROR1 transgenic mice were generated at Genetically Engineered Mouse Modeling facility at the OSU Comprehensive Cancer Center (OSUCCC) as outlined in the material and methods section in supplementary information. Eµ-ROR1 transgenic mouse was crossed with Eµ-TCL1 mouse25 (kindly provided by Carlo Croce, OSU) to generate Eµ-ROR1-TCL1 double transgenic animals, that were confirmed for the expression of both ROR1 and Tcl1 transgenes.
In-vivo experiments
All animal experiments were carried out under protocols approved by OSU Institutional Animal Care and Use Committee (IACUC) modified from our previous report26 and are described in supplementary information.
Statistical analysis of data
All statistical analyses were performed by statisticians in Center for Biostatistics at OSU using SAS 9.3 software (SAS Inc, Cary, NC) as outlined in detail in supplementary information.
RESULTS
OSU-2S mediates PKC dependent cytotoxicity in CLL
OSU-2S mediated potent cytotoxicity in various B cell lines compared to FTY720 (Supplementary Figure S1a). In primary CLL cells OSU-2S caused time, dose {IC50=1.72µM, 95%CI 1.59-1.84µM} and cell density dependent apoptosis (Figure 1a and Supplementary Figure S1b-d). Potent cytotoxicity in prognostically poor IGVH unmutated (Figure 1b), or chromosome 17p (tumor suppressor p53 locus) deleted (Figure 1c) CLL patient cells, in MEC-227 and Raji28 (Supplementary Figure S1e-f) cells that are respectively resistant to fludarabine and rituximab, the two widely used CLL therapies, as well as cytotoxicity of fludarabine resistant/refractory patient derived primary CLL cells (Figure 1d) prompted us to evaluate OSU-2S further. OSU-2S induced comparable levels of PP2A activity in CLL cells similar to the parent compound FTY72010, 14, 29 (Figure 1e). Since PP2A mediated tumor growth suppression and apoptosis through SHP1 in myeloid leukemia treated with FTY720,14 we evaluated if OSU-2S affected the SHP1. Interestingly, OSU-2S treatment resulted in increased phospho SHP1S591 that was co-immunoprecipitated with PP2A (Supplementary Figure S2a-b) as early as 4hrs post-treatment. While OSU-2S consistently induced increased phospho SHP1S591 levels (Figure 2a), there was no change in the levels or enzymatic activity of SHP1 in CLL cells (Supplementary Figure S2c). Consistent with this OSU-2S failed to alter the phosphorylated Src Family Kinase Tyrosine416 (SFKY416), an auto phosphorylation site and a substrate of SHP130 (Supplementary Figure S2d). Moreover, comparison of phospho SHP1S591 levels (5hr post-treatment) and viability (24hr post-treatment) in OSU-2S treated CLL patient cells showed an inverse correlation (Figure 2b). The OSU-2S-induced phospho Serine 591 is located in C-terminal nuclear localization sequence of SHP1 protein.31, 32 Immunoblotting of phospho SHP1S591 and total SHP1 in cytosolic and nuclear extracts of CLL cells treated with OSU-2S revealed abundant phospho SHP1S591 in nuclear fraction which was further confirmed by high resolution confocal immunofluorescence microscopy (Supplementary Figure S2e-f). Protein kinase C (PKC) has been shown to co-associate with SHP1 and phosphorylate SHP1S591 residue.33 Consistent with a role for PKC in OSU-2S induced phosphorylation of SHP1S591 in CLL cells, bisindolylmaleimide (BIS), an inhibitor of PKC that inhibited PKC activator PMA induced phosphorylation of SHP1S591, also partially prevented OSU-2S induced phosphorylation of SHP1S591 (Figure 2a and Supplementary Figure S2g-h) and cytotoxicity (Figure 2c). This was further supported by increase in PKC activity in CLL cells treated with OSU-2S (Figure 2d) and a direct stimulatory effect of OSU-2S on purified PKC in in-vitro kinase assays (Figure 2e).
Figure 1.
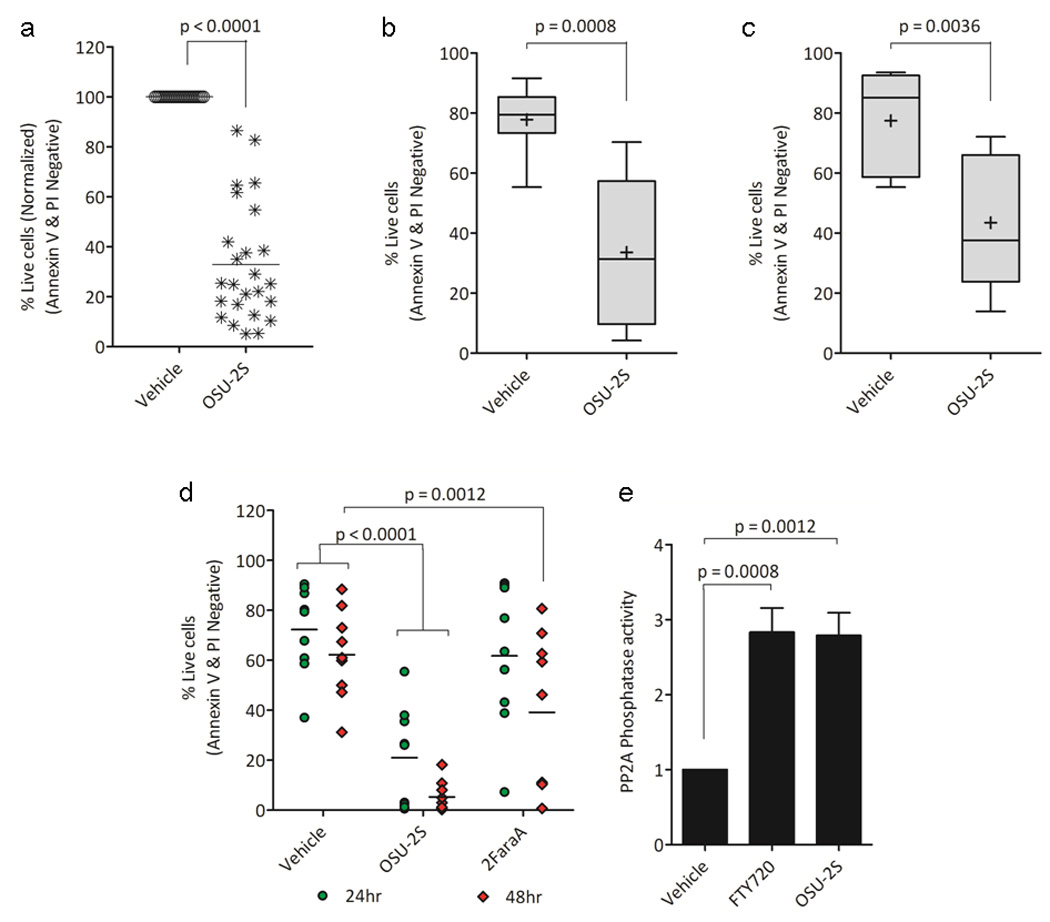
OSU-2S mediates cytotoxicity in CLL primary cells. (a-c) Cytotoxic effects of OSU-2S in CLL. Freshly isolated CD19+ CLL primary cells (1×106/ml) were treated with OSU-2S (2µM) and viability was assessed 24 hours later. (a) Mixed CLL samples (N=25). (B-C) Activity of OSU-2S in prognostically poor CLL patient derived primary cells (b) IGVH unmutated (N=8) and (c) chromosome 17p deleted (N=5) CLL cells. (d) Comparison of OSU-2S and Fludarabine (2FaraA) cytotoxicity in CLL. CLL cells (10×106/ml) were treated with OUS-2S(8µM) or 2FaraA(10µM) and viability was measured at indicated time points (N=9). (e) OSU-2S activates PP2A. CLL cells treated with OSU-2S or FTY720 were used for an in-vitro PP2A phosphatase enzyme assay. Values were normalized to protein levels and vehicle treated control; bars represent SD (N=3).
