Abstract
Objective
Coffee and tea consumption is associated with a decreased type 2 diabetes risk in non-pregnant adults. We examined the relation between first trimester coffee and tea consumption and gestational diabetes mellitus (GDM) risk.
Design
Population-based cohort study.
Setting
Denmark 1996-2002.
Population
Non-diabetic women with singleton pregnancies in the Danish National Birth Cohort (n=71,239).
Methods
Estimated adjusted relative risks (RR) and 95% confidence intervals (95%CI) for the association between first trimester coffee and tea or estimated total caffeine and GDM.
Main outcome measures
GDM ascertained from the National Hospital Discharge Register or maternal interview.
Results
Coffee or tea intake was reported in 81.2% (n=57,882) and GDM complicated 1.3% (n=912) of pregnancies. Among non-consumers, GDM complicated 1.5% of pregnancies. Among coffee drinkers, GDM was highest among women who drank ≥8 cups/d (1.8%) with no significant difference across intake levels (P=.10). Among tea drinkers, there was no difference in GDM across intake levels (1.2%) (P=.98). After adjustment for age, socio-occupational status, parity, prepregnancy body mass index, smoking, and cola, there was suggestion of a protective, but non-significant association with increasing coffee [RR ≥8 vs 0 cups/d=0.89 (95%CI 0.64-1.25)] and tea [RR ≥8 vs 0 cups/d=0.77 (95%CI 0.55-1.08)]. Results were similar by smoking status, except a non-significant 1.45-fold increased risk with ≥8 coffee cups/d for non-smokers. There was a non-significant reduced GDM risk with increasing total caffeine.
Conclusions
Our results suggest that moderate first trimester coffee and tea intake were not associated with GDM increased risk and possibly may have a protective effect.
Keywords: coffee, tea, caffeine, pregnancy, gestational diabetes
Introduction
Coffee and tea are habitually consumed among women of reproductive age and thus their potential effects during pregnancy are of interest.(1) In non-pregnant adults, acute caffeine intake increases insulin resistance; however, regular coffee intake has been associated with a decreased risk for type 2 diabetes.(2) Habitual coffee intake decreases subclinical inflammation and increases adiponectin levels,(3, 4) which may protect against insulin resistance and confer a long-term reduced risk for type 2 diabetes.(5) It is thought that the beneficial effects of coffee act not through caffeine and its metabolites, but through the micronutrients and phenolic compounds, which have antioxidant and prebiotic properties to improve glucose control, insulin sensitivity, and appetite regulation.(6) With lower caffeine levels and additional phenolic compounds,(7) tea consumption has similarly been associated with a reduced risk for type 2 diabetes in non-pregnant adults.(8)
Gestational diabetes mellitus (GDM) is a carbohydrate intolerance first diagnosed during pregnancy. It is unclear whether the mechanism which confers a reduced risk for type 2 diabetes associated with coffee and tea intake outside of pregnancy, acts similarly during pregnancy and is associated with a reduced risk for GDM. Caffeine metabolism slows during pregnancy,(9) which may lead to an increased acute response later in pregnancy. Acute levels of caffeine and paraxanthine, the major metabolite of caffeine, have been associated with increased insulin resistance during pregnancy.(10) Furthermore, there is pronounced peripheral insulin resistance late in pregnancy.(11) Thus the impact of coffee and tea intake during pregnancy may differ from the non-pregnant state because of changes in insulin resistance and metabolism during pregnancy. Because GDM is associated with an increased risk for maternal morbidities, including development of type 2 diabetes, as well as perinatal mortality and morbidities,(12, 13) identification of risk factors or potential preventive factors for GDM are therefore of interest.
Smoking may be an important potential effect modifier of the relation between coffee and GDM risk. Smoking induces the cytochrome P450 enzymes, which can increase caffeine metabolism by almost 100%.(14, 15) Studies of non-pregnant adults report an increased risk for type 2 diabetes among smokers,(16) however, there is no consistent evidence of an association between smoking and GDM.(17)
The aim of this study was to examine the relation between first trimester coffee and tea consumption and the risk of GDM and to investigate whether the association differed by smoking status.
