Abstract
Depression worsens most treatment outcomes in medically ill older adults. Chronic medical illnesses weaken and demoralize patients and compromise their ability to adhere to treatments requiring consistency and effort. Acute medical illnesses create a psychosocial storm that finds patients and their ecosystem unprepared. We describe two intervention models that can be used to target and personalize treatment in depressed, chronically or acutely medically ill older adults.
Personalized Adherence Intervention for Depression and COPD (PID-C) is a model intervention for depressed patients with chronic medical illnesses. It targets patient-specific barriers to treatment engagement and aims to shift the balance in favor of treatment participation. PID-C led to higher remission rates of depression, reduction in depressive symptoms, and reduction in dyspnea-related disability. Addition of problem solving training enables patients to utilize resources available to them and hopefully improve their outcomes.
Ecosystem Focused Therapy (EFT) is a model intervention for depression developing in the context of an acute medical event. It was developed for patients with post-stroke depression (PSD) and targets five areas, part of the “psychosocial storm” originating from the patient’s sudden disability and the resulting change in the patient’s needs and family’s life. A preliminary study suggests that EFT is feasible and efficacious in reducing depressive symptoms and signs and disability in PSD.
Introduction
Late-life depression preferentially affects older adults with comorbid medical illnesses. In community settings, 2% of older adults suffer from depression.(1) In primary care settings, the prevalence is 6–8%, and among long-term care residents it is 12–22.4%. Late-life depression has a modest response to pharmacotherapy, promotes disability, worsens medical outcomes, undermines adherence, and increases expense.(2) Behavioral interventions for depressed medically ill patients, while needed, have been both underdeveloped and underutilized.
Depression afflicts patients with both chronically deteriorating medical illnesses and acute debilitating medical events.(3) In each scenario, patients and families are presented with distinct sets of clinical and psychosocial problems that can serve as treatment targets. Below, we discuss intervention models for depressed patients with chronic obstructive pulmonary disease (COPD), a chronic disease, and patients with post-stroke depression (PSD), an acute medical event. These models could be modified to treat depression within the context of other chronic and acute medical illnesses that share similar characteristics.
COPD is a chronic illness that typifies the challenges faced by depressed, chronically ill patients. More than 20% of COPD patients suffer from at least one episode of major depression, often of longer duration.(3) In addition to high comorbidity, COPD leads to chronic disability, and its rehabilitation and treatment requires active and consistent patient participation. Depressed patients are typically demoralized from the hopelessness and fatigue of depression and the increasing disability of COPD. This impairs their ability to carry out everyday activities and interferes with adherence to efficacious treatments. Antidepressant, while available, safe, and often effective, are undermined by slow onset of their effects and often incomplete remission. This necessitates development of a long term approach to these patients.(3)
Unlike COPD, stroke occurs abruptly and exemplifies the problems of aging adults facing sudden disability after an acute medical event. Stroke afflicts 700,000 Americans each year and more than 20% of them subsequently develop depressive syndromes.(4) Post stroke depression (PSD) develops during the psychosocial and biological storm ensuing after stroke. The “storm” affects the patient and challenges the patient’s ecosystem.(4) The intervention model we describe below is based on a dissection of contributors to the “storm” and interventions targeting each if its components.
Evidence Based Psychotherapies
Therapy models for the treatment of late-life depression exist, although they have been inadequately studied. A recent paper reviewed psychosocial intervention studies using explicit selection criteria: a treatment manual, supervision by experts, raters blind to treatment assignment administering reliable and valid instruments, at least 30 participants per condition, intent to treat analysis and reliance on both statistical and clinical significance.(5) One study of problem solving therapy (PST)(6, 7) and one study of cognitive behavioral therapy(8) reviewed found these treatments more efficacious in reducing depression than an active comparison condition. Interpersonal therapy (IPT) also reduced depressive symptoms more than usual care (9, 10), but had no advantage over usual care in increasing response or remission rates. The only study of supportive therapy that met the above criteria found it less efficacious than PST in reducing depressive symptoms and disability. (6, 7)
Most participants in the studies reviewed were in stable medical health and few, if any, had chronic, deteriorating medical illnesses or a dramatic acute medical event.(5) Accordingly, the interventions targeted the behavioral pathology of depression rather than the distinct behavioral problems and needs arising from the clinical context of severe medical conditions.
Collaborative Care Models
Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) and Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) studied the effectiveness of care management models in depressed, older primary care patients. IMPACT compared usual care to a disease management program, which utilized depression clinical specialists following a stepped-care algorithm including medication or PST modified for the primary care setting. At one year follow-up, the intervention group had lower depression severity, higher rates of response, and higher rates of complete remission of depressive symptoms.(11) The PROSPECT intervention utilized depression care managers and a treatment algorithm with antidepressants as the first line of treatment, and if medications were refused, IPT. This intervention was effective in reducing suicidal ideation, depressive symptoms and increasing remission rates one and two years later.(12) Primary Care Research in Substance Abuse and Mental Health for the Elderly (PRISM-E) compared integrated behavioral health care and enhanced referral care. Results showed that less severe depression responded well to an integrated setting while severe depression responded better to enhanced specialty clinic care.(13)
The above studies demonstrated the value of several care management models in primary care patients. These models are flexible and can be adjusted to the needs of a variety of populations. Care managers working in the community require training on how to recognize treatment targets and guidance in introducing evidence-based therapies addressing the individual patient needs. What follows is an overview of the clinical context of two populations of depressed medically ill older adults and a description of two specialized care management models integrating elements of structured psychotherapies in the service of personalization (Figure 1).
Figure 1.
