Abstract
The hippocampus is one of the most age-sensitive brain regions, yet the mechanisms of hippocampal shrinkage remain unclear. Recent studies suggest that hippocampal subfields are differentially vulnerable to aging and differentially sensitive to vascular risk. Promoters of inflammation are frequently proposed as major contributors to brain aging and vascular disease but their effects on hippocampal subfields are unknown. We examined the associations of hippocampal subfield volumes with age, a vascular risk factor (hypertension), and genetic polymorphisms associated with variation in pro-inflammatory cytokines levels (IL-1β C-511T and IL-6 C-174G) and risk for Alzheimer’s disease (APOEε4) in healthy adult volunteers (N = 80; age = 22-82 years). Volumes of three hippocampal subfields, cornu ammonis (CA) 1-2, CA3-dentate gyrus, and the subiculum were manually measured on high-resolution magnetic resonance images. Advanced age was differentially associated with smaller volume of CA1-2, whereas carriers of the T allele of IL-1β C-511T polymorphism had smaller volume of all hippocampal subfields than CC homozygotes did. Neither of the other genetic variants, nor diagnosis of hypertension was associated with any of the measured volumes. The results support the notion that volumes of age-sensitive brain regions may be affected by pro-inflammatory factors that may be targeted by therapeutic interventions.
Keywords: brain, IL-1beta, IL-6, CA1, APOE, hypertension
Vulnerability of the hippocampus to aging has been demonstrated in multiple studies and one of the most common findings is age-related reduction of hippocampal volume (see Hof and Morrison 1996; Raz and Kennedy 2009; Small et al. 2011, for reviews). However, the magnitude of age differences in hippocampal volume and the rate of hippocampal shrinkage vary widely across samples (e.g., Mu et al. 1999 vs. Sullivan et al. 1995; see Raz 2000 and Raz and Kennedy 2009 for reviews), and the mechanisms underlying age-related changes in the hippocampus remain unclear. Because aging is associated with a dramatic increase in vascular risk (Franklin et al. 1997; Hildrum et al. 2007; Wills et al. 2011) and has been linked to chronic inflammation (Finch and Crimmins 2004; Finch et al. 1969; Grammas 2011), it is plausible that accounting for these factors may clarify the observed discrepancies among studies, elucidate the contributors to individual differences in hippocampal volume, and provide insights into mechanisms of brain aging.
Adverse changes in structural and functional characteristics of the hippocampus have been related to established vascular risk factors such as high arterial blood pressure (e.g., Korf et al. 2004; Qiu et al. 2012; Raz et al. 2005; 2008), insulin resistance (Burns et al. 2012; Rasgon et al. 2011), elevated fasting blood glucose (Cherbuin et al. 2012), high total plasma homocysteine (den Heijer et al. 2003), and high blood concentration of C-reactive protein (Satizabal et al. 2012). In some studies, APOEε4, a polymorphism in a gene that regulates levels of a multifunctional lipid transporter apolipoprotein E (ApoE; Mahley 1988) and conveys an increased risk for vascular (Davignon et al. 1988) and Alzheimer’s (Roses 1996) diseases, has been linked to smaller hippocampal volume (Bender and Raz 2012) and faster hippocampal shrinkage (Moffat et al. 2000).
Until recently, in vivo studies of hippocampal aging did not take into account its cytoarchitectonic and functional heterogeneity (Witter and Amaral 1995). Using the volume of the whole hippocampus may obscure the differential vulnerabilities of its components to specific risk factors, insults, and toxins. For example, one of the hippocampal subfields, cornu ammonis (CA) 1, is particularly sensitive to hypoxia, ischemia, and hyperglycemia whereas CA3 and the dentate gyrus (DG) are usually spared by these events (Petito and Pulsinelli 1984; Pulsinelli et al. 1982; Suyama 1992). In humans, even relatively brief episodes of transient ischemia may result in highly localized CA1 lesions that convey negative cognitive consequences (Bartsch et al. 2011). Thus, vascular risk may affect CA1 more than the other hippocampal subfields. Little is known about the differences in regional hippocampal volumes that may be attributable to genetic indicators of vascular risk. In one study of older healthy adults and Alzheimer’s disease patients, APOEε4 was linked to a smaller volume of the CA3-DG region (Mueller and Weiner 2009), whereas in another sample, an association of the risky allele with CA1 and subiculum (SUB) was observed (Pievani et al. 2011).
Negative vascular events, such as ischemia, are accompanied by increase in neuroinflammation (Kriz and Lalancette-Hébert 2009; Simi et al. 2007), and several pro-inflammatory cytokines have been implicated in disruption of hippocampal function (Williamson and Bilbo 2013). In rodents, induction of chronic inflammation results in significant shrinkage of the hippocampus and adjacent regions that is observable on magnetic resonance imaging (MRI; Hauss-Wegrzyniak et al. 2000). Inflammation, both central and peripheral, may affect hippocampal subfields differentially. Experimental pro-inflammatory manipulations activate microglia, impair synaptic plasticity in CA1, alter glutamate receptor subunit composition, and elevate blood titers of interleukin-1β (IL-1β; Di Filippo et al. 2013). The latter is a pro-inflammatory cytokine associated with brain injury and neurodegenerative disease, including neurological disorders that preferentially target the hippocampus (Di Bona et al. 2008; Kaufmann et al. 2008). Several studies found associations between hippocampal volume and biomarkers of inflammation in non-demented middle-aged and older adults with or without other vascular risk factors. Increase in hippocampal levels of IL-1β accompanies age-related decline in long-term potentiation (LTP; Lynch 1998), and chronic inflammation mediated by release of IL-1β may promote the development of Alzheimer’s disease (Blasko et al. 2004; Di Bona et al. 2008). High circulating levels of pro-inflammatory cytokine interleukin-6 (IL-6) have been associated with smaller hippocampal volume (Marsland et al. 2008; Satizabal et al. 2012). In a recent MRI study of healthy adults, a genetic variant associated with increase in pro-inflammatory cytokine IL-6 was linked to reduced density of gray matter in selected parts of the hippocampus (Baune et al. 2012). However, the volumes of hippocampal subfields that are particularly vulnerable to vascular risk were not assessed in that study.
One of the problems with studying the effects of pro-inflammatory factors on the brain in vivo is that their blood levels cannot be safely manipulated in healthy humans. Measuring peripheral levels of biomarkers does not always inform about their concentration in the brain (Banks 2005; Reynolds 2006). Although the blood-brain barrier (BBB) can be penetrated by many cytokines, especially at its weak points, such as circumventricular organs (Konsman et al. 1999), it is unclear how likely the reverse movement is and therefore, the relationship between brain inflammation and blood levels of IL-1 or IL-6 is far from established (Banks 2005; Banks et al. 1995). For example, human and murine IL-6 as well as murine IL-1β can cross the BBB, but human IL-1β cannot, apparently for the lack of saturable transport mechanism (Banks 2005; Banks et al. 2001). Nonetheless, peripherally circulating cytokines can stimulate pro-inflammatory cytokine production, release, and propagation in the brain (Vitkovic et al. 2000).