Materials and Methods
Study population
The Danish National Birth Cohort (DNBC) is a longitudinal study of pregnant women and their offspring approved by the Danish National Ethics Board. The cohort has been described in detail elsewhere.(18, 19) Briefly, from March 1, 1996 to November 1, 2002 women were recruited across Denmark by general practitioners at their first antenatal visit, of which approximately 60% agreed to participate. Once enrolled, women were interviewed at targeted gestational ages of 12 and 30 weeks and twice after delivery when the child was 6 and 18 months of age. Data were also collected from the Danish Medical Birth Registry and the National Hospital Discharge Register and linked to the interviews using a unique personal code assigned to each citizen in Denmark. The current study utilized information from the first three interviews and these Registries. Institutional review board approval was obtained from the DNBC Steering Committee, the Danish Data Protection Board and the University of Pittsburgh.
A total of 101,033 pregnancies from 91,769 women were recruited to the DNBC. For this analysis we included only each woman's first singleton pregnancy recorded in the DNBC (n=86,453) and further restricted the analysis to women who completed the first two interviews (n=75,941). Women with pre-existing diabetes as identified using the National Hospital Discharge Register International Classification of Disease Code, tenth revision (ICD-10; O24.0, O24.1, O24.3) were excluded (n=268; 0.35%). We further excluded deliveries if any relevant covariates were missing (n=4,434; 5.9%) leaving a total of 71,239 deliveries for analysis. Compared to the women in the final analysis, the women excluded due to missing covariates had different distributions of the relevant exposures and outcome (P<.001). Excluded women were more likely to not consume any coffee (57.0% vs. 55.3%) or tea (40.9% vs. 36.5%) and were more likely to have GDM (2.1% vs. 1.3%).
Exposure
At the first interview women were asked, “How many cups of coffee do you drink daily? (mug=2 cups, 1 pot=8 cups=1L).” If a woman reported drinking <1 cup/d it was coded as 0.5 cup/d. Women were similarly asked about tea. We categorized coffee and tea intake separately (0; 0.5-3; 4-7; ≥8 cups/d) and similar to a prior study we combined daily intake of coffee and tea and estimated the total caffeine intake with 1 cup of coffee or tea estimated to have 100 or 50 mg of caffeine, respectively.(20, 21)
Outcome
A total of 912 cases of GDM were identified from three sources. The primary source of GDM was from the National Hospital Discharge Register (ICD-10, O24.4) (n=546). At the second DNBC interview (∼30 weeks), women were asked if they had diabetes during pregnancy, and if so, the type, resulting in 467 women with GDM. At the first postpartum DNBC interview (∼6 months postpartum) women were asked if diabetes was detected from 30 weeks gestation up to delivery (n=517). Among women with GDM identified from the second or third interview, 51.6% and 61.7% also had GDM identified from the Discharge Register, respectively. For the main analyses we considered cases identified from either the Discharge Register or the interviews (n=912). In secondary analyses we used only GDM cases identified from both the Discharge Register and the interviews (n=404) or from the Discharge Register only (n=546).
Covariable assessment
The following potential confounders were considered based on the potential association with both coffee or tea intake and GDM: maternal age at delivery (16-20, 20-24, 25-29, 30-34, 35-39 y), parity (0, 1, ≥2), self-reported smoking status at the first interview (0, 1-10, ≥11 cigarettes/d), cola intake (0, <1, ≥1 L/wk), prepregnancy body mass index (BMI; kg/m2) calculated from self-reported height and prepregnancy weight (<18.5, 18.5-24.9, 25.0-29.9, 30.0-34.9, 35.0-39.9, ≥40.0 kg/m2), and socio-occupational status (high, middle, low). Socio-occupational status was defined as high, persons with 4 years beyond high school or in management; middle, skilled workers and persons with middle-range training; or low, unskilled workers and unemployed.
Statistical analysis
Chi-square statistics were used for bivariate analyses. We estimated unadjusted and adjusted risk of GDM across levels of coffee and tea intake using modified Poisson regression(22) and performed a test for trend using linear contrasts. Coffee and tea were assessed as categorical variables in main analyses and compared to models with continuous per cup exposure. We also reported estimates stratified by smoking status.