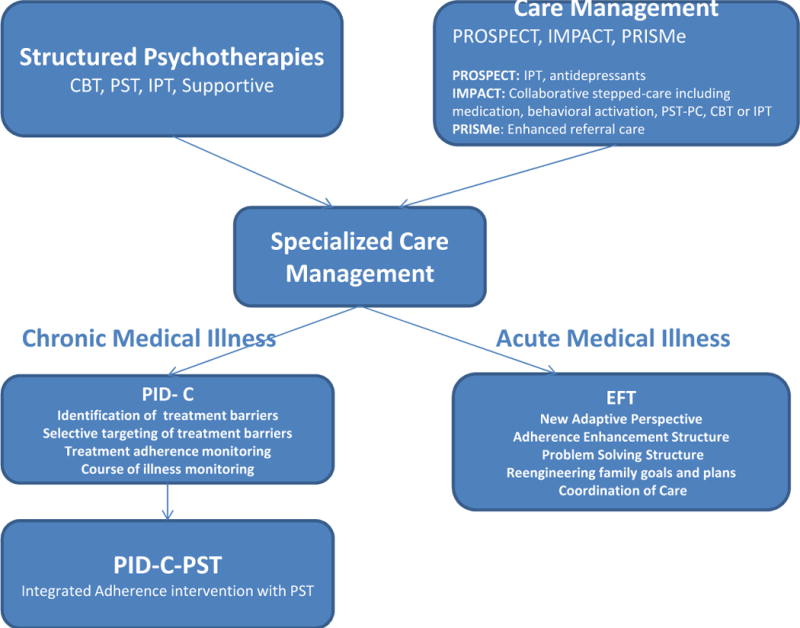
Specialized Care Management: Relationship to Structured Psychotherapies and to Non-Specialized Models of Care Management
Two Models of Care for Depressed Patients with Chronic Medical Illness
Depression in COPD patients exemplifies the clinical complexities encountered in an older, medically ill population. COPD afflicts 16.7% of older men and 12.6% of older women(14), and the prevalence in women and ethnic subpopulations is increasing.(15–18) Since 1990, COPD has been the fourth most frequent cause of death in the United States and the only major chronic disease for which death rates have not declined over the past 30 years.(18)
A recent study documented that depressive symptoms are associated with worse general and pulmonary health and disability in COPD patients.(19)
The American College of Chest Physicians and the American Association of Cardiovascular and Pulmonary Rehabilitation (ACCP/AACVPR) Guideline Panel pointed out that although depression affects a substantial number of COPD patients, depression is not a necessary concomitant of lung disease.(20) In some patients with COPD, there appear to be few, if any, significant psychological symptoms. This suggests that depression is a distinct syndrome in a significant minority of COPD patients that needs to be identified and treated.
The Role of Treatment Adherence
The ACCP/AACVPR Pulmonary Rehabilitation Evidence-Based Guideline states: “… the most important behavioral aspect of pulmonary rehabilitation is the extent to which patients comply with the exercise program or with other medical therapies”.(20) However, treatment adherence is a serious problem for COPD patients; 50% do not adhere to their regimen including taking medications as prescribed, using adequate oxygen at therapeutic level, or following recommended exercise regimes.(20)
The use of pulmonary rehabilitation, including exercise training and psychosocial support, is likely to improve functional exercise capacity and health related quality of life.(21) Patients with severe COPD and depression had a decrease in depressive symptomatology and disability following a comprehensive 15 day rehabilitation program.(22) The main contributors for the efficacy of this program may have been behavioral activation and psychosocial support. However, follow-up data 6 and 14 weeks after discharge from rehabilitation showed there to be no further improvement in these areas.(22)
Depression further increases the likelihood of poor adherence. A recent meta-analytic study concluded that, compared to non-depressed patients, depressed medical patients have 3 times greater odds of being noncompliant with prescribed medications, exercise, diet, health related behavior, vaccination, and appointments.(23) Poor adherence has a consistent negative effect on treatment outcomes.(24–26) Nearly 72% of depressed elderly patients with COPD refuse antidepressant medications and, of those who agreed to treatment, only 50% completed a trial of fluoxetine.(27)
COPD patients’ poor adherence to treatment is not surprising considering the clinical context of patients suffering from severe COPD and major depression. These patients live with daily physical discomfort, a severely compromised lifestyle and often become demoralized by the knowledge of the inevitable deteriorating course of their illness. Depressive symptoms including hopelessness, helplessness, and pessimism further promote resignation and demoralization. These feelings are compounded by a demanding rehabilitation regimen, which requires continuous patient participation. The last things a depressed, demoralized, cognitively compromised and weak COPD patient wants to do are to follow a tiring exercise program, visit treatment centers, and increase social activities.
An Adherence Intervention
In response to concerns about treatment adherence, we developed a personalized adherence intervention (PID-C) aimed at increasing adherence to recommended treatments for COPD and depression. PID-C draws from the Theory of Reasoned Action, in which patients weigh treatment risks and benefits(28) aimed to shift the balance in favor of treatment participation. PID-C is implemented by trained care managers who work with patients and their physicians. Care managers work with patients to identify treatment barriers and to support interventions (e.g., misconceptions about patients’ conditions, misunderstanding of recommendations, misattribution of symptoms, hopelessness, dissatisfaction with treatment, and logistic barriers) with the goal of helping patients’ to work on their exercise regimens and to take antidepressants as prescribed. Care managers also work with patients’ physicians to monitor treatment and the course of illness.(29)
PID-C was a beneficial intervention, resulting in reduced depressive symptomatology, reduced yspnea-related disability, and higher rates of remission compared to patients with major depression and severe COPD in the community.(29) These benefits lasted at least 6 months after the end of the intervention. Adherence to adequately prescribed antidepressants and low level of dyspnea partially mediated the beneficial effect on depression. Exercising at least 2 hours per week and a low severity of depressive symptoms partially mediate the improvement in dyspnea-related disability.
The Need for an Integrated Intervention
Although PID-C has been efficacious, adherence to available antidepressant treatments may be insufficient for some depressed COPD patients. Antidepressants are of limited efficacy, there is a lack of targeted psychotherapies for this population, and the presence of executive and other cognitive dysfunctions in COPD patients may impede treatment response.