Figure 2.
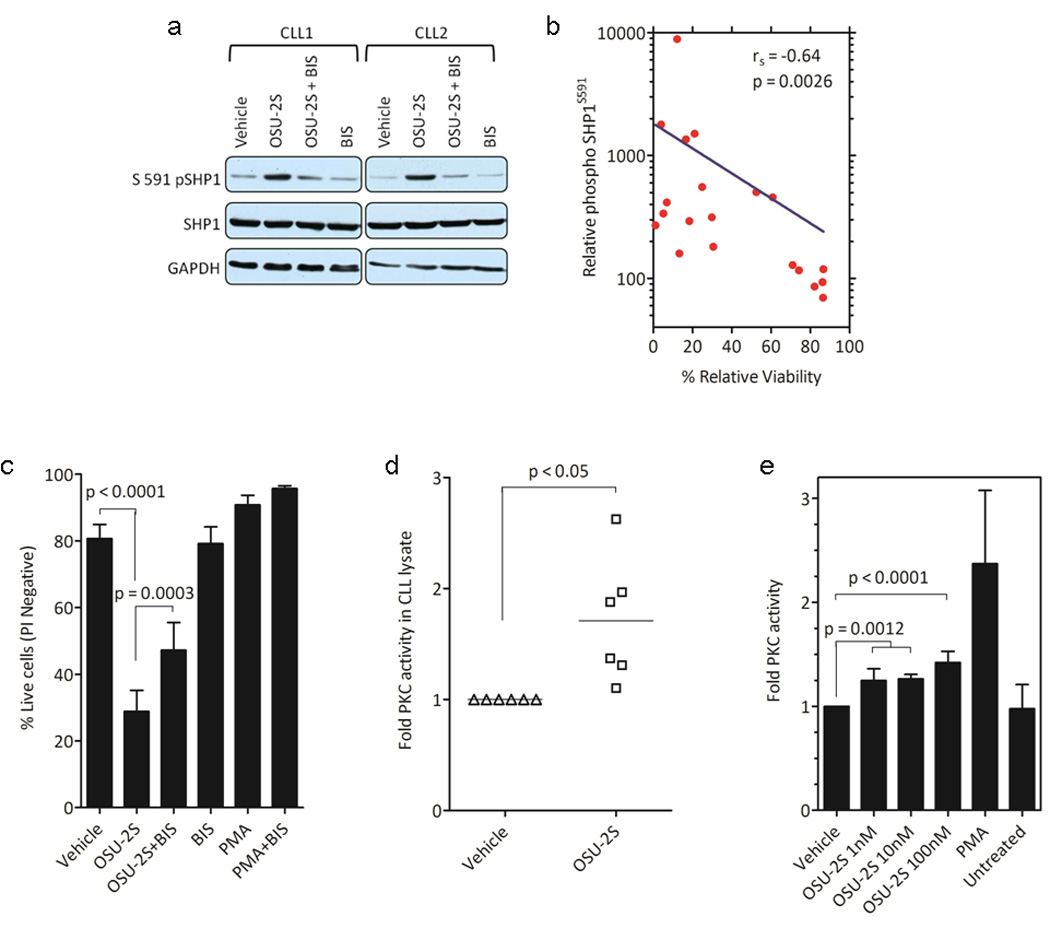
OSU-2S mediates PKC dependent phosphorylation of SHP1S591 in CLL. (a) OSU-2S induces PKC dependent phosphorylation of SHP1S591 in CLL. CLL cells treated with OSU-2S(5 hours) were immunoblotted for phospho SHP1S591. Bisindolylmaleimide (BIS) was used to inhibit PKC. (b) Inverse correlation between phospho SHP1S591 and viability. Spearman rank-order correlation was calculated for phospho SHP1S591 induced after 5 hour by immunoblotting and percent change in the viability at 24 hour time point in response to OSU-2S treatment (N=20). (c) PKC inhibitor BIS reduces phospho SHP1S591 and rescues OSU-2S cytotoxicity. Viability was done at 24 hour time point by PI staining on CLL cells treated with Vehicle or OSU-2S in the presence or absence of PKC inhibitor BIS(2µM) (N=8); bars represent SE. PMA is used as control for BIS. (D-E) OSU-2S activates PKC. (d) PKC activity was assessed in CLL cell lysates collected 4hr after treatment with vehicle or 8µM OSU-2S, (N=6). (e) Addition of OSU-2S to purified rat PKC had a direct stimulatory effect. PMA is used as positive control (N=3).
Need for targeted formulation of OSU-2S
Gene expression studies by microarray analysis of RNA isolated from OSU-2S treated CLL cells revealed at least 260 genes that were altered by >2 fold (p<0.0005) (Figure 3a) compared to vehicle treated controls. These genes represent modulators of cellular function and maintenance; development; growth and proliferation; cell death and survival; and cell cycle (Figure 3b). Ingenuity pathway analysis (IPA) of top 40 genes included BCR signaling pathway components, such as PI3Kγ, PLCγ, and MAP2K6. Consistent with this, OSU-2S treatment reduced BCR activation of CLL cells stimulated with goat F(ab’)2 against human IgA+IgG+IgM (H+L), as identified with reduced activation marker CD86 (Supplementary Figure S3). TCL1A expression, which is involved in the molecular pathogenesis of CLL34 and identified to be down regulated in response to OSU-2S in the gene expression profile was independently confirmed to be significantly down regulated both at the mRNA and protein levels (Figure 3c) with the corresponding up regulation in cFOS and FRA2 two known inhibitory targets of TCL1A.34 Modulation of ubiquitous phosphatases such as PP2A and SHP1 in unintended target cells and normal B cells where these enzymes are necessary for maintaining cellular functions and homeostasis poses serious limitations in clinical settings.
Figure 3.