We also examined the association between GDM risk and estimated total caffeine intake up to 1200 mg non-parametrically with restricted cubic splines. Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms.(23)
We performed multiple sensitivity analyses. In analyses of coffee and tea exposures we limited GDM outcome to women who had GDM indicated in the Discharge Register and also self-reported GDM at either interview (n=404). Second, we limited the analysis only to the women who had GDM indicated in the Discharge Register (n=546). Third, we assessed the impact of missing covariate data by examining the unadjusted associations among all women regardless of their missing covariate data (n=75,673).
We used SAS version 9.3 (SAS Institute, Cary, NC, USA) and considered P-values <.05 significant.
Results
The majority of the study population (81.2%; n=57,882) reported drinking coffee or tea at the first interview, with coffee consumption (44.7%; n=31,860) being less prevalent than tea (63.5%; n=45,208). Among consumers, the median consumption of both coffee and tea was 2 cups/d. Women who drank ≥8 coffee cups/d were the most likely not to drink tea (63.3%), while women who did not drink coffee were the most likely to drink ≥8 tea cups/d (5.0%). Coffee and tea consumption were associated with maternal age, parity, prepregnancy BMI, smoking, socio-occupational status, and cola consumption (Table 1). For example, coffee consumption tended to be lower among younger women and nulliparous women. Women of a low socio-occupational status were more likely to consume higher levels of coffee, but not tea, than women with a high socio-occupational status. Cola consumers were likely to either not consume coffee or drink ≥8 coffee cups/d than non-cola consumers. The proportion of non-smokers decreased linearly with an increase in coffee consumption from 81.2% among non-coffee drinkers to 31.4% among women who drank ≥8 coffee cups/d.
Table 1. Participant characteristics by coffee and tea intake in the Danish National Birth Cohort, 1996-2002.
| Coffee, cups/da | Tea, cups/da | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 (n=39,379) | ½ - 3 (n=22,504) | 4 - 7 (n=6,972) | ≥8 (n=2,384) | 0 (n=26,031) | ½ - 3 (n=32,194) | 4 - 7 (n=9,919) | ≥8 (n=3,095) | |
| Characteristics | % | % | % | % | % | % | % | % |
| Age at delivery, y | ||||||||
| 16-19 | 0.6 | 0.3 | 0.4 | 0.4 | 0.7 | 0.4 | 0.3 | 0.5 |
| 20-24 | 11.3 | 6.3 | 5.1 | 6.0 | 11.2 | 8.4 | 5.7 | 5.6 |
| 25-29 | 43.4 | 35.9 | 28.3 | 25.8 | 40.1 | 40.5 | 34.3 | 28.6 |
| 30-34 | 33.8 | 40.6 | 41.3 | 40.3 | 34.3 | 37.2 | 41.2 | 42.2 |
| 35-39 | 9.8 | 15.1 | 21.8 | 23.7 | 12.2 | 12.1 | 16.5 | 20.2 |
| 40-48 | 1.1 | 1.8 | 3.3 | 3.9 | 1.5 | 1.5 | 2.1 | 3.0 |
| Parity | ||||||||
| 0 | 56.2 | 49.0 | 35.7 | 28.5 | 51.3 | 52.9 | 46.9 | 42.5 |