Antidepressants have been inadequately investigated in depressed COPD patients. Placebo-controlled studies showed that doxepin, desipramine and paroxetine were no better than placebo in improving COPD patients’ moods.(3, 30, 31) Meanwhile, a 12-week placebo-controlled study of depressed or dysthymic COPD patients concluded that nortriptyline is more efficacious than placebo in reducing depressive symptoms, anxiety, respiratory symptoms, overall physical comfort, and day to day functioning.(32)
We hypothesize several reasons for limited efficacy in depressed COPD patients. The efficacy of antidepressants may be low in COPD patients who experience continuous physical discomfort, have a compromised lifestyle, and are exposed to chronic psychosocial adversity.(33) In addition, many COPD patients have cognitive impairments, which are associated with limited response to antidepressant agents. A review of literature by ACCP/AACVPR concluded that many COPD patients have executive dysfunction resulting in inability to think flexibly, perform complex perceptual motor maneuvers, and engage in perceptual discrimination.(20, 34–36) Several studies, including ours, suggest that executive dysfunction and brain abnormalities associated with executive dysfunction compromise treatment response to antidepressants.(37–41)
Sparse literature suggests that cognitive behavioral interventions reduce symptoms of anxiety and depression in COPD patients.(42, 43) The ACCP/AACVPR Pulmonary Rehabilitation Evidence-Based Guideline states that “evidence does not support the benefits of short-term psychosocial interventions as single therapeutic modalities, but longer-term interventions may be beneficial.”
An Integrated Intervention Model
The literature reviewed above indicates that the interactions between depression, disability, and poor treatment adherence contribute to behavioral and physical problems in COPD patients. We argue that PST integrated with our PID-C treatment adherence intervention can target the triad of depression, disability, and treatment non-adherence. This may disrupt the spiraling decline of these patients, reduce their suffering, and improve their quality of life. We base this assertion on findings documenting that: 1. PST can reduce depressive symptoms and disability in various groups with major depression; 2. PST has been effectively modified to reduce depression and disability in those with cognitive abnormalities similar to those displayed by COPD patients; and 3. PST incorporated within the PID-C intervention would target adherence to COPD treatment.
PST targets depression by enhancing patients’ problem solving skills to manage everyday problems and major life events. This is accomplished by providing structure for approaching problems and negotiating their daily lives. Research to date has shown that PST reduces symptoms of depression and disability in elderly patients with major depression, medical patients, and medically ill elderly patients.(44–46) We also reported that PST reduces depressive symptoms and disability in depressed older adults.(44, 47) In addition, patients whose depression improved with PST had increased problem solving skills and less disability.
PST has never been systematically studied with depressed COPD patients. Despite this, the evidence from other studies indicates that PST, when appropriately modified, improves functioning in patients with cognitive impairment equal to or greater than, COPD patients. An example is the efficacy of PST in mildly retarded adults.(48) Furthermore, PST in schizophrenic patients, a group with greater executive dysfunction, improves initiation and completion of tasks.(49–51)
The PST approach can help identify and manage key problems contributing to chronic life adversity, while PID-C provides the therapist with a structured approach to evaluate and understand reasoning for inadequate treatment adherence. The PID-C protocol could resolve some adherence problems using basic interventions including clarification, education, and direct instruction. Other adherence problems may require a more targeted PST intervention leading to the development of behavioral skills. PID-C and PST synergistically address the various reasons that depressed COPD patients are non-adherent to treatment.
In addition to their action on adherence, PST and PID-C also directly address symptoms of depression and disability. Behavioral activation and increased exposure to positive events have a beneficial impact on psychomotor slowing and anhedonia. This is evident in patients that successfully manage their illnesses; they obtain a sense of empowerment and an increase in their self-esteem. As the PST approach is learned, patients utilize these skills to manage other life problems, including social isolation and relations with family and friends. The positive experience of accomplishing solutions to daily problems introduces a sense of hope. This promotes a desire to further manage problems arising from dyspnea and disability, thus improving overall function.
An Intervention Model for Depressed Patients Suffering an Acute Medical Event
Acute disabling medical events are common in late life and are often followed by depression. Stroke is one such illness, and post-stroke depression (PSD) affects a large number of stroke patients. This dramatic medical event confers disability and psychosocial consequences that require treatment attention.
PSD has been described by medical writers since the 5th century BC and perhaps earlier, but modern psychiatry began to focus on PSD about 30 years ago after seminal work by Johns Hopkins investigators.(52–54) Combined studies of 2,178 patients hospitalized for acute stroke showed that 22% developed major depression and 17% developed minor depression.(55) Women are almost twice as likely as men to be diagnosed with major depression after stroke.(56) Other risk factors for PSD include level of disability from the stroke, personal and family history of a psychiatric disorder, and additional medical illnesses.(57–59)
The Clinical Picture of PSD
The signs and symptoms of PSD are similar to those of idiopathic depression, but PSD does not come alone. PSD is consistently shown to be associated with cognitive impairment, disability, and stroke severity.(60) Compared to non-depressed stroke patients, PSD patients are more likely to have impairments in executive functions, problem solving, psychomotor speed, attention, and memory.(61) Furthermore, greater depression severity is associated with cognitive impairment and a prior diagnosis of a psychiatric disorder in female stroke victims.(56) Impairment in activities of daily living is the single strongest correlate of depression after stroke.(59) Additionally, 83% of studies show that depression happening soon after stroke is a predictor of greater functional impairment at followup, ranging from 6 months to 2 years.(59, 62) This suggests that depression and disability are intertwined. PSD and disability are correlated with poor quality of life, social support, physical functioning, self-esteem, perceived control, and pessimism in stroke patients.(63, 64)
Alternatively, PSD patients that remit within the first few months have a greater recovery in activities of daily living.(65, 66)
Initial studies suggested that post-stroke depression is most common in patients with left anterior lesions and that the lesion proximity to the frontal pole is correlated with the severity of depression.(8) Subsequent studies showed that stroke in other sites of the brain pose a comparable risk for depression.(67) Nonetheless, physical disability, stroke severity and cognitive impairment were consistently associated with depression.(60)
Most stroke survivors receive intensive initial post-stroke rehabilitation (i.e., physical, occupational, and speech therapy) at acute and sub-acute facilities, or at home. However, this initial attention drops off after the first month. On discharge, patients are given demonstrations and advice for home modifications but most patients do not fully master these instructions. They may not be motivated, and rehabilitation therapists have limited time and scheduling flexibility with inadequate training on how to approach the behavioral consequences of depression and its impact on the family environment. The combination of physical limitations, lack of motivation and energy, hopelessness, family disruption, and possible transportation difficulties lead to missed appointments and poor adherence to home exercises. Home-based care by professional, trained clinicians targeting these challenges is critical at a time when neural repair is most active.(68–71)
Antidepressants in PSD
It has been suggested that antidepressants are effective in PSD,(59) and a recent study showed that escitalopram may even prevent the development of depression.(55) However, a meta-analysis of 13 agents showed only a weak effect of antidepressants in PSD.(72) A recent large study showed that antidepressants may increase the risk of ischemic and hemorrhagic stroke(73) confirming earlier reports(74–76) and suggesting that perhaps antidepressants should not be used in PSD patients.