The challenge of manipulating pro-inflammatory factors in humans can be overcome to some extent by employing a Mendelian randomization (Katan 1986; Lawlor et al. 2008). In this approach, the researchers take advantage of known genetic variants such as single nucleotide polymorphisms (SNPs) that predispose individuals to a certain level of inflammatory response and influence concentration of the pro-inflammatory biomarkers on both sides of the BBB. The existence of several SNPs in genes that are relevant to IL-1β and IL-6 provides an opportunity for implementing such an approach.
Baseline systemic levels of IL-1β are controlled by the eponymous gene, a functional SNP of which IL-1β C-511T predisposes its carriers to an increased pro-inflammatory response (Hurme and Santtila 1998). Homozygocity for the risky T allele of IL-1β C-511T has been linked to larger volume of frontal and parietal white matter hyperintensities in healthy adults (Raz et al. 2012), ventriculomegaly in schizophrenia (Meisenzahl et al. 2001), and reduced brain volume in bipolar disorder (Papiol et al. 2005; 2008). To the best of our knowledge, the effect of genetically induced variation in IL-1β levels on hippocampal volume in healthy humans is unknown.
Circulating levels of IL-6 vary across allelic variants of IL-6 C-174G, which has been implicated in longevity (Di Bona et al. 2008) and stroke (Tso et al. 2007); although the evidence of its involvement in Alzheimer’s disease has not been replicated (Ravaglia et al. 2006). At the time of this writing, we are unaware of studies relating genetic variants in IL-6 to hippocampal volume.
Until recently, research on the human hippocampus was limited by an inability to clearly demarcate hippocampal regions on MRI. Introduction of high-resolution MRI methods (Mueller et al. 2007) permitted reliable estimation of regional hippocampal subfield volumes in vivo. Studies employing such methods reported smaller CA1-2 volumes in older adults compared to their younger counterparts, with smaller differences in other hippocampal regions (Mueller et al. 2011; Mueller et al. 2007; Mueller and Weiner, 2009). However, the association between CA1 volume and age may reflect the effects of arterial hypertension, a common age-related vascular risk factor (Shing et al. 2011).
In light of the reviewed evidence, the goals of this study were to examine age differences in the volume of the medial temporal lobe structures - three hippocampal subfields—CA1-2, CA3-DG, and subiculum and the entorhinal cortex and to gauge the extent to which these volumes are affected by hypertension and two pro-inflammatory genetic variants: IL1β C-511T and IL-6C-174G, as well as a common risk factor for Alzheimer’s and vascular diseases, APOEε4.
Method
Participants
The participants, who were part of an ongoing longitudinal study of brain and cognitive aging, were recruited through advertising in the local media, were interviewed and provided informed consent in accord with University Institutional Review Board guidelines. The sample consisted of 80 adult volunteers (75% women; 18% African-American). The participants in this study completed a health status questionnaire and reported no neurological, cardiovascular, psychiatric, or endocrine disease. However, some of them exhibited a certain degree of vascular risk. Twenty-one participants (26%) reported a diagnosis of hypertension and were taking anti-hypertensive medication, and 14 (18%) took statins. However, we have no data on compliance with medication regimen. An additional six participants (8%) had elevated blood pressure that placed them in the hypertensive range, and they were classified, for the purpose of analyses, as hypertensive. According to the self-report, the majority of participants led an active life-style, with 69 (86%) exercising at least once a week, with the median being 3.5 days of exercise per week. Eleven participants (14%) smoked tobacco. All participants were native English speakers and high-functioning residents of urban and suburban communities, with high scores on the mini-mental state examination (MMSE; Folstein et al. 1975; cut-off = 26) and a mean number of formal schooling years that corresponded to a four-year college degree (see Table 1).
Table 1.
Descriptive statistics of the sample.
| Variable | Mean | SD | Range | CV | Normality test p (Shapiro-Wilk) |
|---|---|---|---|---|---|
| Age (years) | 57.84 | 14.27 | 22 - 82 | 0.25 | 0.044 |
| Education (years) | 16.28 | 2.45 | 12 - 22 | 0.15 | 0.007 |
| MMSE | 28.94 | 1.05 | 26 - 30 | 0.04 | 0.000 |
| CES-D | 3.85 | 3.39 | 0 - 15 | 0.88 | 0.000 |
| Fasting blood glucose (mg/dl) | 87.90 | 10.01 | 69 - 123 | 0.11 | 0.011 |
| Systolic blood pressure (mmHg) | 122.41 | 12.13 | 88 - 152 | 0.10 | 0.906 |
| Diastolic blood pressure (mmHg) | 75.52 | 6.94 | 60 - 95 | 0.09 | 0.612 |
The participants completed a questionnaire to rule out current symptoms of depression (CES-D; Radloff 1977; cut-off = 15). All participants were right-handed (handedness score > 75%; Oldfield 1971). The participants were screened for vision problems (Optec 2000 Vision Tester, Stereo Optical Co., Inc., Chicago, IL) and speech-range hearing deficits (MA27 Screening Audiometer, Maico Diagnostics, Eden Prairie, MN). Trained technicians measured blood pressure with an auscultatory method using diastole phase V for identification of diastolic pressure (Pickering et al. 2005). Blood pressure was measured with the participant comfortably seated in a quiet room, on three separate days, normally one to two weeks apart. The values were averaged across the measurement occasions. None of the participants had a diagnosis of diabetes and all were confirmed normoglycemic by a fasting blood glucose test. For sample descriptive statistics, see Table 1.
MRI Acquisition
The following sequences relevant to this study were acquired as part of a one-hour protocol on a 3T Siemens Verio (Siemens Medical AG, Erlangen, Germany) full-body magnet with a 12-channel receive-only Siemens TIM head coil. To measure the intracranial volume (ICV), we acquired a high-resolution T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with the following parameters: TR = 1680 ms; TE = 3.51 ms; TI = 900 ms; flip angle = 9°, pixel bandwidth = 180 Hz/pixel, GRAPPA acceleration factor PE = 2; voxel size 0.67 mm × 0.67 mm × 1.34 mm. For regional hippocampal measures, we acquired a high-resolution proton density-weighted turbo spin echo (PD-TSE) sequence in the coronal plane, oblique to the long axis of the hippocampus, with the following parameters: TR = 7150 ms; TE = 17 ms; flip angle = 120°; pixel bandwidth = 96 Hz/pixel; turbo factor 11, voxel size = 0.4 mm × 0.4 mm × 2.0 mm, FOV = 280 × 512 mm; 30 slices; elliptical filter and fat saturation – on; no GRAPPA; we limited the FOV to a smaller 2D cross-section to allow for faster acquisition.
Post-Processing and Manual Demarcation of Anatomical Regions
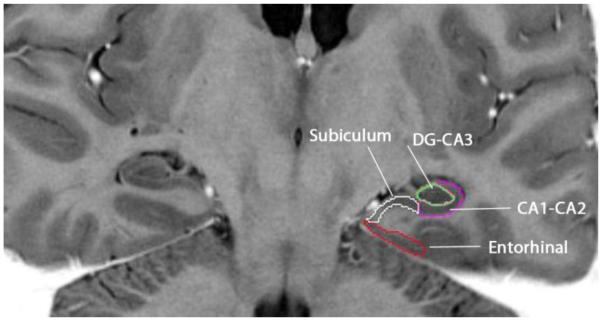
Rules for tracing the hippocampal subfields and entorhinal cortex (EC) were adapted from Shing et al. (2011) as modified from Mueller et al. (2007) and Mueller and Weiner (2009), with one difference: the border between SUB and CA1-2 followed the tissue contrast gradient rather than a vertical line. See Figure 1 for an example of region of interest (ROI) tracings. Using Analyze 11.0 software (Mayo Clinic, Rochester, MN), two independent raters (A.M.D. and A.R.B) manually demarcated regional boundaries with a stylus on a 21-inch digitizing tablet (Wacom Cintiq). Inter-rater reliability was confirmed by an intra-class correlation coefficient for independent raters (ICC(2); Shrout and Fleiss 1979) of at least .90 for the bilateral total volume for each region. Regions included EC, SUB, CA1, CA2, CA3-4, and DG.