| 1 | 31.9 | 35.3 | 39.3 | 37.9 | 33.6 | 33.5 | 35.7 | 34.8 |
| ≥2 | 11.9 | 15.7 | 25.0 | 33.6 | 15.1 | 13.6 | 17.4 | 22.8 |
| Prepregnancy BMI, kg/m2 | ||||||||
| <18.5 | 4.2 | 4.5 | 4.9 | 5.8 | 4.5 | 4.4 | 4.2 | 4.1 |
| 18.5-24.9 | 66.8 | 71.5 | 68.9 | 64.3 | 65.0 | 70.3 | 70.8 | 69.0 |
| 25.0-29.9 | 20.3 | 17.3 | 19.2 | 21.8 | 20.9 | 18.4 | 18.1 | 19.5 |
| 30.0-34.9 | 6.3 | 5.1 | 5.4 | 6.3 | 7.0 | 5.1 | 5.1 | 5.5 |
| 35.0-39.9 | 1.9 | 1.2 | 1.2 | 1.4 | 2.0 | 1.3 | 1.4 | 1.7 |
| ≥40.0 | 0.7 | 0.5 | 0.4 | 0.4 | 0.8 | 0.5 | 0.4 | 0.3 |
| Cigarettes, per d | ||||||||
| 0 | 81.2 | 72.3 | 52.4 | 31.4 | 68.0 | 78.5 | 76.2 | 67.7 |
| 1-10 | 14.8 | 22.6 | 34.8 | 38.7 | 23.9 | 17.1 | 18.4 | 23.1 |
| >10 | 4.0 | 5.2 | 12.8 | 29.9 | 8.1 | 4.4 | 5.4 | 9.2 |
| Socio-occupational statusb | ||||||||
| High | 53.0 | 57.3 | 47.3 | 37.4 | 46.2 | 56.3 | 60.4 | 58.1 |
| Middle | 38.3 | 35.3 | 41.3 | 44.3 | 42.5 | 36.1 | 32.8 | 33.0 |
| Low | 8.7 | 7.4 | 11.4 | 18.3 | 11.3 | 7.6 | 6.8 | 8.9 |
| Cola, L/wk | ||||||||
| 0 | 34.0 | 33.5 | 33.4 | 35.2 | 34.3 | 32.7 | 34.6 | 38.3 |
| <1 | 47.1 | 53.0 | 51.3 | 46.5 | 46.0 | 52.2 | 50.4 | 45.3 |
| ≥1 | 18.9 | 13.5 | 15.4 | 18.3 | 19.7 | 15.1 | 15.0 | 16.4 |
| Tea, cups/d | ||||||||
| 0 | 33.9 | 33.3 | 52.8 | 63.3 | -- | -- | -- | -- |
| ½ - 3 | 46.0 | 51.1 | 29.2 | 22.8 | -- | -- | -- | -- |
| 4 - 7 | 15.0 | 12.5 | 14.0 | 9.6 | -- | -- | -- | -- |
| ≥8 | 5.0 | 3.2 | 4.0 | 4.4 | -- | -- | -- | -- |
| Coffee, cups/d | ||||||||
| 0 | -- | -- | -- | -- | 51.3 | 56.3 | 59.6 | 64.2 |
| ½ - 3 | -- | -- | -- | -- | 28.8 | 35.7 | 28.2 | 23.5 |
| 4 - 7 | -- | -- | -- | -- | 14.1 | 6.3 | 9.9 | 9.0 |
| ≥8 | -- | -- | -- | -- | 5.8 | 1.7 | 2.3 | 3.4 |
Abbreviations: BMI, body mass index
Coffee and tea intake significantly different across all characteristics (P<.001).
Socio-occupational status: high, persons with 4 years beyond high school or in management; middle, skilled workers and persons with middle-range training; low, unskilled workers and unemployed.
GDM complicated 1.3% (n=912) of pregnancies. Women with GDM were slightly older [mean (SD), 31.8 (4.6) vs. 30.4 (4.3) years, P<.001], had a higher prepregnancy BMI [27.4 (5.9) vs. 23.5 (4.2) kg/m2, P<.001), were less likely to be nulliparous (40.6 vs. 51.1%, P<.001), more often a non-smoker (70.7 vs. 73.9%, P<.001), had a low socio-occupational status (42.1 vs. 53.4%, P<.001) and did not consume cola (30.6 vs. 33.9%, P<.001). GDM complicated 1.5% of pregnancies among women who did not consume either coffee or tea. Among coffee drinkers, GDM prevalence was highest among women who drank ≥8 coffee cups/d (1.8%), but there was no significant difference across levels of coffee intake (P=.10). Among tea drinkers, there was no difference in the prevalence of GDM across levels of tea intake (1.2%) (P=.98).