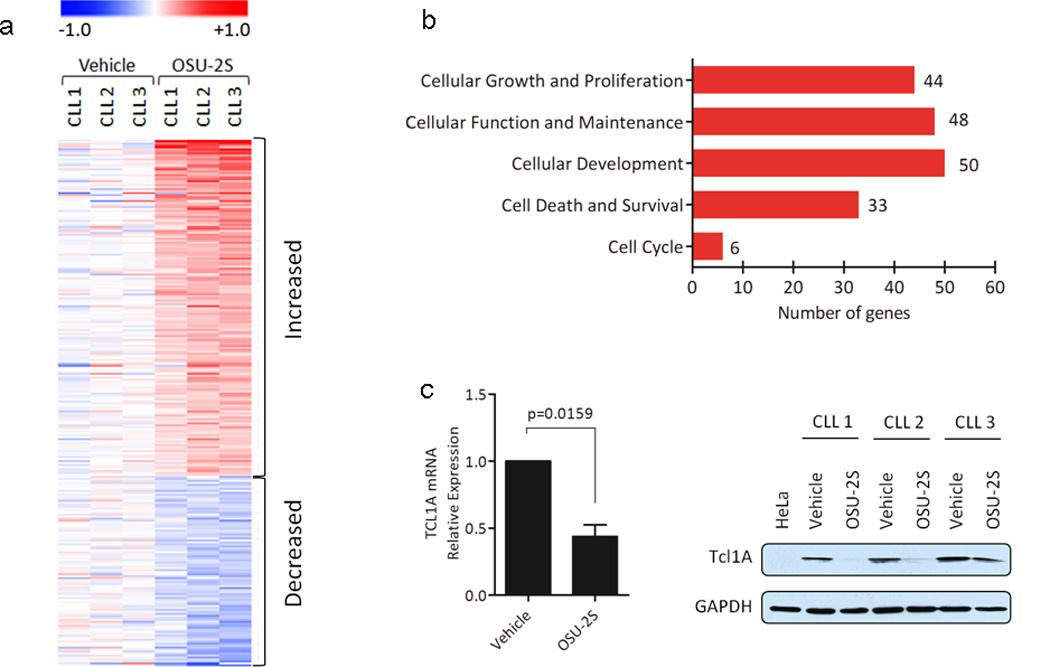
Gene expression analysis of CLL cells treated with OSU-2S. (a) Heat map of 266 differently expressed genes in OSU-2S treated CLL. RNA was isolated from CLL cells (N=3) treated with vehicle or OSU-2S(8µM) at 16 hours and subjected to microarray analyses using GeneChip Human Genome U133 plus 2.0 (Affymetrix, GPL570). After statistical analyses, genes that changed by ≥ 2 fold and p<0.0005 in response to OSU-2S treatment were used for constructing a heat map. (b) Top five molecular and cellular functions revealed through Ingenuity Pathway analysis (IPA) of genes modulated by OSU-2S in CLL. (c) OSU-2S down modulates TCL1A. RNA was extracted from vehicle or OSU-2S treated (16hr) CLL patients cells (N=7) and TCL1A expression relative to 18S was determined by real-time RT-PCR analysis and values are normalized to vehicle control; bars represent SD. Immunoblot of TCL1A in CLL cells treated with vehicle or OSU-2S for 24 hours is also shown.
ROR1 targeted immunonanoparticle formulation of OSU-2S
To target hROR1 surface protein in CLL cells, we considered anti-hROR1 mAb 2A2 (2A2-IgG)35 for immunonanoparticle formulation. The specificity analysis revealed 2A2-IgG stained only leukemic cells from CLL patients but not healthy donor B cells (Supplementary Figure S4a-c). Therefore, targeting ROR1 in CLL patients spares normal B cells, a robust advantage compared to other B cell targeting antibodies such as CD20 or CD19. In addition to specific targeting to leukemic cells, high rate of internalization of 2A2-IgG compared to anti-CD19 or anti-CD37 antibodies was observed in CLL cells (Figure 4a). The confocal microscopic analysis of 2A2-IgG mediated internalization as early as 15 minutes at 37°C in CLL cells is shown in supplementary figure S4d. Selective binding and internalization of 2A2-IgG in CLL cells prompted us to evaluate 2A2-ILPs for targeted delivery of OSU-2S in CLL. 2A2-ILPs loaded with calcein fluorescence molecule co-stained only CD19+ B cells but not CD3+ T cells in PBMC isolated from CLL patients (Supplementary Figure S4e), demonstrating selective binding and uptake of 2A2-ILPs by hROR1+ CLL cells. Furthermore, normal B cells did not uptake any 2A2-ILP calcein consistent with the lack of expression of hROR1 in normal B cells (Supplementary Figure S4f). CLL cells were dominantly selected by 2A2-ILP calcein while normal B cells or T cells did not uptake 2A2-ILPs as evident from mean fluorescent intensities (Figure 4b). It has been previously reported that 2A2-IgG alone was not able to induce significant direct cytotoxicity, complement dependent cytotoxicity or antibody dependent cellular cytotoxicity in primary CLL cells or hROR1 positive cell lines.35 In contrast to cross-linking effect of anti-CD19 and anti-CD37 ILPs that resulted in direct cytotoxicity,24 evaluation of hROR1+ cell lines, CLL cells or hROR1-ve normal B cells treated with 2A2-IgG, 2A2-IgG cross-linked with αFc antibody or 2A2-ILPs did not induce cytotoxicity in hROR1+ cell lines or CLL cells (Supplementary Figure S5a-b), thus providing a strong rationale for encapsulation of OSU-2S in 2A2-ILPs for ROR1 targeted delivery in CLL (Supplementary Table 1). CLL cells and normal B cells did not exhibit differences in responses to the treatment with free OSU-2S, OSU-2S-LP or IgG-OSU-2S-ILP (with non-targeting mouse IgG). However, CLL cells but not normal B cells were efficiently killed by hROR1 targeted 2A2-OSU-2S-ILP (Figure 4c-d).
Figure 4.