Fig 1.
Demarcation of hippocampal subfields: the SUB (white), CA1-2 (purple), CA3-DG (green) and the EC (red). The image is in radiological orientation.
To improve visualization, the image intensities were inverted to mimic an inversion-recovery T1-weighted image. However, given the limited visualization of anatomical boundaries between subfields, CA3-4 and DG were collapsed into a single region (CA3-DG), as were CA1 and 2 (CA1-2; see Figure 1). All regions were traced in both hemispheres. Ranges were allowed to differ by starting slice based on anatomical hemispheric differences. To ensure separation between the hippocampus and the amygdala, the hippocampal subfield range began with the slice on which the head of the hippocampus was no longer visible.
EC
The EC was traced on six contiguous slices, beginning five slices anterior to the starting slice of the hippocampal subfield range. The end of the SUB defined the superior medial boundary and the opening of the collateral sulcus was the inferior lateral boundary. Reliability for this region was ICC(2) = .99.
SUB
The SUB was traced on three slices beginning on the starting slice. The region was traced from the end of the CA1 to the dorsal EC boundary, which was determined by extending a diagonal line from the medial border of the DG. Reliability for this region was ICC(2) = .93.
CA1-2
The CA1-2 region was traced on three slices beginning on the starting slice. The boundary circled the CA3-DG, extending medially from the dorsal border to the SUB. Reliability for this region was ICC(2) = .91.
CA3-DG
This region was traced on the three contiguous slices with the other subfields. The ovoid region was traced within the hyperintense border between CA1-2. Reliability for this region was ICC(2) = .93.
ICV
The ICV was calculated using the brain extraction tool (BET; Smith 2002) in FSL 4.1 using the T1-weighted images. In order to minimize inclusion of non-brain tissue such as eyes, periorbital fat, or neck muscle and fat, we first applied standard-space masking (standard_space_roi; Keihaninejad et al. 2010). The cranium was demarcated in BET by applying a fractional intensity threshold of 0.2 without gradient; the method used the ‘–A’ flag for estimation of the skull from the masked image with the betsurf option (Jenkinson et al. 2005). ICV values were sampled from the outer skull mask output by betsurf, and an experienced operator visually inspected the results. These settings were determined following comparison of hand-tuned BET output on a subsample.
The volumes of all measured ROIs were corrected for ICV via a linear equation: Volumeadj = Volumerawi – b(ICVi – Mean ICV), where Volumeadj is the adjusted regional volume, Volumerawi is the original volume for an individual, b is the slope of the ROI volume regressed on ICV, and Mean ICV is the sample mean of ICV. In this correction, we divided the ICV values by 1000 to equalize the scales of the hippocampus and ICV.
Genotyping
We used methods and assays described in previous publications (e.g., Raz et al. 2012). DNA was isolated from buccal samples obtained in mouthwash. Isolation was performed using a Gentra Autopure LS with the standard buccal cell protocol. For genotyping quality control, 37% direct repeats and DNA sequencing for verification were performed. Both control DNA and no-template controls were used.
IL-1β C-511T (rs16944)
Polymorphism for IL-1β C-511T was interrogated with the 5′-nuclease assay using a Taqman SNP Genotyping assay (Applied Biosystems, Foster City, CA, USA). Genotyping success rate was 99%. The allelic distribution of the IL-1β C-511T polymorphism contained 34% CC homozygotes (n = 27), 47% C/T heterozygotes (n = 38), and 15 (19%) TT homozygotes. Hardy-Weinberg (HW) equilibrium for IL1βC-511T was not violated: χ2 = 0.06, p = .80. CC homozygotes were older than CT heterozygotes, who in turn were older than TT homozygotes: F(2,78) = 3.77, p = .027. This difference could reflect a selection bias: older persons with pro-inflammatory allele T might have been less likely to maintain good health and pass the screening criteria for the study. The age difference between the allelic groupings of this SNP was controlled by having age included in all analyses. No differences were noted in MMSE scores (F < 1) or education: F(2,78) = 1.59, p = .21. Although the sample combined participants from different ancestral populations, we found no difference in allele distribution between African-Americans and Caucasians: χ2 (2) = 1.59, p = .45.
IL-6 C-174G (rs1800795)
The IL-6 C-174G polymorphism was interrogated with the 5′-nuclease assay using standard TaqMan conditions and Genotyping Assay (Applied Biosystems, Foster City, CA, USA). The primers and probes for the assay were 5′-CGACCTAAGCTGCACTTTTCC -3′, 5′- GGGCTGATTGGAAACCTTATTAAGATTG -3′, VIC- CCTTTAGCATGGCAAGAC -NFQ and FAM- CCTTTAGCATCGCAAGAC -NFQ. Genotyping had a success rate of 98% and revealed that 30 (37%) participants were heterozygous (CG), 10 (13%) were homozygous for the G allele (GG) and 40 (50%) were homozygous for the C allele (CC). The distribution of the alleles conformed to the HW equilibrium: χ2 = 1.30, p = .25. There were no significant differences among the ApoE allelic groupings in age (F < 1), education (F = 1.57, p = .21), or MMSE (F = 1.69, p = .19). The allele frequencies did not differ between Caucasian and African-American subgroups of the sample: χ2 (2) = 0.96, p = .62.
ApoE ε variants
ApoE polymorphisms (rs429358 and rs7412) were preamplified with forward 5′-CAATGCTACCGAGTTTTCTTCC-3′ and reverse primers 5′-TTCAGATTCTTCACAGATGCGTA-3′ in a 25 μl reaction containing 2.5 mmol/l MgCl2, 0.5 μmol/l of the primers, 1.25 U AmpliTaq Gold polymerase, and 200 μmol/l dTTPs. The mixture was denatured at 95°C for 10 minutes and amplification achieved by 15 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. One μl of this reaction was subsequently used for rs429358 and rs7412 5′-nuclease assays under standard conditions. The primers and probes for the rs429358 assay were 5′-GCGGGCACGGCTGT-3′, 5′-GCTTGCGCAGGTGGGA-3′, VIC-CATGGAGGACGTGTGC-NFQ and FAM-ATGGAGGACGTGCGC-NFQ. The primers and probes for the rs7412 assay were 5′-TCCGCGATGCCGATGAC-3′, 5′-CCCCGGCCTGGTACAC-3′, VIC-CAGGCGCTTCTGC-NFQ and FAM-CAGGCACTTCGC-NFQ. There were 9 ε2 carriers (no homozygotes), 53 ε3 homozygotes and 21 ε4 carriers (three of them homozygotes); there were three ε2/ε4 heterozygotes. For ε4 vs. ε3 and ε2 vs. ε3 alleles, HW equilibrium was maintained: χ2 = 1.86, p = .17 and χ2 = 2.32, p = .13, respectively. Because of very low frequency of non-ε3 genotypes, two groups were used for comparisons: ε4 carriers (n = 21, 26%) vs. non- ε4 genotypes (n = 59, 74%). There were no significant differences among the ApoE allelic groupings in age (F = 1.82, p = .17), education (F = 1.27, p = .29), or MMSE (F < 1). There was no difference in ε4 frequency between the Caucasian and African-American subgroups of the sample: χ2 (1) = 0.1, p = .94.