Psychosocial Interventions
Psychosocial interventions for PSD have received less attention than pharmacotherapy. A 2008 Cochrane review identified 4 psychotherapy studies:(72) One used problem solving therapy (PST),(77) one used Cognitive Behavioral Therapy,(78) one used motivational interviewing,(79) and one was a broadly defined psychosocial intervention.(80) In three of these studies, the control group was usual care(77, 79, 80) and one used both usual care and attention-control comparison groups.(78) This meta-analysis concluded that there was no evidence that psychotherapy is beneficial in a PSD population.(72)
A recent study added brief behavioral therapy to antidepressant drug therapy as part of standard care to patients with PSD.(81) The intervention resulted in higher remission rates than “usual care” at the end of the intervention and at a 12 month follow-up.(81). Although this study did not control for “exposure” to therapists (the comparison group was “usual care”), the results are encouraging and serve as a signal of the potential value of psychosocial interventions.
The Model and Goals of Ecosystem Focused Therapy
We conceptualized Ecosystem Focused Therapy (EFT) in the context of our model of late-life depression,(2) and we postulate two primary pathways to PSD. First, that stroke increases vulnerability to depression by compromising frontolimbic circuitry through the ischemic event itself(59, 82) and the ensuing inflammatory(83, 84) and resculpting(85) brain responses. Second, the “psychosocial storm,” which we will describe magnifies many of these events (86–100) and provides an additional biological burden to this cascade of depressogenic influences.
EFT focuses on the “psychosocial storm”, which originates both from the patient’s sudden disability and the subsequent change in patient’s needs and family life.(4) The “psychosocial storm” can be conceptualized as a result of the interaction between the severity of the patient’s clinical state and the strengths of the patient’s “ecosystem”. Patients become deskilled after a stroke for a multitude of reasons, including the abrupt loss of strength, coordination, language, and executive functions, behavioral disorganization, lack of motivation, fear for their life, and hopelessness. This, compounded by the demanding rehabilitation and lack of readiness, leads to an increase in stress, feelings of helplessness, and resultant depression.(101–104) The family experiences a similar disturbance and is forced to alter their usual goals and tasks to account for the newly disabled stroke victim. They must now assist in daily routines and help to facilitate (e.g. organizing, driving, waiting) physical, occupational, and speech therapies. The burden is not limited to family members that live with the PSD patient.(4) Negativity and resignation experienced by the PSD patient often transmits to those expected to help and immobilizes them. Without guidance, even the most committed family members may not find a way to help effectively and can become a hindrance to the patient’s recovery.(4) Conversely, physical, occupational, and speech therapists must be aware of the “ecosystem” and coordinate with families to limit disruptions. These problems must be addressed early after the stroke, as there is a time window during which neural repair is most active.(68–71)
EFT is consistent with the classical social science concept of biographical disruption, which refers to the substantial and directive influence that illness can have over the course of a person’s life. (105) The “psychosocial storm”, and the ensuing diversity of treatments and tasks that need to be undertaken, the timing and tempo associated with each treatment and task, all contribute to the experience of a disrupted daily life to the individual’s and the family’s overall biography.
As mentioned above, the “psychosocial storm” interacts with the stroke’s ischemic event. The vascular lesion and repair response, inflammation, and plasticity, interact with the neurobiological responses to the “psychosocial storm”. These neurobiological responses include: inflammation, reactive oxygen species, dendritic remodeling, neurogenesis, and altered functional connectivity. We propose that the stroke’s biological responses and the neurobiological responses to the “psychosocial storm” affect the predisposing factors (frontolimbic compromise and heredity) and the mediating mechanisms (hypometabolism of the dorsal neocortex and hypermetabolism of the ventral limbic structures) of PSD.
The goals of EFT are to enable PSD patients to develop new perspectives and adaptation skills and to change their “ecosystems” to accommodate to their new states.(4) This way, home-delivered EFT can offer an optimal chance at adaptation by promoting adherence to rehabilitation and other treatments as well as increasing “behavioral activation” and perceived “self-efficacy,” thus reducing depression. The presumed mechanisms of rehabilitation therapies after stroke include enhancement of brain plasticity resulting in a new functional architecture.(106) Accordingly, behavioral enrichment may aid in functional recovery and enhance the benefits of physical, occupational and speech therapies.(107)
EFT targets critical contributors to the “psychosocial storm” experienced by the PSD patient and his/her family through five components: 1) It helps the patient and family develop an action-oriented new perspective about the patient’s recovery and state. Using a language that they can understand and accept, the therapist explains the prognosis of PSD, the expected disability, the role of rehabilitation, and the possible gratifying activities available to the patients at present and in the future, thus instilling hope for recovery. 2) Aided by a manualized strategy set, the EFT therapist guides the patient to develop an adherence enhancement structure mitigating the effects of resignation, executive dysfunction, and motivational disturbance through action plans. The family and/or professional caregivers participate based on need and availability. 3) The EFT therapist provides a problem solving structure to help the patient address solvable problems pertinent to daily functioning and to increasing the patient’s engagement in rewarding activities (behavioral activation). 4) The EFT therapist provides guidance in helping family members reengineer goals and modify their involvement and plans to accommodate to the patient’s disability, thus maximizing adherence to rehabilitation (facilitating arrangements). 5) The EFT therapist manages care with the patient’s other therapists to formulate a collaborative approach aiming to motivate the patient and help the patient and family develop a plan for participation in rehabilitation and for use of community resources, e.g., support groups and recreational services for the physically challenged. When combined, the components of EFT may confer a sense of competence and increase engagement in rewarding activities.(4)
We are encouraged by the results of a preliminary study of 24 patients comparing EFT to Education on Stroke and Depression (ESD). In the EFT group, 66.7% of subjects achieved remission of depression, whereas 16.7% of the ESD group remitted. Furthermore, in patients’ receiving EFT, improvement in disability was associated with greater improvement in depressive symptoms.(108)
Personalization
Clearly, not every PSD patient needs all five EFT components equally at all times. The changing recovery trajectory and needs of the patient and his/her “ecosystem” make the personalization of EFT a critical component of effective treatment. Each session emphasizes the most pertinent components which are guided by structured questions about persisting or emerging problems.(4) The selection of patient homework assignments and the guidance offered to the other people in the patient’s “ecosystem” follow the Rehabilitation Medicine Principle: “Motivation is highest and performance is optimal when a behavioral goal is set above the current level of performance but within reach”.(109) This way, each EFT session offers on-time and on-target “needed EFT components”, thus optimizing the chance to preempt adverse PSD outcomes.