In the fully adjusted model we tested for but did not detect a multiplicative interaction between coffee and tea intake (P=.58) and thus we report the risk of GDM separately for coffee and tea intake. Increasing levels of coffee and tea intake suggested a decreased risk of developing GDM, but all were non-significant including a linear trend test (Table 2). When analyzed as continuous exposures, risk ratios per cup of coffee or tea were suggestive of a protective association for GDM risk, but non-significant for both coffee (adjusted RR=0.97; 95% CI 0.95, 1.00) and tea (adjusted RR=0.98; 95% CI 0.96, 1.01).
Table 2. First trimester coffee and tea consumption and adjusted risk ratios (95% confidence intervals) for gestational diabetes mellitus in the Danish National Birth Cohort, 1996-2002.
| Overall (n=71,239) | Stratified by Smoking Status | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Smokers (n=52,629) | Smokers (n=18,610) | ||||||||||||||
| Cups/ d | GDM Casesa | (%) | aRRb | 95% CI | Pc | GDM Casesa | (%) | aRRd | 95% CI | Pc | GDM Casesa | (%) | aRRd | 95% CI | Pc |
| Coffee | .16 | .05 | .91 | ||||||||||||
| 0 | 500 | (1.27) | 1.00 | Referent | 389 | (1.22) | 1.00 | Referent | 111 | (1.50) | 1.00 | Referent | |||
| ½ – 3 | 280 | (1.24) | 0.97 | 0.84, 1.13 | 196 | (1.21) | 0.97 | 0.81, 1.15 | 84 | (1.35) | 0.99 | 0.75, 1.32 | |||
| 4 – 7 | 90 | (1.29) | 0.81 | 0.64, 1.02 | 41 | (1.12) | 0.76 | 0.55, 1.05 | 49 | (1.48) | 0.86 | 0.61, 1.22 | |||
| ≥8 | 42 | (1.76) | 0.89 | 0.64, 1.25 | 19 | (2.54) | 1.45 | 0.92, 2.30 | 23 | (1.41) | 0.69 | 0.44, 1.09 | |||
| Tea | .31 | .24 | .79 | ||||||||||||
| 0 | 381 | (1.46) | 1.00 | Referent | 249 | (1.41) | 1.00 | Referent | 132 | (1.58) | 1.00 | Referent | |||
| ½ – 3 | 376 | (1.17) | 0.91 | 0.79, 1.05 | 296 | (1.17) | 0.94 | 0.79, 1.11 | 80 | (1.16) | 0.81 | 0.62, 1.07 | |||
| 4 – 7 | 118 | (1.19) | 0.88 | 0.72, 1.08 | 77 | (1.02) | 0.78 | 0.60, 1.01 | 41 | (1.74) | 1.16 | 0.82, 1.64 | |||
| ≥8 | 37 | (1.20) | 0.77 | 0.55, 1.08 | 23 | (1.10) | 0.75 | 0.49, 1.14 | 14 | (1.40) | 0.81 | 0.47, 1.40 | |||
Abbreviations: aRR, adjusted risk ratio; CI, confidence interval; GDM, gestational diabetes mellitus.
GDM cases identified from Discharge Register or self-reported on either DNBC interview (n=912).
Model adjusted for coffee or tea consumption (categorical), as appropriate, age (categorical), socio-occupational status (categorical), parity (categorical), prepregnancy body mass index (categorical), smoking (categorical) and cola intake (categorical).
Represents P for linear trend.
Model adjusted for coffee or tea consumption (categorical), as appropriate, age (categorical), socio-occupational status (categorical), parity (categorical), prepregnancy body mass index (categorical), and cola intake (categorical).
While non-smokers overall were less likely to develop GDM than smokers (1.2% vs. 1.4%, P=.03) the GDM prevalence differed slightly by coffee and tea intake. Among non-smokers GDM occurred most often among women who drank ≥8 cups/d (2.5%) with an adjusted relative risk of 1.45 compared to non-coffee drinkers (95% CI 0.92, 2.30) (Table 2). Among smokers GDM risk consistently decreased as coffee intake increased, but never reached significance. In regard to tea intake the risk of GDM was highest among non-consumers (1.4%) and while all adjusted risk ratios were below 1, none were statistically significant. Among smokers there was no consistent pattern in GDM risk by level of tea intake.