Statistical Analyses
A general linear models (GLM) were fitted to the data. In the models, the adjusted volume of three hippocampal subfields or EC volume served as a the dependent variable, subfield was a repeated measures factor, and age (centered at the sample mean) was a continuous independent variable and sex, IL-1β C-511T (CC, CT, or TT), IL-6 (CC, CG or GG), APOE (ε4 carriers vs. non-carriers) and diagnosis of hypertension (yes, no) were categorical factors. All bivariate interactions were tested and if found non-significant (p > .15), removed from the model. Due to a small number of participants with specific combinations of alleles, we could not test higher-level interactions within the same model. All models contained first-order interactions among all predictors. We used Hyuhn-Feldt correction to adjust the probability levels of all interactions that involved repeated measures for violation of the sphericity assumption. The GLM analyses were followed by univariate analyses of simple effects, and testing the differences among levels of the categorical variables with Fisher’s Least Significant Difference test.
Results
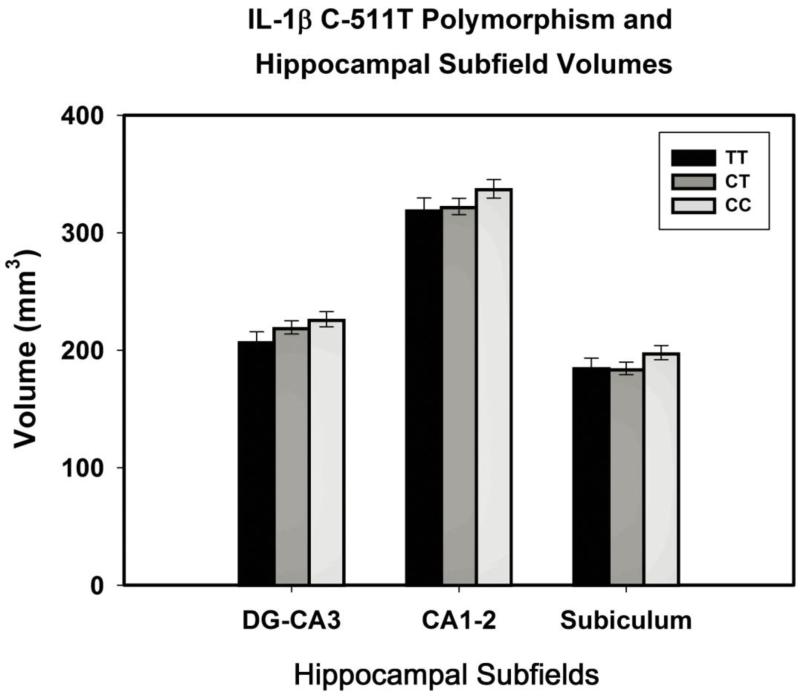
The analyses revealed main effects of Age (F(1,71) = 4.37, p = .04) and IL-1β polymorphism (F(2,71) = 3.87, p = .025), with no significant effects of sex, diagnosis of hypertension, IL-6, APOEε4 or interactions of ROI with polymorphisms, diagnosis of hypertension, and sex (all F < 1). Post-hoc evaluation of the allele differences in the combined volume of all ROIs showed that C homozygotes had significantly larger hippocampal subfield volumes in comparison to the heterozygotes and T homozygotes: p = .023 and .015, respectively (Fisher test, see Fig. 2).
Fig 2.
IL-1β C-511T polymorphism and hippocampal subfield volumes. The CC group has significantly larger hippocampal subfield volumes than CT and TT do: p = .023 and .015, respectively. Subfield volumes are adjusted for ICV (see text for details).
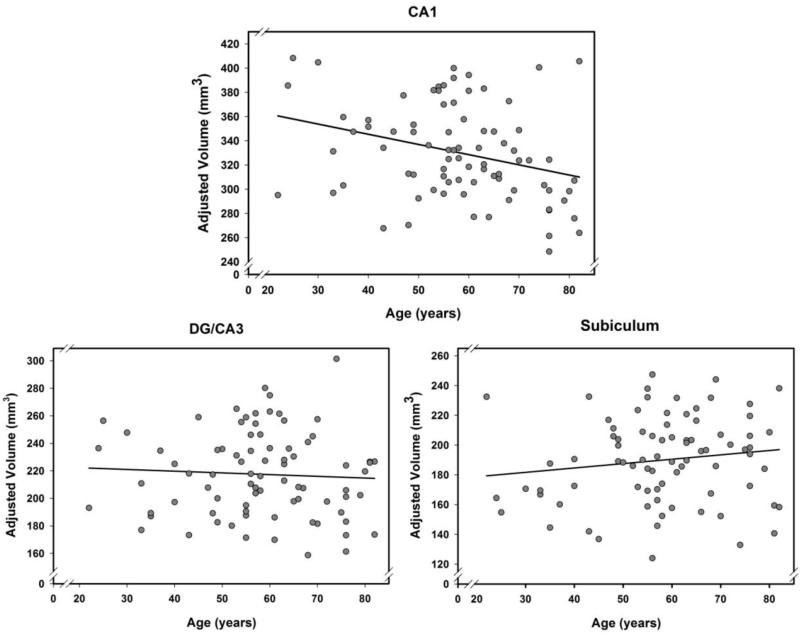
In addition to the main effect of Age, there was a significant ROI × Age interaction: F(2, 142) = 3.44, p = .04. The interaction reflected age-related differences confined to the CA1-2 subfield only: a correlation of volume with age, r = −.31, p = .006; Bonferroni-adjusted p = .03. There were no associations between volume and age noted in the SUB (r = .14, p = .20) and the CA3-DG region (r = .07, p = .61). See Figure 3 for scatter plots and regressions of each subfield volume on age. All analyses were repeated for the EC and revealed no significant effects (all F < 1).
Fig 3.
Associations between age and hippocampal subfield volumes. Only CA1 shows significant age differences: p = .006; no associations of volume with age in the SUB (p = .20) and CA3-DG subfields (p = .61). All regional volumes are adjusted for ICV (see text for details).
Discussion
There are two main findings in this study. The first is the association between genetic predisposition for higher levels of pro-inflammatory cytokine IL-1β and hippocampal subfield volumes in healthy adults. Carriers of the T allele, especially T homozygotes, of the IL-1β C-511T polymorphism exhibited significantly smaller volumes of the hippocampal subfields CA1-2, CA3-DG, and SUB, compared to C homozygotes who were likely to have lesser pro-inflammatory activity. Thus, whereas IL-1β is an important means of defense against infection (Pedra et al. 2009), its increased release into the system, including the brain, may promote processes associated with hindered development or enhanced atrophy of the hippocampus. It will take a longitudinal study on a lifespan sample to determine which of these processes may play a role in the observed association. Notably, this genetic association is specific to the IL-1β C-511T polymorphism and not another pro-inflammatory genetic variant (IL-6) or an established risk factor for Alzheimer’s disease (APOEε4). The effect of IL-1β C-511T polymorphism did not vary across hippocampal subfields. Such uniformity may reflect the dense but relatively even distribution of IL-1β receptors in the hippocampus, although somewhat greater concentration of these receptors has been noted in the DG (Ban et al. 1991).