Conclusion
The modest response of late-life depression to pharmacotherapy necessitates use of behavioral interventions. The intervention models for patients with depression and either chronic COPD or acute stroke highlight the development of treatments based on dissection of problems encountered by patients and their ecosystems. Awareness of specific common problem constellations has two advantages. First, it enables clinicians to focus and structure their assessments. Second, accurate problem identification in the individual patient and his/her ecosystem provides a clear plan for offering sharply targeted interventions. The end result is a structured personalization of interventions driven by the needs of the patient and his/her ecosystem.
We developed the PID-C and EFT behavioral intervention models at a time in which increasing longevity and large numbers of patients with chronic and acute illnesses challenge the healthcare system. In mental health, this problem is reaching critical proportions because of the high numbers of aging baby boomers and the fact that the baby boomers are afflicted by depression more than past cohorts.(10)
Evidence-based interventions are rarely used and sustained in the community.(110) There are several barriers to intervention implementation.(111, 112) One such barrier in the complexity of behavioral interventions and the fact that they are beyond the clinical skills of many clinicians working in community settings.(113–116) Depression of older adults with severe debilitating conditions or acute disabling medical events requires adaptation of existing care management models to the complex clinical context of these patients. This process is often beyond the skill level of professionals likely to offer care management in the community. Moreover, community implementation of an intervention is a complex process requiring buy-in by organizations and programmatic changes. Important elements of interventions that promote implementation are effectiveness, reimbursement, acceptance by patients, ease of use by clinicians, time to learn and routinize intervention use, compatibility with routine care, observable outcomes use of the intervention and its outcomes, and the ability of community organizations to test them and drop them at low cost.(117, 118)
Arguably, the PID-C and EFT have potential for implementation. Each intervention was designed and explicitly structured after long interactions and input by community clinicians with the goal of meeting criteria for simplicity, cost, and compatibility with routine care. They were developed for social workers and nurses after consideration of their skill sets. These professionals are common providers of initial care for late-life depression in rehabilitation settings, home-health care, and the community. Assuming adequate evidence of feasibility of training, low therapist skill drift, and efficacy, these interventions have the potential to be broadly used by many professionals and to become accessible to many depressed medically ill older adults.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jimmy N. Avari, Instructor in Psychiatry, Weill Cornell Medical College
George S. Alexopoulos, S.P. Tobin and A.M. Cooper Professor, Director, Weill Cornell Institute of Geriatric Psychiatry
References
- 1.Reynolds CF, 3rd, Lebowitz BD, Schneider LS. The NIH Consensus Development Conference on the Diagnosis and Treatment of Depression in Late Life: an overview. Psychopharmacology bulletin. 1993;29:83–85. [PubMed] [Google Scholar]
- 2.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos GS, Raue PJ, Sirey JA, et al. Developing an intervention for depressed, chronically medically ill elders: a model from COPD. Int J Geriatr Psychiatry. 2008;23:447–453. doi: 10.1002/gps.1925. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos GS, Wilkins VM, Marino P, et al. Ecosystem focused therapy in poststroke depression: a preliminary study. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiosses DN, Leon AC, Arean PA. Psychosocial interventions for late-life major depression: evidence-based treatments, predictors of treatment outcomes, and moderators of treatment effects. Psychiatr Clin North Am. 2011;34:377–401. doi: 10.1016/j.psc.2011.03.001. , viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arean PA, Raue P, Mackin RS, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167:1391–1398. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 2011;68:33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimoda K, Robinson RG. The relationship between poststroke depression and lesion location in long-term follow-up. Biol Psychiatry. 1999;45:187–192. doi: 10.1016/s0006-3223(98)00178-4. [DOI] [PubMed] [Google Scholar]
- 9.Shuba MF, Gurkovskaia AV, Buryi VA. Electrogenesis and contraction of smooth muscle taenia coli kept in a solution with elevated concentration of potassium ions. Fiziologicheskii zhurnal SSSR imeni I M Sechenova. 1982;68:1367–1375. [PubMed] [Google Scholar]
- 10.Hasin DS, Goodwin RD, Stinson FS, et al. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 11.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. Jama. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 12.Alexopoulos GS, Reynolds CF, 3rd, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166:882–890. doi: 10.1176/appi.ajp.2009.08121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krahn DD, Bartels SJ, Coakley E, et al. PRISM-E: comparison of integrated care and enhanced specialty referral models in depression outcomes. Psychiatr Serv. 2006;57:946–953. doi: 10.1176/ps.2006.57.7.946. [DOI] [PubMed] [Google Scholar]
- 14.Verbrugge LM, Patrick DL. Seven chronic conditions: their impact on US adults’ activity levels and use of medical services. Am J Public Health. 1995;85:173–182. doi: 10.2105/ajph.85.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Light RW, Merrill EJ, Despars JA, et al. Prevalence of depression and anxiety in patients with COPD. Relationship to functional capacity. Chest. 1985;87:35–38. doi: 10.1378/chest.87.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Gillum RF. Chronic obstructive pulmonary disease in blacks and whites: pulmonary function norms and risk factors. J Natl Med Assoc. 1991;83:393–401. [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Current estimates from the National Health Interview Survey. USDHHS (PHS); 1993. [Google Scholar]