Limiting the GDM cases to those identified both in the Discharge Register and the interviews (n=404) (Table S1) or the Discharge Register (n=546) (Table S2) revealed similar associations between coffee and tea intake overall and when stratified by smoking with one exception. When only cases from the Discharge Register were used, drinking ≥8 coffee cups/d compared to no coffee was associated with a stronger and significantly increased risk for GDM among non-smokers (RR=2.25; 95% CI 1.35, 3.75).
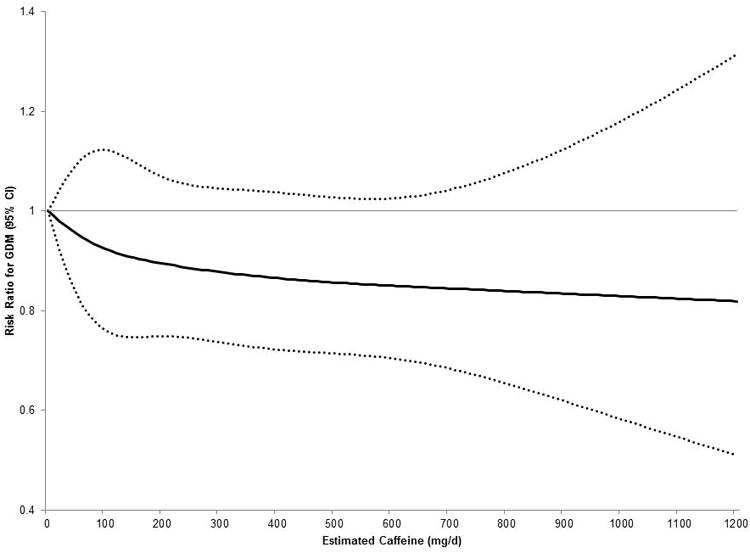
When analyzed as the estimated total caffeine intake (median 100 mg/d; interquartile range 25-300 mg/d) from both coffee and tea, a similar overall pattern with GDM risk was observed. In adjusted analyses there was a non-significant decreased risk of GDM with increasing caffeine intake (Figure 1).
Figure 1. Adjusted association between estimated total caffeine intake and risk of gestational diabetes mellitus in the Danish National Birth Cohort, 1996-2002.
Lastly, because many women were excluded from our analysis due to missing covariate data (n=4,434), we examined the unadjusted associations between coffee and tea and the risk for GDM among all women meeting the inclusion criteria (n=75,673) and observed similar results (data not shown).
Discussion
Main findings
First trimester coffee or tea consumption was not consistently or significantly associated with GDM risk in this large cohort where coffee and tea were commonly consumed. While most risk ratios were below 1, indicating a potential protective association, the strength of the associations were small. When stratified by maternal smoking, drinking ≥8 coffee cups/d tended to increase GDM risk among nonsmokers, but decrease GDM risk among smokers, although this was not observed among tea drinkers. A small, but reduced risk of GDM was consistently suggested with increasing tea consumption.
Strengths and Limitations
GDM cases were collected from three sources. The Danish National Patient Registry collects discharge diagnoses for hospitalizations in Denmark. Although low (1.3%), the GDM prevalence in the DNBC was consistent with other reports of low prevalence of GDM in Northern European countries.(24) In Denmark only high-risk women are routinely screened for GDM,(24) but selective and universal screenings may have similar sensitivities.(25) In a study of type 2 diabetes, good positive predictive values from the Denmark Registry data were observed, however this does not necessarily apply to GDM.(26) In our study we used GDM cases identified from the Discharge Register and supplemented it with maternal report from the second trimester and postpartum DNBC interview. A total of 74% of the GDM cases found in the Discharge Register were verified in the interview data. In supplemental analyses we further evaluated the robustness of our findings by examining the associations using GDM cases captured from the Discharge Register and the interview or the Discharge Register only and observed similar findings. However, we do not know the quality of the GDM diagnosis, but any misclassification would likely be non-differential. While changes in the criteria for diagnosis of GDM have changed over time, it is unclear how the differences in the underlying prevalence of GDM and the severity of the disease may affect the association with coffee and tea intake. Also we have no reason to suspect that the underlying biology of the association between coffee and tea intake and GDM would be different if the underlying prevalence were higher.