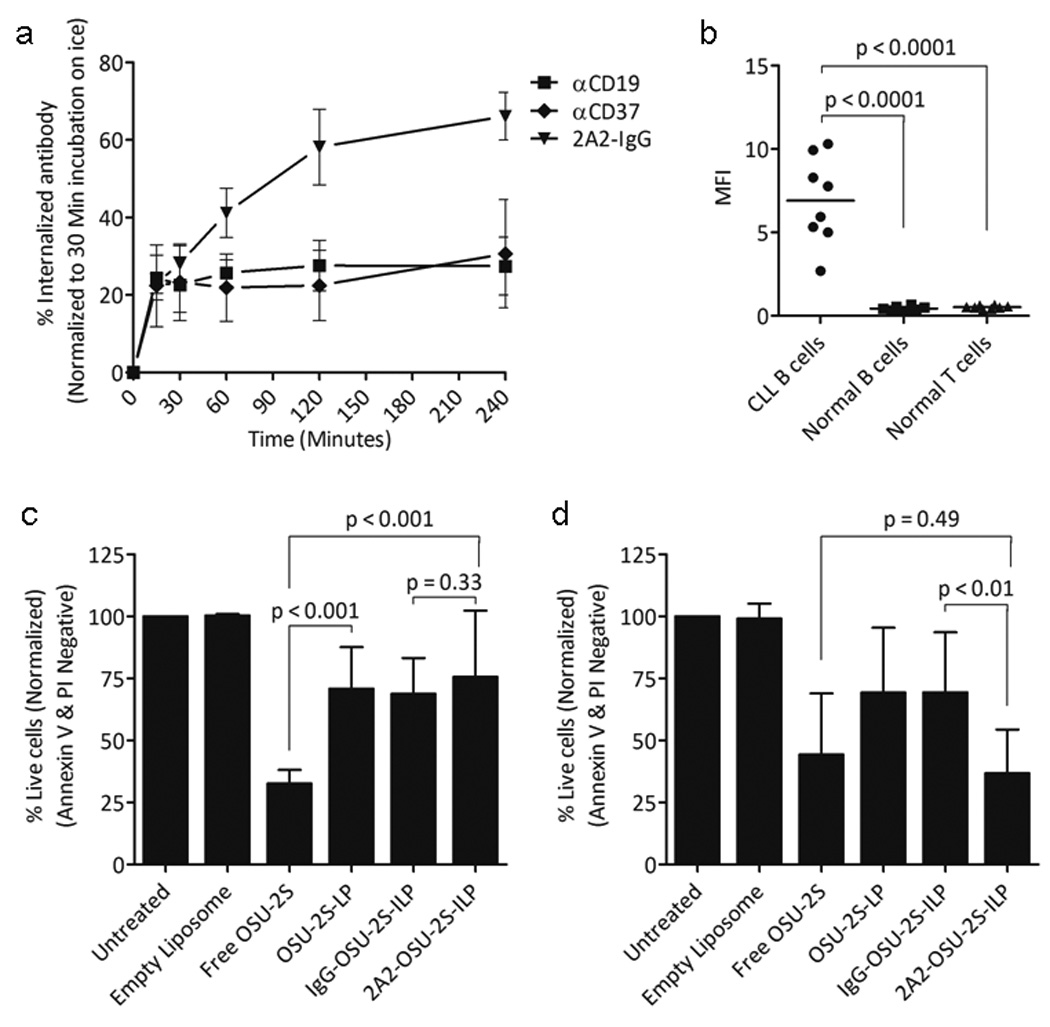
Immunonanoparticle 2A2-ILPs targeting ROR1+ CLL cells for OSU-2S drug delivery. (a) Internalization curve for 2A2-IgG bound ROR1 in CLL cells. Internalization involving time-dependent increase in mean fluorescent intensity (MFI) of labeled antibody on CLL cells treated with 2A2-IgG, αCD19 or αCD37 is shown. Bars represent mean ± SD, P<0.001 (N=7). (b) Internalization of 2A2-ILP. MFI showing the binding and uptake of 2A2-ILP calcein by CLL cells, normal B cells and T cells (N=8, P<0.0001). (c-d) 2A2-OSU-2S-ILP mediates specific cytotoxicity in CLL cells. 1×106 normal B cells from healthy donors or CLL cells were incubated with different formulation at 5µM of OSU-2S and 0.1µg/ml mAbs for 24hr before viability was analyzed by flow cytometry (mean ± SEM). (c) No difference in cytotoxicity between 2A2-OSU-2S-ILP and IgG-OSU-2S-ILP formulations was found for normal B cells (N=4). (d) 2A2-OSU-2S-ILP significantly induced improved cytotoxicity in CLL cells compared to non-targeting IgG-OSU-2S-ILP (N=8, P<0.01).
Generation of spontaneous leukemia model for in-vivo evaluation of ROR1 targeted therapy
For in-vivo evaluation of hROR1 targeted OSU-2S, we generated Eµ-ROR1 transgenic mouse expressing hROR1 exclusively in B cells using a transgenic construct containing the IgH promoter/enhancer elements upstream of hROR1 cDNA21 (Supplementary Figure S6a-b). The transgenic line was identified by transgene specific PCR primers and confirmed by Southern blotting . B cell specific hROR1 transgenic protein expression in the splenocytes was confirmed by flow cytometry using hROR1 antibody in combination with B (anti-B220) and T (anti-CD3) cell specific markers (Supplementary Figure S6c-e). 2A2-ILPs loaded with FAM ODN fluorescence molecule showed selective binding of 2A2-ILPs to hROR1+B220+ splenocytes from hROR1 transgenic mouse but not age matched non-transgenic mouse that are hROR1-ve B220+ (Figure 5a-b). Administration of 2A2-OSU-2S-ILP in Eµ-ROR1 single transgenic animals resulted in specific depletion of hROR1+ B cells in the peripheral blood (Figure 6a). To recapitulate the human CLL disease with surface hROR1 antigen, the hROR1 transgenic mouse was crossed with a CLL mouse model expressing human TCL1 transgene25 to generate Eµ-ROR1-TCL1 double transgenic mice. The development of CLL disease burden in these mice characterized by accumulation surface hROR1+B220+CD5+ leukemic cells in peripheral blood, spleen and bone marrow was confirmed and the splenic hROR1+B220+CD5+ leukemic cells are shown in figure 6b. Further splenocytes from hROR1+ Eµ-ROR1-TCL1 (Figure 6c) but not Eµ-TCL1 (Figure 6d) mice were significantly killed by 2A2-OSU-2S-ILP in ex-vivo cultures. To target leukemic B cells and to avoid off target effects and adverse reaction if any, we used hROR1 targeted 2A2-OSU-2S-ILP in Eµ-ROR1-TCL1 double transgenic splenocytes engraftment model for therapy evaluation. Engraftment of B220+CD5+hROR1+ leukemic splenocytes originating from Eµ-ROR1-TCL1 double transgenic mice into C57BL/6 mice developed aggressive leukemia (WBC counts >10×103/µl) with hROR1+B220+CD5+ cells in peripheral blood. These mice were randomly grouped into 2A2-OSU-2S-ILP, IgG-OSU-2S-ILP, OSU-2S-LP or 2A2-Empty-ILP treatment cohorts and dosed 5 days every week for 4 weeks by intra-peritoneal injection of respective drug formulations. The leukemic burden assessed by staining peripheral blood for CD45+B220+CD5+ cells every other week revealed decreased leukemic burden and prolonged median survival (82 days) in 2A2-OSU-2S-ILP treated animals compared to 2A2-Empty-ILP group (22 days) (Figure 6e-f).
Figure 5.