- 18.Petty TL. Scope of the COPD problem in North America: early studies of prevalence and NHANES III data: basis for early identification and intervention. Chest. 2000;117:326S–331S. doi: 10.1378/chest.117.5_suppl_2.326s. [DOI] [PubMed] [Google Scholar]
- 19.Felker B, Katon W, Hedrick SC, et al. The association between depressive symptoms and health status in patients with chronic pulmonary disease. Gen Hosp Psychiatry. 2001;23:56–61. doi: 10.1016/s0163-8343(01)00127-x. [DOI] [PubMed] [Google Scholar]
- 20.ACCP/AACVPR.: Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based guidelines. American College of Chest Physicians. American Association of Cardiovascular and Pulmonary Rehabilitation Pulmonary Rehabilitation Guidelines Panel. Chest. 1997;112:1363–1396. [PubMed] [Google Scholar]
- 21.Lacasse Y, Guyatt GH, Goldstein RS. The components of a respiratory rehabilitation program: a systematic overview. Chest. 1997;111:1077–1088. doi: 10.1378/chest.111.4.1077. [DOI] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, Sirey JA, Raue PJ, et al. Outcomes of depressed patients undergoing inpatient pulmonary rehabilitation. Am J Geriatr Psychiatry. 2006;14:466–475. doi: 10.1097/01.JGP.0000199381.98971.d1. [DOI] [PubMed] [Google Scholar]
- 23.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 24.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Rovelli M, Palmeri D, Vossler E, et al. Noncompliance in organ transplant recipients. Transplant Proc. 1989;21:833–834. [PubMed] [Google Scholar]
- 26.DiMatteo MR, Hays RD, Gritz ER, et al. Patient adherence to cancer control regimens: scale development and initial validation. Psychol Assess. 1993;5:102–112. [Google Scholar]
- 27.Yohannes AM, Connolly MJ, Baldwin RC. A feasibility study of antidepressant drug therapy in depressed elderly patients with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2001;16:451–454. doi: 10.1002/gps.461. [DOI] [PubMed] [Google Scholar]
- 28.Ajzden I. The directive influence of attitudes on health behavior. In: Gollwitzer PM, Bargh JA, editors. The psychology of action:linking cognition and motivation to behavior. New York: The Guilford Press; 1996. [Google Scholar]
- 29.Alexopoulos GS, Kiosses DN, Sirey JA, et al. Personalised intervention for people with depression and severe COPD. The British journal of psychiatry : the journal of mental science. 2013;202:235–236. doi: 10.1192/bjp.bp.112.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon GH, Michiels TM, Mahutte CK, et al. Effect of desipramine on control of ventilation and depression scores in patients with severe chronic obstructive pulmonary disease. Psychiatry Res. 1985;15:25–32. doi: 10.1016/0165-1781(85)90036-8. [DOI] [PubMed] [Google Scholar]
- 31.Light RW, Merrill EJ, Despars J, et al. Doxepin treatment of depressed patients with chronic obstructive pulmonary disease. Arch Intern Med. 1986;146:1377–1380. [PubMed] [Google Scholar]
- 32.Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33:190–201. doi: 10.1016/S0033-3182(92)71995-1. [DOI] [PubMed] [Google Scholar]
- 33.Alexopoulos GS. LV: Depression comorbidity with COPD. Psychiatr Ann. 2004:289–295. [Google Scholar]
- 34.Fix AJ, Golden CJ, Daughton D, et al. Neuropsychological deficits among patients with chronic obstructive pulmonary disease. Int J Neurosci. 1982;16:99–105. doi: 10.3109/00207458209147610. [DOI] [PubMed] [Google Scholar]
- 35.Grant I, Heaton RK, McSweeny AJ, et al. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982;142:1470–1476. [PubMed] [Google Scholar]
- 36.Incalzi RA, Gemma A, Marra C, et al. Chronic obstructive pulmonary disease. An original model of cognitive decline. Am Rev Respir Dis. 1993;148:418–424. doi: 10.1164/ajrccm/148.2.418. [DOI] [PubMed] [Google Scholar]
- 37.Leuchter AF, Dunkin JJ, Lufkin RB, et al. Effect of white matter disease on functional connections in the aging brain. J Neurol Neurosurg Psychiatry. 1994;57:1347–1354. doi: 10.1136/jnnp.57.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickie I, Scott E, Mitchell P, et al. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- 39.Lesser IM, Boone KB, Mehringer CM, et al. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 40.Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- 41.Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 42.Kunik ME, Braun U, Stanley MA, et al. One session cognitive behavioural therapy for elderly patients with chronic obstructive pulmonary disease. Psychological medicine. 2001;31:717–723. doi: 10.1017/s0033291701003890. [DOI] [PubMed] [Google Scholar]
- 43.de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84:1154–1157. doi: 10.1016/s0003-9993(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 44.Areán PA, Perri MG, Nezu AM, et al. Comparative effectiveness of social problem-solving therapy and reminiscence therapy as treatments for depression in older adults. J Consult Clin Psychol. 1993;61:1003–1010. doi: 10.1037//0022-006x.61.6.1003. [DOI] [PubMed] [Google Scholar]
- 45.Mynors-Wallis L, Davies I, Gray A, et al. A randomised controlled trial and cost analysis of problem-solving treatment for emotional disorders given by community nurses in primary care. The British journal of psychiatry : the journal of mental science. 1997;170:113–119. doi: 10.1192/bjp.170.2.113. [DOI] [PubMed] [Google Scholar]
- 46.Mynors-Wallis L. Problem-solving treatment: evidence for effectiveness and feasibility in primary care. Int J Psychiatry Med. 1996;26:249–262. doi: 10.2190/0HVY-CD2F-0KC7-FVTB. [DOI] [PubMed] [Google Scholar]
- 47.Alexopoulos GS, Raue P, Areán P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11:46–52. [PubMed] [Google Scholar]
- 48.Nezu CM, Nezu AM, Areán P. Assertiveness and problem-solving training for mildly mentally retarded persons with dual diagnoses. Res Dev Disabil. 1991;12:371–386. doi: 10.1016/0891-4222(91)90033-o. [DOI] [PubMed] [Google Scholar]
- 49.Liberman RP, Corrigan PW. Designing new psychosocial treatments for schizophrenia. Psychiatry. 1993;56:238–249. discussion 250–233. [PubMed] [Google Scholar]