We did not replicate the negative effect of the APOEε4 variant on any of the hippocampal subfields, and there are several possible explanations for this discrepancy. Notably, unlike the studies that reported such an association (Mueller and Weiner 2009; Pievani et al. 2011), we did not include Alzheimer’s disease patients and cognitively impaired persons in our sample. It is unclear whether healthy carriers of the APOEε4 variant have smaller hippocampal volumes. Several reports of negative effects of APOEε4 on the hippocampal volume in healthy participants (Chiang et al. 2011; Cohen et al. 2001; den Heijer et al. 2012; Lind et al. 2006; Lu et al. 2011; Moffat et al. 2000) are balanced by studies that failed to find such an association (Adamson et al. 2010; Cherbuin et al. 2008; Ferencz et al. 2013; Hostage et al. 2013; Lemaître et al. 2005; Richter-Schmidinger et al. 2011; Troyer et al. 2012), with some finding the effect only for the homozygotes (Crivello et al. 2010).
The second finding is differential age-related variation in the volume of CA1-2 subfields of the human hippocampus. This finding is in accord with the extant literature (Kerchner et al 2013; Mueller et al. 2007; Shing et al. 2011). However, we did not observe a significant effect of hypertension, which was previously reported to explain age-related differences in CA1-2 volumes (Shing et al. 2011), and the reasons for this discrepancy are unclear. It is possible that the influence of hypertension on CA1 is more pronounced at older age (in the Shing et al. sample, the age range was 70-78 years) or that the variance that was attributed to hypertension in that sample was spread among the other vascular risk factors considered. It is also possible that the effect of hypertension was weakened by selection bias in an optimally healthy sample. We can only speculate that participants who maintained overall good health in spite of diagnosed hypertension might have possessed beneficial genetic or developmental characteristics that were not measured and included in the model, but could offset the negative effects of vascular risk. Thus, the differential vulnerability of CA1 to vascular risk in otherwise healthy adults remains indeterminate.
Heightened sensitivity of CA1 to aging observed in this study is in agreement with several previous reports (Mueller et al. 2007; 2011; Mueller and Weiner 2009; Shing et al. 2011). However, there are some contradictory findings. Mueller and colleagues (2011) reported age differences in CA1 and CA3-DG. A recent study reported no age differences in CA1 and significant age differences in CA3 and DG/CA4 (Pereira et al. 2014). A semi-automated segmentation approach used in that study differed from ours (based on Mueller et al. 2007) in several respects: it used images with lower in-plane resolution of 1 mm2 vs. 0.16 mm2, had lower reliability of measurements (and, of note, differentially low reliability of CA1 volumes), and used different rules for region aggregation (combined CA2 with CA3, and not with CA1; and DG only with CA4, and not with CA3) using an approach developed by van Leemput and colleagues (2009). In another study, SUB but not CA1 volume was associated with age (La Joie et al. 2010). In that study, region demarcation rules differed from those in Mueller et al. (2007) especially in defining the boundary separating SUB from CA1. Notably, in all of these studies, the participant age range was narrower than in our study. Thus, it is unclear whether the described discrepancies stem from differences in studied populations or from methodological variations.
The meaning of volume differences observed on MRI is not yet clearly established. Although the loss of neurons in Alzheimer’s disease correlates highly with reduction in hippocampal volume (Bobinski et al. 2000), it is not necessarily true that volume differences reflect neuronal attrition in normal adults. In normal mammalian brains, MRI-based volume estimates track closely to fluctuations in neuropil volume (Qiu et al. 2013). Thus, until more precise in vivo estimates of cytoarchitectonic changes in the hippocampus are available, the neurobiological meaning of the observed differences in hippocampal subfield volumes remains unclear.
The mechanism of the observed associations between the pro-inflammatory risk allele and regional brain volumes is unknown. Brain response to inflammation is mediated mainly by microglia, and probably more so in older organisms. In normal aging, which is accompanied by elevated basal levels of IL-1β and IL-6, microglia are “primed” to respond with enhanced proliferation to rising cytokines levels (Jurgens and Johnson 2012). In response to experimental induction of systemic inflammation, microglia expressing IL-1β emerge in the hippocampi of older but not younger rodents, and LTP suppression ensues (Liu et al. 2012). However, the complex role of microglia and pro-inflammatory cytokines in dynamic morphology of CA1 and DG is still not settled (Ekdahl et al. 2009; Harry and d’Hellencourt 2003; Koo and Duman 2008; Sugawara et al. 2002; Wolf et al. 2002). Although the association of microgliosis with loss of neurons and neuropil in hippocampal subfields has been observed in rodents undergoing kainite treatment (Wolf et al. 2002), the neurobiology underlying differences in volumes measured on relatively coarse MR images is uncertain. Genetic predisposition towards increased pro-inflammatory response is a risk factor for Alzheimer’s disease (Griffin and Mrak 2002), and may be associated with augmented microgliosis, although thus far neuropathological evidence of such connection has been found only for the IL-1α polymorphism (Hayes et al. 2004).
The results of this study should be interpreted in the context of its limitations. First, an important caveat is that this is a cross-sectional study and therefore cannot elucidate age-related changes and individual differences therein. Pro-inflammatory cytokines of the interleukin family play many diverse roles in development, maintenance and aging of the brain, and in particular, the hippocampus. Because of their involvement in plasticity, synaptic pruning, and neurogenesis (Arisi 2014; Graeber et al. 2011; Huang and Sheng 2010; Yirmiya and Goshen 2011), it is premature to infer what aspect of IL-1β is central to the influence on hippocampal volume observed in this cross-sectional study. Although several longitudinal studies have confirmed the progressive (and probably accelerated) nature of age-related hippocampal shrinkage (see Raz and Kennedy 2009 for a review), at the time of this writing, there are no longitudinal studies of hippocampal subfield volumes in normal adults.
The composition of the sample employed in this study limits generalization of the findings. The participants had higher levels of education, greater levels of activity, and lower risk for multiple age-related diseases than typical adults, especially the elderly drawn from an unscreened general population. On the other hand, in such a selective sample, the likelihood of confounding effects of multiple health risks on hippocampal volumes is reduced by comparison to the typical community samples.
The size of the sample available for this study did not allow investigation of second-order interactions among the genetic variants, or between the latter and vascular risk biomarkers. The interaction that we examined could have been rendered non-significant by low statistical power. As this study is a part of an ongoing investigation, we hope for better statistical power in future analyses and follow-ups.
The Mendelian randomization approach used in this study should be qualified by a relatively small sample size. As we did not assess blood levels of IL-1β or IL-6, we could not ascertain that in this sample, the genetic randomization indeed resulted in manipulation of the levels of this pro-inflammatory cytokine.