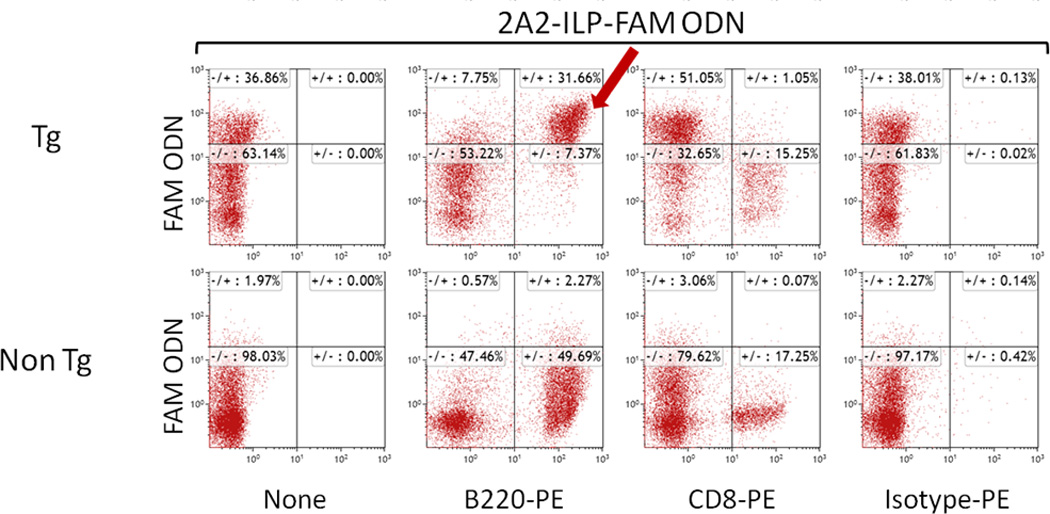
Immunonanoparticle 2A2-ILPs selectively bind to hROR1+ B cells from Eµ-ROR1 transgenic mice. Splenocytes (1×106) from Eµ-ROR1 transgenic(Tg) or age matched non-transgenic(Non Tg) mice were treated with 2A2-ILP FAM ODN for 30 minutes at 37°C, washed and stained with mouse B220-PE or CD8-PE. Flowcytometric analysis revealed FAM ODN signal only in B220+ Eµ-ROR1 transgenic mice but not to B cells from non-transgenic mice.
Figure 6.
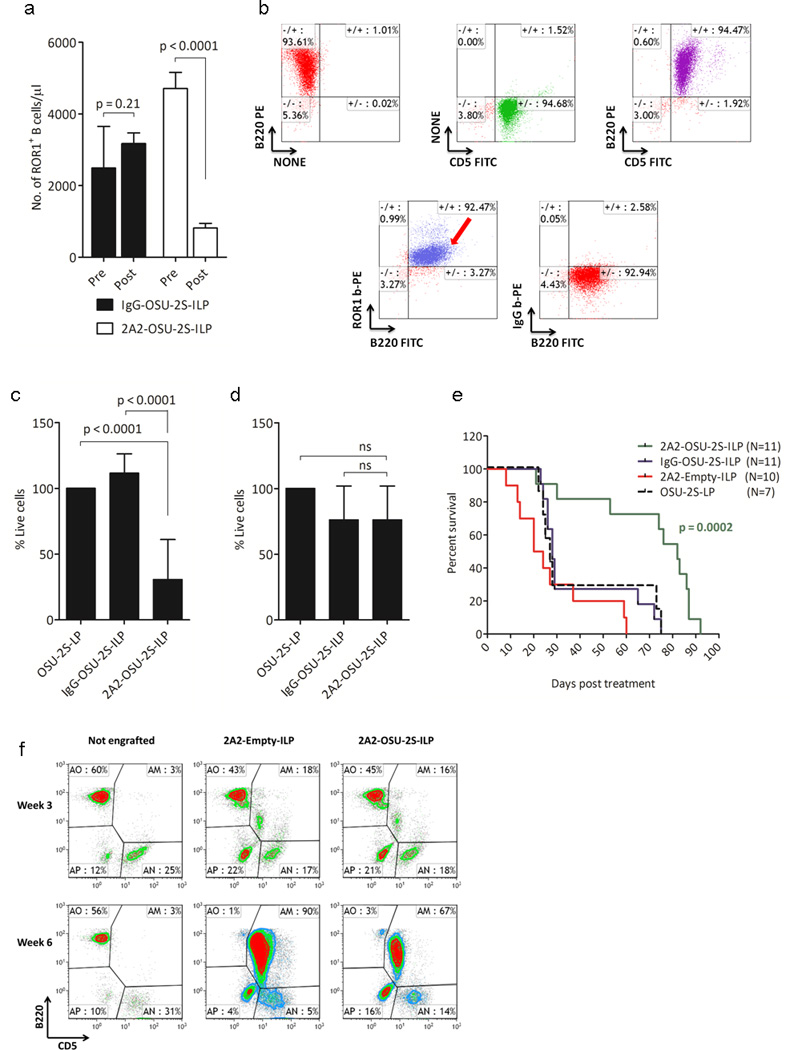
ROR1 targeted delivery of OSU-2S prolongs survival in hROR1+ CLL mouse model. (a) Specific depletion of ROR1+ B cells in peripheral blood of Eµ-ROR1 single transgenic animals after three doses of 2A2-OSU-2S-ILP intravenous treatment (10mg/kg every other day). (b) Flowcytometric dot plot of hROR1+ splenic cells from Eµ-ROR1-TCL1 animal with leukemia. (c-d) Comparison of specific cytotoxicity of 2A2-OSU-2S-ILP in Eµ-ROR1-TCL1(N=8) (c) and Eµ-TCL1(N=6) (d) mice derived splenocytes cultured ex-vivo with different ILPs(5µg/ml) and viability was assessed after 16hr by flowcytometry. (e-f) ROR1 targeted delivery of OSU-2S prolongs survival in hROR1+ CLL mouse model. (e) Survival curve of Eµ-ROR1-TCL1 splenocytes engrafted mice treated with 2A2-OSU-2S-ILP(N=11); IgG-OSU-2S-ILP(N=11), OSU-2S-LP(N=7) or 2A2-Empty-ILP(N=10). (f) Peripheral blood analysis of leukemic burden in the engrafted mice by flowcytometry.
DISCUSSION
Chemotherapeutics remains a choice of treatment for several malignant diseases. Here we describe a CLL tumor antigen ROR1 targeted delivery of immunonanoparticle carrying a novel non-immunosuppressive phosphatase activating FTY720 derivative OSU-2S with potent cytotoxicity against leukemic B cells sparing normal B cells. These studies have focused on rational preclinical development of FTY720 derivative OSU-2S, its in-vitro and in-vivo mechanistic and therapeutic evaluations using novel targeted immunoliposomal delivery formulations and humanized mouse models of leukemia developed for this purpose. Despite promising in-vitro and in-vivo activity of FTY720 against a variety of solid tumors and heme-malignancies,10, 11, 13–15 our attempts to repurpose FTY720, a clinically approved drug for relapsing multiple sclerosis, for oncology indications were hindered by its immunosuppressive property involving T cell homing to lymph nodes. Hence, OSU-2S was rederived to distinguish it from the parent FTY720 compound in 3 aspects:16 i) Unlike FTY720, OSU-2S is not phosphorylated by sphingosine kinase-2 in in-vitro systems; ii) synthetic phosphorylated OSU-2S failed to internalize S1PRs in cell lines expressing S1PR but is internalized when treated with FTY720 or phosphorylated FTY720; iii) administration of OSU-2S to mice did not cause drastic peripheral blood T cell count reduction in contrast to FTY720 which engender lymph node homing of T cells (Supplementary Figure S7).