- 50.Tarrier N, Sharpe L, Beckett R, et al. A trial of two cognitive behavioural methods of treating drug-resistant residual psychotic symptoms in schizophrenic patients. II. Treatment-specific changes in coping and problem-solving skills. Soc Psychiatry Psychiatr Epidemiol. 1993;28:5–10. doi: 10.1007/BF00797826. [DOI] [PubMed] [Google Scholar]
- 51.Heinssen RK, Liberman RP, Kopelowicz A. Psychosocial skills training for schizophrenia: lessons from the laboratory. Schizophr Bull. 2000;26:21–46. doi: 10.1093/oxfordjournals.schbul.a033441. [DOI] [PubMed] [Google Scholar]
- 52.Robinson RG, Price TR. Post-stroke depressive disorders: a follow-up study of 103 patients. Stroke. 1982;13:635–641. doi: 10.1161/01.str.13.5.635. [DOI] [PubMed] [Google Scholar]
- 53.Robinson RG, Starr LB, Kubos KL, et al. A two-year longitudinal study of post-stroke mood disorders: findings during the initial evaluation. Stroke. 1983;14:736–741. doi: 10.1161/01.str.14.5.736. [DOI] [PubMed] [Google Scholar]
- 54.Robinson RG, Kubos KL, Starr LB, et al. Mood disorders in stroke patients. Importance of location of lesion. Brain. 1984;107(Pt 1):81–93. doi: 10.1093/brain/107.1.81. [DOI] [PubMed] [Google Scholar]
- 55.Robinson RG, Jorge RE, Moser DJ, et al. Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. Jama. 2008;299:2391–2400. doi: 10.1001/jama.299.20.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paradiso S, Robinson RG. Gender differences in poststroke depression. J Neuropsychiatry Clin Neurosci. 1998;10:41–47. doi: 10.1176/jnp.10.1.41. [DOI] [PubMed] [Google Scholar]
- 57.Leentjens AF, Aben I, Lodder J, et al. General and disease-specific risk factors for depression after ischemic stroke: a two-step Cox regression analysis. Int Psychogeriatr. 2006;18:739–748. doi: 10.1017/S1041610206003486. [DOI] [PubMed] [Google Scholar]
- 58.Tenev VT, Robinson RG, Jorge RE. Is family history of depression a risk factor for poststroke depression? Meta-analysis. Am J Geriatr Psychiatry. 2009;17:276–280. doi: 10.1097/JGP.0b013e3181953b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson RG, Spalletta G. Poststroke depression: a review. Can J Psychiatry. 2010;55:341–349. doi: 10.1177/070674371005500602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- 61.Kauhanen M, Korpelainen JT, Hiltunen P, et al. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke. 1999;30:1875–1880. doi: 10.1161/01.str.30.9.1875. [DOI] [PubMed] [Google Scholar]
- 62.Parikh RM, Robinson RG, Lipsey JR, et al. The impact of post-stroke deprssion on recovery in activities of daily living over two year follow-up. Arch Neurol. 1990;47:785–789. doi: 10.1001/archneur.1990.00530070083014. [DOI] [PubMed] [Google Scholar]
- 63.Teoh V, Sims J, Milgrom J. Psychosocial predictors of quality of life in a sample of community-dwelling stroke survivors: a longitudinal study. Top Stroke Rehabil. 2009;16:157–166. doi: 10.1310/tsr1602-157. [DOI] [PubMed] [Google Scholar]
- 64.Raju RS, Sarma PS, Pandian JD. Psychosocial problems, quality of life, and functional independence among Indian stroke survivors. Stroke. 2010;41:2932–2937. doi: 10.1161/STROKEAHA.110.596817. [DOI] [PubMed] [Google Scholar]
- 65.Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke. 2001;32:113–117. doi: 10.1161/01.str.32.1.113. [DOI] [PubMed] [Google Scholar]
- 66.Bilge C, Kocer E, Kocer A, et al. Depression and functional outcome after stroke: the effect of antidepressant therapy on functional recovery. Eur J Phys Rehabil Med. 2008;44:13–18. [PubMed] [Google Scholar]
- 67.Mitiuk II, Karavanov GG, Buryi VT. Device for stimulating lympho-venous drainage from the lower extremities. Klinicheskaia khirurgiia. 1982:59–61. [PubMed] [Google Scholar]
- 68.Green J. Brain Reorganization After Stroke. Top Stroke Rehabil. 2003;10:1–20. doi: 10.1310/H65X-23HW-QL1G-KTNQ. [DOI] [PubMed] [Google Scholar]
- 69.Teasell R, Bayona NA, Bitensky J. Plasticity and reorganization of the brain post stroke. Top Stroke Rehabil. 2005;12:11–26. doi: 10.1310/6AUM-ETYW-Q8XV-8XAC. [DOI] [PubMed] [Google Scholar]
- 70.Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14:369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- 71.Carmichael ST. Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke. 2008;39:1380–1388. doi: 10.1161/STROKEAHA.107.499962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hackett ML, Anderson CS, House A, et al. Interventions for treating depression after stroke. Cochrane Database Syst Rev. 2008:CD003437. doi: 10.1002/14651858.CD003437.pub3. [DOI] [PubMed] [Google Scholar]
- 73.Wu CS, Wang SC, Cheng YC, et al. Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry. 2011;168:511–521. doi: 10.1176/appi.ajp.2010.10071064. [DOI] [PubMed] [Google Scholar]
- 74.Chen Y, Guo JJ, Patel NC. Hemorrhagic stroke associated with antidepressant use in patients with depression: does degree of serotonin reuptake inhibition matter? Pharmacoepidemiol Drug Saf. 2009;18:196–202. doi: 10.1002/pds.1699. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Guo JJ, Li H, et al. Risk of cerebrovascular events associated with antidepressant use in patients with depression: a population-based, nested case-control study. Ann Pharmacother. 2008;42:177–184. doi: 10.1345/aph.1K369. [DOI] [PubMed] [Google Scholar]
- 76.Smoller JW, Allison M, Cochrane BB, et al. Antidepressant use and risk of incident cardiovascular morbidity and mortality among postmenopausal women in the Women’s Health Initiative study. Arch Intern Med. 2009;169:2128–2139. doi: 10.1001/archinternmed.2009.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Towle D, Lincoln N, Mayfield L. Evaluation of social work on depression after stroke. Clinical Rehabilitation. 1989;3:89–96. [Google Scholar]
- 78.Lincoln NB, Flannaghan T. Cognitive behavioral psychotherapy for depression following stroke: a randomized controlled trial. Stroke. 2003;34:111–115. doi: 10.1161/01.str.0000044167.44670.55. [DOI] [PubMed] [Google Scholar]
- 79.Watkins CL, Auton MF, Deans CF, et al. Motivational interviewing early after acute stroke: a randomized, controlled trial. Stroke. 2007;38:1004–1009. doi: 10.1161/01.STR.0000258114.28006.d7. [DOI] [PubMed] [Google Scholar]