We did not assess the amyloid burden in this sample. Induction of systemic inflammation in mice results in accumulation of beta-amyloid (Aβ) in the hippocampus followed by cognitive deficits that can be reversed by anti-inflammatory treatment (Guo et al. 2002; Lee at al. 2008). Examining the associations between pro-inflammatory response, hippocampal volume, and Aβ deposits is an important undertaking for future studies. However, the lack of APOEε4 effects suggests that differences in amyloid deposits are unlikely to explain the observed differences.
Although the parcellation of the hippocampus that we used in this study is an improvement over the whole-hippocampus measures used in the vast majority of studies, it is still a coarse one. Our method did not allow for separation of CA1 from CA2, and most importantly, of DG from CA3. Moreover, even within CA3, there may be distinct structural and functional regions (Kesner 2007) that cannot be demarcated on any of the available MR images. Solution of this problem will have to await greater accessibility of super-high field magnets, e.g. 7T devices that allow significantly better in-plane resolution.
The goal of this study was the comparison of the associations of age and pro-inflammatory genetic variants with regional hippocampal volumes. At the time of this writing, we had no data on the total hippocampal volume and the volumes of other age-sensitive brain regions in this sample. Similar negative effects of various pro-inflammatory factors on the brain structure were observed in the subcortical white matter (Raz et al. 2012; Satizabal et al. 2012) and gray matter volume (Satizabal et al. 2012). Thus, whereas age showed a differential effect on CA1-2 subfield, specificity of the observed effects of pro-inflammatory factors is unclear.
Finally, whereas this study highlights the possible influence of pro-inflammatory cytokine on the hippocampal volume, it sheds no light on what potential influence this association may have on cognitive performance. This study was a part of an ongoing longitudinal investigation and the high-resolution hippocampus imaging protocol was added after the participants underwent one or more waves of cognitive testing, with variable delays between tests and MRI scans. Thus, we could not at this time examine the associations between the volume of hippocampal subfields, inflammation, and cognition.
In summary, genetic predisposition for enhanced pro-inflammatory activity is linked to smaller volume of the hippocampal subfields. The effects are independent of age, sex, diagnosis of hypertension and other genetic risk factors. Inflammation may be, therefore, an age-independent modifier of hippocampal size and not an integral part of an “inflammaging” (Franceschi et al. 2000) process. Nonetheless, reduced hippocampal size is associated with poor performance on memory tasks in older adults (Raz and Kennedy 2009) and prevention of hippocampal shrinkage may be a worthy goal. Neuroinflammation is a modifiable phenomenon and can be ameliorated by a variety of pharmacological and physical means, including exercise, which can specifically reduce IL-1β levels in the hippocampus (Gomes da Silva et al. 2013). Thus, inflammation, especially in persons with a known genetic predisposition, may be a worthwhile target of therapeutic intervention.
Ethical Standards
The human study contained herein was approved by the Institutional Review Board and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided informed consent in accord with University Institutional Review Board guidelines.
Acknowledgements
The authors gratefully acknowledge their funding sponsor, the National Institutes of Health, grant number R37 AG-11230.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Naftali Raz, Department of Psychology & Institute of Gerontology, Wayne State University, 87 E. Ferry St., 226 Knapp Building, Detroit, MI 48202.
Ana M. Daugherty, Department of Psychology & Institute of Gerontology, Wayne State University, 87 E. Ferry St., 226 Knapp Building, Detroit, MI 48202
Andrew R. Bender, Institute of Gerontology, Wayne State University, 87 E. Ferry St., 226 Knapp Building, Detroit, MI 48202
Cheryl L. Dahle, Institute of Gerontology, Wayne State University, 87 E. Ferry St., 226 Knapp Building, Detroit, MI 48202
Susan Land, Department of Obstetrics and Gynecology, Wayne State University, 275 East Hancock, C.S. Mott Center, Detroit, MI 48201.
References
- Adamson M, Landy KM, Duong S, Fox-Bosetti S, Ashford JW, Murphy GM, Weiner M, Taylor JL. Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiol Aging. 2010;31:1059–1063. doi: 10.1016/j.neurobiolaging.2008.07.017. doi: 10.1016/j.neurobiolaging.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisi GM. Nervous and immune systems signals and connections: Cytokines in hippocampus physiology and pathology. Epilepsy Behav. 2014:S1525–5050. doi: 10.1016/j.yebeh.2014.01.017. 00030-00034. doi: 10.1016/j.yebeh.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Receptors for interleukin-1 (alpha and beta) in mouse brain: Mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21–30. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, La Scola ME, Morley JE. Intravenous human interleukin-1 alpha impairs memory processing in mice: Dependence on blood-brain barrier transport into posterior division of the septum. J Pharmacol Exp Ther. 2001;299:536–541. [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Banks WA. Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr Pharm Des. 2005;11:973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Döhring J, Rohr A, Jansen O, Deuschl G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci U S A. 2011;108:17562–17567. doi: 10.1073/pnas.1110266108. doi: 10.1073/pnas.1110266108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Konrad C, Grotegerd D, Suslow T, Birosova E, Ohrmann P, Bauer J, Arolt V, Heindel W, Domschke K, Schöning S, Rauch AV, Uhlmann C, Kugel H, Dannlowski U. Interleukin-6 gene (IL-6): A possible role in brain morphology in the healthy adult brain. J Neuroinflammation. 2012;9:125. doi: 10.1186/1742-2094-9-125. doi: 10.1186/1742-2094-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE ε4 carriers: The contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50:704–714. doi: 10.1016/j.neuropsychologia.2011.12.025. doi: 10.1016/j.neuropsychologia.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: The role of microglia and astrocytes. Aging Cell. 2004;3:169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, Rusinek H, Wisniewski HM. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- Burns JM, Honea RA, Vidoni ED, Hutfles LJ, Brooks WM, Swerdlow RH. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim Biophys Acta. 2012;1822:333–339. doi: 10.1016/j.bbadis.2011.06.011. doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Anstey KJ, Sachdev PS, Maller JJ, Meslin C, Mack HA, Wen W, Easteal S. Total and regional gray matter volume is not related to APOE*E4 status in a community sample of middle-aged individuals. J Gerontol A Biol Sci Med Sciences. 2008;63:501–504. doi: 10.1093/gerona/63.5.501. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Sachdev P, Anstey KJ. Higher normal fasting plasma glucose is associated with hippocampal atrophy: The PATH Study. Neurology. 2012;79:1019–1026. doi: 10.1212/WNL.0b013e31826846de. doi: 10.1212/WNL.0b013e31826846de. [DOI] [PubMed] [Google Scholar]
- Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang ST, Thompson PM, Reiman EM, Jack CR, Jr, Fox NC, Jagust WJ, Harvey DJ, Beckett LA, Gamst A, Aisen PS, Petersen RC, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative Impact of apolipoprotein E4-cerebrospinal fluid β-amyloid interaction on hippocampal volume loss over 1 year in mild cognitive impairment. Alzheimers Dement. 2011;7:514–520. doi: 10.1016/j.jalz.2010.12.010. doi: 10.1016/j.jalz.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57:2223–2228. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- Crivello F, Lemaître H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage. 2010;53:1064–1069. doi: 10.1016/j.neuroimage.2009.12.116. doi: 10.1016/j.neuroimage.2009.12.116. [DOI] [PubMed] [Google Scholar]
- Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126:170–175. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- den Heijer T, van der Lijn F, Ikram A, Koudstaal PJ, van der Lugt A, Krestin GP, Vrooman HA, Hofman A, Niessen WJ, Breteler MM. Vascular risk factors, apolipoprotein E, and hippocampal decline on magnetic resonance imaging over a 10-year follow-up. Alzheimers Dement. 2012;8:417–425. doi: 10.1016/j.jalz.2011.07.005. doi: 10.1016/j.jalz.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Di Bona D, Plaia A, Vasto S, Cavallone L, Lescai F, Franceschi C, Licastro F, Colonna-Romano G, Lio D, Candore G, Caruso C. Association between the interleukin-1beta polymorphisms and Alzheimer’s disease: A systematic review and meta-analysis. Brain Res Rev. 2008;59:155–163. doi: 10.1016/j.brainresrev.2008.07.003. doi: 10.1016/j.brainresrev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampà C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de lure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis. 2013;52:229–236. doi: 10.1016/j.nbd.2012.12.009. doi: 10.1016/j.nbd.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Ferencz B, Laukka EJ, Lövdén M, Kalpouzos G, Keller L, Graff C, Wahlund LO, Fratiglioni L, Bäckman L. The influence of APOE and TOMM40 polymorphisms on hippocampal volume and episodic memory in old age. Front Hum Neurosci. 2013;7:198. doi: 10.3389/fnhum.2013.00198. doi: 10.3389/fnhum.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Foster JR, Mirsky AE. Ageing and the regulation of cell activities during exposure to cold. J Gen Physiol. 1969;54:690–712. doi: 10.1085/jgp.54.6.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin WT, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Gomes da Silva S, Simões PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graça Naffah-Mazzacoratti M, Arida RM. Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflammation. 2013;10:61. doi: 10.1186/1742-2094-10-61. doi: 10.1186/1742-2094-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB, Li W, Rodriguez MI. Role of microglia in CNS inflammation. FEBS Lett. 2011;585:3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer’s disease. J Leukoc Biol. 2002;72:233–238. [PMC free article] [PubMed] [Google Scholar]
- Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: Implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JT, Yu J, Grass D, de Beer FC, Kindy MS. Inflammation-dependent cerebral deposition of serum amyloid a protein in a mouse model of amyloidosis. J Neurosci. 2002;22:5900–5909. doi: 10.1523/JNEUROSCI.22-14-05900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry GJ, d’Hellencourt CL. Dentate gyrus: Alterations that occur with hippocampal injury. Neurotoxicology. 2003;24:343–356. doi: 10.1016/S0161-813X(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Galons JP, Wenk GL. Quantitative volumetric analyses of brain magnetic resonance imaging from rat with chronic neuroinflammation. Exp Neurol. 2000;165:347–354. doi: 10.1006/exnr.2000.7469. [DOI] [PubMed] [Google Scholar]
- Hayes A, Green EK, Pritchard A, Harris JM, Zhang Y, Lambert JC, Chartier-Harlin MC, Pickering-Brown SM, Lendon CL, Mann DM. A polymorphic variation in the interleukin 1A gene increases brain microglial cell activity in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1475–1477. doi: 10.1136/jnnp.2003.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health. 2007;7:220. doi: 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. Hippocampal and neocortical involvement in normal brain aging and dementia: Morphological and neurochemical profile of the vulnerable circuits. J Am Geriatr Soc. 1996;44:857–864. doi: 10.1111/j.1532-5415.1996.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Hostage CA, Roy Choudhury K, Doraiswamy PM, Petrella JR, Alzheimer’s Disease Neuroimaging Initiative Dissecting the gene dose-effects of the APOE ε4 and ε2 alleles on hippocampal volumes in aging and Alzheimer’s disease. PLoS One. 2013;8:e54483. doi: 10.1371/journal.pone.0054483. doi: 10.1371/journal.pone.0054483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZB, Sheng GQ. Interleukin-1beta with learning and memory. Neurosci Bull. 2010;26:455–468. doi: 10.1007/s12264-010-6023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1β genes. Eur J Immunol. 1998;28:2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, Smith S. BET2: MR-based estimation of brain, skull and scalp surfaces; Proceedings from the Eleventh Annual Meeting of the Organization for Human Brain Mapping; Toronto, Ontario, Canada. 2005. [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp Neurol. 2012;233:40–48. doi: 10.1016/j.expneurol.2010.11.014. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan MB. Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet. 1986;1:507–508. doi: 10.1016/s0140-6736(86)92972-7. [DOI] [PubMed] [Google Scholar]
- Kauffman MA, Moron DG, Consalvo D, Bello R, Kochen S. Association study between interleukin 1 beta gene and epileptic disorders: A HuGe review and meta-analysis. Genet Med. 2008;10:83–88. doi: 10.1097/GIM.0b013e318161317c. doi:10.1097/GIM.0b013e318161317c. [DOI] [PubMed] [Google Scholar]
- Keihaninejad S, Heckemann RA, Fagiolo G, Symms MR, Hajnal JV, Hammers A. A robust method to estimate the intracranial volume across MRI field strengths (1.5T and 3T) Neuroimage. 2010;50:1427–1437. doi: 10.1016/j.neuroimage.2010.01.064. doi: 10.1016/j.neuroimage.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Bernstein JD, Fenesy MC, Deutsch GK, Saranathan M, Zeineh MM, Rutt BK. Shared vulnerability of two synaptically-connected medial temporal lobe areas to age and cognitive decline: a seven tesla magnetic resonance imaging study. J Neurosci. 2013;33:16666–16672. doi: 10.1523/JNEUROSCI.1915-13.2013. doi: 10.1523/JNEUROSCI.1915-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–548. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: The Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- Kriz J, Lalancette-Hébert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117:497–509. doi: 10.1007/s00401-009-0496-1. doi: 10.1007/s00401-009-0496-1. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Harbord RM, Sternej AC, Timpson N, Davey Smith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- La Joie R, Fouquet M, Mézenge F, Landeau B, Villain N, Mevel K, Pélerin A, Eustache F, Desgranges B, Chételat G. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. Neuroimage. 2010;53:506–514. doi: 10.1016/j.neuroimage.2010.06.024. doi: 10.1016/j.neuroimage.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître H, Crivello F, Dufouil C, Grassiot B, Tzourio C, Alpérovitch A, Mazoyer B. No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage. 2005;24:1205–1213. doi: 10.1016/j.neuroimage.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu Z, Hayashi Y, Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012;216:133–142. doi: 10.1016/j.neuroscience.2012.04.050. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]