Congruent with FTY720, OSU-2S activated PP2A in CLL cells. Interestingly, OSU-2S induced association of PP2A and phospho SHP1S591 in CLL cells. Since SHP1 dependent PP2A mediated tumor growth suppression and apoptosis by reduced BCR/ABL expression and activity was established in CML,5 we sought to see if OSU-2S affected the enzyme activity of SHP1. While OSU-2S failed to modulate the SHP1 phosphatase activity, it induced phosphorylation of SHP1S591 at the putative PKC substrate motif (K/RXS*XK/R)36 on SHP1. Consistent with our speculation on the role for PKC in OSU-2S induced phosphorylation, concentrations of PKC inhibitor BIS that reduced the PMA induced PKC activity also inhibited OSU-2S induced phosphorylation of SHP1S591 in CLL cells. This was also supported by increase in PKC activity in CLL cells. More importantly OSU-2S had a direct stimulatory effect on purified PKC in our in-vitro kinase assays. These finding were consistent with literature evidence suggesting PKC as a relevant phosphorylating kinase of SHP1S591 residue.33, 37 Experimental conditions reducing the phosphorylation of SHP1 also significantly prevented the cytotoxicity of OSU-2S implying the potential role of SHP1 in OSU-2S mediated apoptosis. The expression of SHP1 is drastically lowered in many leukemias and lymphomas due to DNA promoter hyper methylation implying negative role of SHP-1 in development of leukemia/lymphoma.38 Moreover, restoration of SHP1 in leukemia having low expression or devoid of SHP1 resulted in sensitization or direct death of tumor cells.39–41 Further, it was recently shown that SHP1 is directly involved in promoting death signals in CLL cells after ligation of tetraspanin CD37.42 Our preliminary experiments indicated that cell lines expressing low levels of SHP1 are less sensitive to OSU-2S and when reconstituted with SHP1 became sensitive to OSU-2S only when SHP1S591 was phosphorylated, altogether confirming the importance of SHP1S591 phosphorylation in OSU-2S cytotoxicity. We did not find change in enzyme activity of phosphorylated SHP1S591 in CLL. This was supported by established cell type specific roles of S591 including increase or decrease in enzyme activity and nuclear localization signals. Phorbol ester had been shown to induce SHP1 activation with serine phosphorylation in HL-60 pro-myelocytic cells accompanied by growth arrest.43 However, in our study we saw no change in the enzyme activity, but nuclear localization of phospho SHP1S591 and cell death after treatment with OSU-2S. It is possible that nuclear translocated SHP1 might act on nuclear substrates or interact with other nuclear proteins regulating transcription to bring about cell death in our model.
The novel strategy to enhance the efficiency and specificity of OSU-2S drug delivery in CLL using hROR1 targeting ILP has promising clinical implications.ROR1 identified as oncofetal protein is expressed during embryogenesis and in some malignancies including CLL cells but absent in mature normal B cells.17, 18, 44 Recent discovery of ROR1 expression in B cell precursor, hematogones may indicate toxic potential of 2A2-OSU-2S-ILP on these population.45 But it should be noted that mature normal B cells or CD34+ precursors lack ROR1 and hematogones have low ROR1 expression and occur in very low frequencies compared to CLL. Furthermore, recent reports using different ROR1 antibodies indicate that ROR1 expression and its phosphorylation are directly correlated with disease severity in CLL and can enhance leukemogenesis in CLL.46–48 Thus, the unique expression pattern of ROR1 in CLL cells makes it an attractive target for therapy. This has led to development of various ROR1 targeted therapeutics including anti-ROR1 antibodies raised against different epitopes of ROR1 and have different cytotoxic potential.35, 48–51 Moreover, Fc domain engineering of anti-ROR1 antibodies are undertaken to enhance the effector functions like antibody dependent cell-mediated cytotoxicity(ADCC), complement dependent cytotoxicity(CDC) or properties of antibody-drug conjugate(ADC). The recently reported ROR1 directed chimeric antigenic receptor (ROR1-CAR) designed using 2A2-ScFv on T cell demonstrating potent anti-leukemic property against ROR1+ cells is noteworthy.52 Consistent with previous reports, our results demonstrated that anti-hROR1 mAb, 2A2-IgG selectively binds to CLL cells, is internalized efficiently, and does not induce cytotoxicity as a single reagent or in the presence of antibody or liposomal cross linker.35 Further, due to the limited cell surface density, hROR1 is considered preferably a target for armed rather than naked mAbs, though other anti-hROR1 mAbs inducing apoptosis in CLL have been reported.48, 50 It is conceivable that incorporation of cytotoxic anti-hROR1 mAbs as well as the use of human or humanized anti-hROR1 mAbs in ILP formulations has potential clinical utility.
The liposomal packaging of OSU-2S reduced the risk of other cells being exposed to the drug, however the minimal effect of OSU-2S-LP on normal B cell (Figure 4c & Supplementary Figure S8) may be due to prolonged exposure of these cells to lingering OSU-2S-LP particles which could have caused the particles to be fusing to normal B cells. Moreover, in targeted therapy 2A2-OSU-2S-ILP particles would be consumed by malignant cells and induce cytotoxicity and therefore fewer particles would be available for normal cells to get exposed and additional dosing can be used to eliminate the malignant cells if needed. Additionally, the concentration of targeted particles could be lowered and optimized in a range that can be toxic only to malignant cells for which extensive pharmacokinetic/pharmacoanalytical studies are needed and to confirm the integrity of the liposomes. Interestingly, 2A2-OSU-2S-ILP promoted comparable levels of cytotoxicity as CD20-OSU-2S-ILP in ROR1+CD20+ CLL cells (Supplementary Figure S9). Moreover, OSU-2S did not change the expression pattern of ROR1 in CLL which would support that OSU-2S neither lowers the target for 2A2-OSU-2S-ILP nor it enhances the ROR1 expression mediated survival in CLL cells (Supplementary Table 2). While cell surface CD19 did not changed after OSU-2S treatment in CLL, there was mild decrease in CD20 surface protein although statistically insignificant (Supplementary Table 3). These findings would support that OSU-2S does not alter the targets CD19 or CD20 which can be used for salvage therapy in CLL.