- 80.Zhao H-W, Zhou C-X, Su X-L, et al. Effect of mental intervention on post-stroke depression and rehabilitation of neurological function. Chinese Journal of Clinical Rehabilitation. 2004;8:2408–2409. [Google Scholar]
- 81.Mitchell PH, Veith RC, Becker KJ, et al. Brief psychosocial-behavioral intervention with antidepressant reduces poststroke depression significantly more than usual care with antidepressant: living well with stroke: randomized, controlled trial. Stroke. 2009;40:3073–3078. doi: 10.1161/STROKEAHA.109.549808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson RG. The Clinical Neuropsychiatry of Stroke. Cambridge, England: Cambridge University Press; 2006. [Google Scholar]
- 83.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang J, Cheng Q. Etiological mechanisms of post-stroke depression: a review. Neurol Res. 2009;31:904–909. doi: 10.1179/174313209X385752. [DOI] [PubMed] [Google Scholar]
- 85.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 86.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 87.Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garcia-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 2008;32:1136–1151. doi: 10.1016/j.neubiorev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011 doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Munhoz CD, Garcia-Bueno B, Madrigal JL, et al. Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res. 2008;41:1037–1046. doi: 10.1590/s0100-879x2008001200001. [DOI] [PubMed] [Google Scholar]
- 91.Cook F, Ciorciari J, Varker T, et al. Changes in long term neural connectivity following psychological trauma. Clin Neurophysiol. 2009;120:309–314. doi: 10.1016/j.clinph.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 92.Frank MG, Baratta MV, Sprunger DB, et al. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 93.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Marle HJ, Hermans EJ, Qin S, et al. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- 95.Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport. 2006;17:863–867. doi: 10.1097/01.wnr.0000221827.03222.70. [DOI] [PubMed] [Google Scholar]
- 96.Czeh B, Perez-Cruz C, Fuchs E, et al. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter? Behav Brain Res. 2008;190:1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 97.Jayatissa MN, Bisgaard C, Tingstrom A, et al. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- 98.Radley JJ, Johnson LR, Janssen WG, et al. Associative Pavlovian conditioning leads to an increase in spinophilin-immunoreactive dendritic spines in the lateral amygdala. Eur J Neurosci. 2006;24:876–884. doi: 10.1111/j.1460-9568.2006.04962.x. [DOI] [PubMed] [Google Scholar]
- 99.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev. 2008;57:561–570. doi: 10.1016/j.brainresrev.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- 102.Kraaij V, Arensman E, Spinhoven P. Negative life events and depression in elderly persons: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2002;57:P87–94. doi: 10.1093/geronb/57.1.p87. [DOI] [PubMed] [Google Scholar]
- 103.Moos RH, Brennan PL, Schutte KK, et al. Older adults’ coping with negative life events: common processes of managing health, interpersonal, and financial/work stressors. Int J Aging Hum Dev. 2006;62:39–59. doi: 10.2190/ENLH-WAA2-AX8J-WRT1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Areán P, Alexopoulos G, Chu J. Cognitive Behavioral Case Management for Depressed Low-income Older Adults. In: Gallagher-Thompson D, Steffen A, Thompson L, editors. Handbook of Behavioral and Cognitive Therapies with Older Adults. New York: Springer; 2008. pp. 219–232. [Google Scholar]
- 105.Bury M. Chronic illness as biographical disruption. Sociology of health & illness. 1982;4:167–182. doi: 10.1111/1467-9566.ep11339939. [DOI] [PubMed] [Google Scholar]
- 106.Ward NS. Neural plasticity and recovery of function. Prog Brain Res. 2005;150:527–535. doi: 10.1016/S0079-6123(05)50036-0. [DOI] [PubMed] [Google Scholar]
- 107.Ward NS. Plasticity and the functional reorganization of the human brain. Int J Psychophysiol. 2005;58:158–161. doi: 10.1016/j.ijpsycho.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Alexopoulos GS, Wilkins VM, Marino P, et al. Ecosystem focused therapy in poststroke depression: a preliminary study. Int J Geriatr Psychiatry. 2012;27:1053–1060. doi: 10.1002/gps.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ecology and the aging process. The Psychology of Adult Development and Aging. 1973 [Google Scholar]
- 110.Goldman HH, Azrin ST. Public policy and evidence-based practice. Psychiatr Clin North Am. 2003;26:899–917. doi: 10.1016/s0193-953x(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 111.Aarons GA, Glisson C, Green PD, et al. The organizational social context of mental health services and clinician attitudes toward evidence-based practice: a United States national study. Implement Sci. 2012;7:56. doi: 10.1186/1748-5908-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Green AE, Fettes DL, Aarons GA. A Concept Mapping Approach to Guide and Understand Dissemination and Implementation. J Behav Health Serv Res. 2012 doi: 10.1007/s11414-012-9291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mellman LA, Beresin E. Psychotherapy competencies: development and implementation. Acad Psychiatry. 2003;27:149–153. doi: 10.1176/appi.ap.27.3.149. [DOI] [PubMed] [Google Scholar]
- 114.Spruill J, Rozensky RH, Stigall TT, et al. Becoming a competent clinician: basic competencies in intervention. J Clin Psychol. 2004;60:741–754. doi: 10.1002/jclp.20011. [DOI] [PubMed] [Google Scholar]
- 115.Gehart D. The core competencies and MFT education: practical aspects of transitioning to a learning-centered, outcome-based pedagogy. J Marital Fam Ther. 2011;37:344–354. doi: 10.1111/j.1752-0606.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- 116.Sburlati ES, Schniering CA, Lyneham HJ, et al. A model of therapist competencies for the empirically supported cognitive behavioral treatment of child and adolescent anxiety and depressive disorders. Clin Child Fam Psychol Rev. 2011;14:89–109. doi: 10.1007/s10567-011-0083-6. [DOI] [PubMed] [Google Scholar]
- 117.Rogers E. Diffusion of Innovation. 4. New York: Free Press; 1995. [Google Scholar]
- 118.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]