- Lind J, Larsson A, Persson J, Ingvar M, Nilsson LG, Bäckman L, Adolfsson R, Cruts M, Sleegers K, Van Broeckhoven C, Nyberg L. Reduced hippocampal volume in non-demented carriers of the apolipoprotein E epsilon4: Relation to chronological age and recognition memory. Neurosci Lett. 2006;396:23–27. doi: 10.1016/j.neulet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- Lu PH, Thompson PM, Leow A, Lee GJ, Lee A, Yanovsky I, Parikshak N, Khoo T, Wu S, Geschwind D, Bartzokis G. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. JAlzheimers Dis. 2011;23:433–442. doi: 10.3233/JAD-2010-101398. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Age-related impairment in long-term potentiation in hippocampus: A role for the cytokine, interleukin-1 beta? Prog Neurobiol. 1998;56:571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Mahley R. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–640. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. doi: 10.1016/j.biopsych. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenzahl EM, Rujescu D, Kirner A, Giegling I, Kathmann N, Leinsinger G, Maag K, Hegerl U, Hahn K, Möller HJ. Association of an interleukin-1beta genetic polymorphism with altered brain structure in patients with schizophrenia. Am J Psychiatry. 2001;158:1316–1319. doi: 10.1176/appi.ajp.158.8.1316. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 2000;55:134–136. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR Am J Neuroradiol. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Chao LL, Berman B, Weiner MW. Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4T. Neuroimage. 2011;56:851–857. doi: 10.1016/j.neuroimage.2011.03.028. doi: 10.1016/j.neuroimage.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, Weiner MW. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28:719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus. 2009;19:558–564. doi: 10.1002/hipo.20614. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papiol S, Molina V, Desco M, Rosa A, Reig S, Gispert JD, Sanz J, Palomo T, Fañanás L. Ventricular enlargement in schizophrenia is associated with a genetic polymorphism at the interleukin-1 receptor antagonist gene. Neuroimage. 2005;27:1002–1006. doi: 10.1016/j.neuroimage.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Papiol S, Molina V, Desco M, Rosa A, Reig S, Sanz J, Palomo T, Fañanás L. Gray matter deficits in bipolar disorder are associated with genetic variability at interleukin-1 beta gene (2q13) Genes Brain Behav. 2008;7:796–801. doi: 10.1111/j.1601-183X.2008.00421.x. doi: 10.1111/j.1601-183X.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009;21:10–16. doi: 10.1016/j.coi.2009.01.006. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Valls-Pedret C, Ros E, Palacios E, Falcón C, Bargalló N, Bartrés-Faz D, Wahlund LO, Westman E, Junque C. Regional vulnerability of hippocampal subfields to aging measured by structural and diffusion MRI. Hippocampus. 2014;24:403–414. doi: 10.1002/hipo.22234. doi: 10.1002/hipo.22234. [DOI] [PubMed] [Google Scholar]
- Petito CK, Pulsinelli WA. Delayed neuronal recovery and neuronal death in rat hippocampus following severe cerebral ischemia: Possible relationship to abnormalities in neuronal processes. J Cereb Blood Flow Metab. 1984;4:194–205. doi: 10.1038/jcbfm.1984.28. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of Professional and Public Education of the American Heart Association Council on high blood pressure research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- Pievani M, Galluzzi S, Thompson PM, Rasser PE, Bonetti M, Frisoni GB. APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer’s disease. Neuroimage. 2011;55:909–919. doi: 10.1016/j.neuroimage.2010.12.081. doi: 10.1016/j.neuroimage.2010.12.081. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: A neuropathologic study in the rat. Neurology. 1982;32:1239–1246. doi: 10.1212/wnl.32.11.1239. [DOI] [PubMed] [Google Scholar]
- Qiu C, Zhang Y, Bronge L, Herlitz A, Aspelin P, Bäckman L, Fratiglioni L, Wahlund LO. Medial temporal lobe is vulnerable to vascular risk factors in men: A population-based study. Eur J Neurol. 2012;19:876–883. doi: 10.1111/j.1468-1331.2011.03645.x. doi: 10.1111/j.1468-1331.2011.03645.x. [DOI] [PubMed] [Google Scholar]
- Qiu LR, Germann J, Spring S, Alm C, Vousden DA, Palmert MR, Lerch JP. Hippocampal volumes differ across the mouse estrous cycle, can change within 24 hours, and associate with cognitive strategies. Neuroimage. 2013;83:593–598. doi: 10.1016/j.neuroimage.2013.06.074. doi: 10.1016/j.neuroimage.2013.06.074. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging. 2011;32:1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. doi:10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaglia G, Paola F, Maioli F, Martelli M, Montesi F, Bastagli L, Bianchin M, Chiappelli M, Tumini E, Bolondi L, Licastro F. Interleukin-1beta and interleukin-6 gene polymorphisms as risk factors for AD: A prospective study. Exp Gerontol. 2006;41:85–92. doi: 10.1016/j.exger.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition - II. Erlbaum; New Jersey: 2000. pp. 1–90. [Google Scholar]
- Raz N, Kennedy KM. A systems approach to the aging brain: Neuroanatomic changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the aging brain. Oxford University Press; New York: 2009. pp. 43–70. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Yang Y, Dahle CL, Land S. Volume of white matter hyperintensities in healthy adults: Contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta. 2012;1822:361–369. doi: 10.1016/j.bbadis.2011.08.007. doi: 10.1016/j.bbadis.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, Lewczuk P, Sidiropoulos C, Kneib T, Perneczky R, Doerfler A, Kornhuber J. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2011;118:249–257. doi: 10.1007/s00702-010-0539-8. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E and Alzheimer’s disease. A rapidly expanding field with medical and epidemiological consequences. Ann N Y Acad Sci. 1996;802:50–57. doi: 10.1111/j.1749-6632.1996.tb32598.x. [DOI] [PubMed] [Google Scholar]
- Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- Shing YL, Rodrigue KM, Kennedy KM, Fandakova Y, Bodammer N, Werkle-Bergner M, Lindenberger U, Raz N. Hippocampal subfield volumes: Age, vascular risk, and correlation with associative memory. Front Aging Neurosci. 2011;3:2. doi: 10.3389/fnagi.2011.00002. doi: 10.3389/fnagi.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans. 2007;35:1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Lewén A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19:85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- Suyama K. Changes of neuronal transmission in the hippocampus after transient ischemia in spontaneously hypertensive rats and the protective effects of MK-801. Stroke. 1992;23:260–266. doi: 10.1161/01.str.23.2.260. [DOI] [PubMed] [Google Scholar]
- Troyer AK1, Murphy KJ, Anderson ND, Craik FI, Moscovitch M, Maione A, Gao F. Associative recognition in mild cognitive impairment: Relationship to hippocampal volume and apolipoprotein E. Neuropsychologia. 2012;50:3721–8. doi: 10.1016/j.neuropsychologia.2012.10.018. doi: 10.1016/j.neuropsychologia.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Tso AR, Merino JG, Warach S. Interleukin-6 174G/C polymorphism and ischemic stroke: A systematic review. Stroke. 2007;38:3070–3075. doi: 10.1161/STROKEAHA.107.492231. [DOI] [PubMed] [Google Scholar]
- van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19:549–557. doi: 10.1002/hipo.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Bilbo SD. Chemokines and the hippocampus: A new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G, Hardy R. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440. doi: 10.1371/journal.pmed.1000440. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos GT, editor. The rat nervous system. 2nd edn. Academic Press; California: 1995. pp. 443–493. [Google Scholar]
- Wolf OT, Dyakin V, Patel A, Vadasz C, de Leon MJ, McEwen BS, Bulloch K. Volumetric structural magnetic resonance imaging (MRI) of the rat hippocampus following kainic acid (KA) treatment. Brain Res. 2002;934:87–96. doi: 10.1016/s0006-8993(02)02363-6. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]