We have tested the activity OSU-2S as a single agent in a novel CLL mouse model (Supplementary Figure S10). In order to test the drug in a clinically relevant setting and to avoid drug adverse reaction, if any, we decided to deliver OSU-2S using an ILP formulation that could selectively target leukemic B cells in a mouse model of CLL. To accomplish this, we generated Eµ-ROR1 transgenic mice and crossed with the Eµ-TCL1 leukemia mouse model. These double transgenic mice exhibited CLL like disease later in life (>9 months) with additional expression of hROR1 in B220+CD5+ leukemic cells. Since these mice express hROR1 and to conduct an in-vivo study in a uniform leukemic model, we adapted the leukemic cells suitable for allograft model by passaging the leukemic clones in wild type background mice. By confirming the tumor burden in peripheral blood after engrafting hROR1+ leukemic splenocytes in experimental animals, we then started treatment with OSU-2S ILP targeting hROR1 which resulted in cytotoxicity specific for leukemic cells. The OSU-2S immunonanoparticle formulation that selectively targets hROR1+ leukemic CLL but not normal B cells in-vitro and in a humanized CLL mouse model rendering therapeutic benefit adds credence to the approach described here and can be extended to other therapeutic agents and ROR1+ malignancies including mantle cell lymphoma.
Supplementary Material
ACKNOWLEDGMENTS
The authors are thankful to the patients who supported this research by providing their blood. This work was funded by Leukemia & Lymphoma Society- Specialized Center of Research (SCOR) in leukemia grant LLS 7004-11, NCI-P50-CA140158, Harry Mangurian Foundation, D. Warren Brown Foundation, Robert J Anthony Fund and NSF grant NSEC EEC-0914790.
Footnotes
AUTHOR CONTRIBUTIONS: R.M. planned, designed and performed majority of the in-vitro and in-vivo research, analyzed data, wrote the initial and subsequent drafts of the paper and approved the final version of the paper. Y.M. synthesized and optimized targeted liposomes, designed and performed research, analyzed data, wrote the initial drafts of the paper. F.F. generated and characterized the ROR1 and ROR1xTCL1 double transgenic mice, assisted with the experiments. C.C., J.W., Y.Z., Y.W., and B.Y. synthesized and characterized targeted liposomes, helped with experiments. R.Y. synthesized OSU-2S. X.M. and L.Y. participated in designing sample sizes for each experiment, performed the statistical analysis. J.C.B., J.F., J.J., and L.A. contributed to the CLL patient care, patient sample acquisition and characterization, translation insight to the experimental designs. S.B., C.R., M.A.P., R.J.L. and C.S.C. and L.J.L. provided necessary reagents, supported components of the research. N.M oversaw the study, sought funding, participated in experimental design and generation of mouse models, data interpretation.
CONFLICT OF INTEREST: A provisional patent has been filed for the use of OSU-2S to treat hematologic malignancies. The authors declare no additional competing financial interests.
Supplementary information is available at Leukemia’s website.
REFERENCES
- 1.Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. doi: 10.1182/blood-2016-08-737650. [DOI] [PubMed] [Google Scholar]
- 2.Binet JL, Lepoprier M, Dighiero G, Charron D, D’Athis P, Vaugier G, et al. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer. 1977;40:855–864. doi: 10.1002/1097-0142(197708)40:2<855::aid-cncr2820400239>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Danilov AV. Targeted therapy in chronic lymphocytic leukemia: past, present, and future. Clinical therapeutics. 2013;35:1258–1270. doi: 10.1016/j.clinthera.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davids MS, Brown JR. Targeting the B Cell Receptor Pathway in Chronic Lymphocytic Leukemia. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2012.695781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Raghunath PN, Vonderheid E, Odum N, Wasik MA. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter. Am J Pathol. 2000;157:1137–1146. doi: 10.1016/S0002-9440(10)64629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oka T, Yoshino T, Hayashi K, Ohara N, Nakanishi T, Yamaai Y, et al. Reduction of hematopoietic cell-specific tyrosine phosphatase SHP-1 gene expression in natural killer cell lymphoma and various types of lymphomas/leukemias : combination analysis with cDNA expression array and tissue microarray. Am J Pathol. 2001;159:1495–1505. doi: 10.1016/S0002-9440(10)62535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oka T, Ouchida M, Koyama M, Ogama Y, Takada S, Nakatani Y, et al. Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res. 2002;62:6390–6394. [PubMed] [Google Scholar]
- 9.Johan MF, Bowen DT, Frew ME, Goodeve AC, Reilly JT. Aberrant methylation of the negative regulators RASSFIA, SHP-1 and SOCS-1 in myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol. 2005;129:60–65. doi: 10.1111/j.1365-2141.2005.05412.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Alinari L, Chen CS, Yan F, Dalton JT, Lapalombella R, et al. FTY720 shows promising in vitro and in vivo preclinical activity by downmodulating Cyclin D1 and phospho-Akt in mantle cell lymphoma. Clin Cancer Res. 2010;16:3182–3192. doi: 10.1158/1078-0432.CCR-09-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, et al. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893–6903. doi: 10.1182/blood-2011-06-363879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua CW, Lee DT, Ling MT, Zhou C, Man K, Ho J, et al. FTY720 a fungus metabolite inhibits in vivo growth of androgen-independent prostate cancer. Int J Cancer. 2005;117:1039–1048. doi: 10.1002/ijc.21243. [DOI] [PubMed] [Google Scholar]
- 14.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, et al. FTY720 a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung JH, Lu YS, Wang YC, Ma YH, Wang DS, Kulp SK, et al. FTY720 induces apoptosis in hepatocellular carcinoma cells through activation of protein kinase C delta signaling. Cancer Res. 2008;68:1204–1212. doi: 10.1158/0008-5472.CAN-07-2621. [DOI] [PubMed] [Google Scholar]
- 16.Omar HA, Chou CC, Berman-Booty LD, Ma Y, Hung JH, Wang D, et al. Antitumor effects of OSU-2S a nonimmunosuppressive analogue of FTY720 in hepatocellular carcinoma. Hepatology. 2011;53:1943–1958. doi: 10.1002/hep.24293. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Baskar S, Kwong KY, Hofer T, Levy JM, Kennedy MG, Lee E, et al. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2008;14:396–404. doi: 10.1158/1078-0432.CCR-07-1823. [DOI] [PubMed] [Google Scholar]
- 18.Daneshmanesh AH, Mikaelsson E, Jeddi-Tehrani M, Bayat AA, Ghods R, Ostadkarampour M, et al. Ror1 a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int J Cancer. 2008;123:1190–1195. doi: 10.1002/ijc.23587. [DOI] [PubMed] [Google Scholar]
- 19.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature reviews. Drug discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 21.Chen HC, Byrd JC, Muthusamy N. Differential role for cyclic AMP response element binding protein-1 in multiple stages of B cell development differentiation and survival. J Immunol. 2006;176:2208–2218. doi: 10.4049/jimmunol.176.4.2208. [DOI] [PubMed] [Google Scholar]
- 22.Dyrskjot L, Thykjaer T, Kruhoffer M, Jensen JL, Marcussen N, Hamilton-Dutoit S, et al. Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet. 2003;33:90–96. doi: 10.1038/ng1061. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Lu Y, Piao L, Wu J, Liu S, Marcucci G, et al. Targeting human clonogenic acute myelogenous leukemia cells via folate conjugated liposomes combined with receptor modulation by all-trans retinoic acid. Int J Pharm. 2010;402:57–63. doi: 10.1016/j.ijpharm.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu B, Mao Y, Yuan Y, Yue C, Wang X, Mo X, et al. Targeted drug delivery and cross-linking induced apoptosis with anti-CD37 based dual-ligand immunoliposomes in B chronic lymphocytic leukemia cells. Biomaterials. 2013;34:6185–6193. doi: 10.1016/j.biomaterials.2013.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci U S A. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckwith KA, Frissora FW, Stefanovski MR, Towns WH, Cheney C, Mo X, et al. The CD37-targeted antibody-drug conjugate IMGN529 is highly active against human CLL and in a novel CD37 transgenic murine leukemia model. Leukemia. 2014 doi: 10.1038/leu.2014.32. (10.1038/leu.2014.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassano C, Mactier S, Mulligan SP, Belov L, Huang P, Christopherson RI. Cladribine and Fludarabine Nucleoside Change the Levels of CD Antigens on B-Lymphoproliferative Disorders. International journal of proteomics 2010. 2010:964251. doi: 10.1155/2010/964251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czuczman MS, Olejniczak S, Gowda A, Kotowski A, Binder A, Kaur H, et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol. 2003;138:1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somani AK, Bignon JS, Mills GB, Siminovitch KA, Branch DR. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 1997;272:21113–21119. doi: 10.1074/jbc.272.34.21113. [DOI] [PubMed] [Google Scholar]
- 31.Craggs G, Kellie S. A functional nuclear localization sequence in the C-terminal domain of SHP-1. J Biol Chem. 2001;276:23719–23725. doi: 10.1074/jbc.M102846200. [DOI] [PubMed] [Google Scholar]
- 32.He D, Song X, Liu L, Burk DH, Zhou GW. EGF-stimulation activates the nuclear localization signal of SHP-1. J Cell Biochem. 2005;94:944–953. doi: 10.1002/jcb.20307. [DOI] [PubMed] [Google Scholar]
- 33.Brumell JH, Chan CK, Butler J, Borregaard N, Siminovitch KA, Grinstein S, et al. Regulation of Src homology 2-containing tyrosine phosphatase 1 during activation of human neutrophils. Role of protein kinase C. J Biol Chem. 1997;272:875–882. doi: 10.1074/jbc.272.2.875. [DOI] [PubMed] [Google Scholar]
- 34.Pekarsky Y, Palamarchuk A, Maximov V, Efanov A, Nazaryan N, Santanam U, et al. Tcl1 functions as a transcriptional regulator and is directly involved in the pathogenesis of CLL. Proc Natl Acad Sci U S A. 2008;105:19643–19648. doi: 10.1073/pnas.0810965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baskar S, Wiestner A, Wilson WH, Pastan I, Rader C. Targeting malignant B cells with an immunotoxin against ROR1. mAbs. 2012;4:349–361. doi: 10.4161/mabs.19870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 37.Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal. 2005;17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12. doi: 10.1016/s0378-1119(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 39.Han Y, Amin HM, Frantz C, Franko B, Lee J, Lin Q, et al. Restoration of shp1 expression by 5-AZA-2’-deoxycytidine is associated with downregulation of JAK3/STAT3 signaling in ALK-positive anaplastic large cell lymphoma. Leukemia. 2006;20:1602–1609. doi: 10.1038/sj.leu.2404323. [DOI] [PubMed] [Google Scholar]
- 40.Wu C, Guan Q, Wang Y, Zhao ZJ, Zhou GW. SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. J Cell Biochem. 2003;90:1026–1037. doi: 10.1002/jcb.10727. [DOI] [PubMed] [Google Scholar]
- 41.Paling NR, Welham MJ. Role of the protein tyrosine phosphatase SHP-1 (Src homology phosphatase-1) in the regulation of interleukin-3-induced survival proliferation and signalling. The Biochemical journal. 2002;368:885–894. doi: 10.1042/BJ20021054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lapalombella R, Yeh YY, Wang L, Ramanunni A, Rafiq S, Jha S, et al. Tetraspanin CD37 Directly Mediates Transduction of Survival and Apoptotic Signals. Cancer Cell. 2012;21:694–708. doi: 10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Z, Shen SH, Fischer EH. Phorbol ester-induced expression phosphorylation and translocation of protein-tyrosine-phosphatase 1C in HL-60 cells. Proc Natl Acad Sci U S A. 1994;91:5007–5011. doi: 10.1073/pnas.91.11.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borcherding N, Kusner D, Liu GH, Zhang W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein & cell. 2014 doi: 10.1007/s13238-014-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broome HE, Rassenti LZ, Wang HY, Meyer LM, Kipps TJ. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk Res. 2011;35:1390–1394. doi: 10.1016/j.leukres.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daneshmanesh AH, Porwit A, Hojjat-Farsangi M, Jeddi-Tehrani M, Tamm KP, Grander D, et al. Orphan receptor tyrosine kinases ROR1 and ROR2 in hematological malignancies. Leuk Lymphoma. 2013;54:843–850. doi: 10.3109/10428194.2012.731599. [DOI] [PubMed] [Google Scholar]
- 47.Hojjat-Farsangi M, Khan AS, Daneshmanesh AH, Moshfegh A, Sandin A, Mansouri L, et al. The tyrosine kinase receptor ROR1 is constitutively phosphorylated in chronic lymphocytic leukemia (CLL) cells. PLoS One. 2013;8:e78339. doi: 10.1371/journal.pone.0078339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Widhopf GF, 2nd, Cui B, Ghia EM, Chen L, Messer K, Shen Z, et al. ROR1 can interact with TCL1 and enhance leukemogenesis in Emu-TCL1 transgenic mice. Proc Natl Acad Sci U S A. 2014;111:793–798. doi: 10.1073/pnas.1308374111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hojjat-Farsangi M, Ghaemimanesh F, Daneshmanesh AH, Bayat AA, Mahmoudian J, Jeddi-Tehrani M, et al. Inhibition of the receptor tyrosine kinase ROR1 by anti-ROR1 monoclonal antibodies and siRNA induced apoptosis of melanoma cells. PLoS One. 2013;8:e61167. doi: 10.1371/journal.pone.0061167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Daneshmanesh AH, Hojjat-Farsangi M, Khan AS, Jeddi-Tehrani M, Akhondi MM, Bayat AA, et al. Monoclonal antibodies against ROR1 induce apoptosis of chronic lymphocytic leukemia (CLL) cells. Leukemia. 2012;26:1348–1355. doi: 10.1038/leu.2011.362. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Baskar S, Kwong KY, Kennedy MG, Wiestner A, Rader C. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS One. 2011;6:e21018. doi: 10.1371/journal.pone.0021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