This Danish cohort of pregnant women offered the unique ability to assess the association between coffee and tea with GDM as these beverages were commonly consumed in the first trimester. Nonetheless, we were unable to assess all sources of caffeine intake, or take into account different brewing methods, cup sizes, different strengths, or distinguish decaffeinated coffee or type of tea. While decaffeinated coffee is rarely consumed in Denmark, both herbal and black tea are commonly consumed. Therefore in the estimated caffeine intake analysis there was likely a greater degree of misclassification due to tea than coffee intake and leading to possibly an overestimate of total caffeine. Although cola intake was strongly associated with GDM in bivariate analyses and we adjusted for cola in our models we did not further examine this relationship due to the limited information on the level of intake, type of sweetener or caffeine levels. We did not have any information on women's coffee and tea intake prior to pregnancy. Some women may reduce their consumption due to aversion and nausea,(27) so while coffee intake was collected prospectively and we adjusted for many important confounding variables, it may have been impacted by other maternal factors. Lastly, while this Danish cohort was well suited for this research our results may not be fully generalizable to all women in Denmark nor women in other countries with a difference racial background or patterns of consumption. Not all recruited women agreed to participate in the DNBC, but this should not have impacted the internal validity of our estimates.(28, 29) Nonetheless, some women were excluded from our analysis due to missing covariates and these women had a slightly different pattern of coffee and tea consumption and a higher prevalence of GDM. However, we performed a sensitivity analysis repeating our unadjusted analyses among all women and observed similar findings.
Interpretation
The widely reported association between coffee consumption and a decreased risk for type 2 diabetes in non-pregnant adults served as an impetus for this research focused on pregnant women and the association with GDM.(5) A prior study of U.S. women found that compared to non-consumers pre-pregnancy consumption of 0.5-7 caffeinated coffee cups/wk was associated with a reduced risk for GDM, but observed no significant association with >7 cups/wk, and similar to our study, observed no significant association when first trimester intake was also considered.(30) The different results for type 2 diabetes may be due to the length of time for the exposure to have an impact. Alternatively, insulin resistance during pregnancy tends to be peripheral rather than hepatic and thus the mechanisms may be different.(11) Interestingly, in a trial of pregnant women early in their third trimester, caffeine intake was associated with impaired insulin sensitivity in women with GDM, but not in controls without GDM, suggesting that there may be a different response to caffeine intake among women who develop GDM.(31)
Because cigarette smoking induces the cytochrome P450 enzymes, which can increase caffeine metabolism,(32) we examined for effect measure modification by smoking. Consumption of ≥8 coffee cups/d was associated with an increased GDM risk among non-smokers and a decreased risk among smokers, indicating that caffeine or its metabolites are an important factor; however this potential increased risk was not observed when we estimated total caffeine intake from both coffee and tea. It is further plausible that there may be residual confounding among women who consume high levels of coffee.
Our results are reassuring that first trimester coffee and tea intake <8 cups/d was not associated with an increased risk for GDM and possibly may have a protective effect. This further supports the American College of Obstetrics and Gynecology guidelines that women can consume one cup of coffee a day without an increased risk of miscarriage or preterm delivery.(33) While a randomized trial designed to reduce caffeine consumption during pregnancy observed no adverse impact of caffeine on birthweight or length of gestation,(34) observational studies have reported an association between high intake of caffeine and spontaneous abortion(35) and coffee and late fetal death.(20) Therefore our findings should be interpreted in the context of a wide-range of potential obstetric outcomes when counseling pregnant women.
Conclusions
In this large prospective cohort study, moderate coffee and tea consumption in the first trimester were not associated with an increased risk for GDM. Although results were in a similar direction, unlike the non-pregnant state where coffee and tea are associated with a reduced risk of type 2 diabetes, we did not observe a similar significant reduction in GDM risk.
Supplementary Material
Acknowledgments
None.
Funding: The Danish National Research Foundation has established the Danish Epidemiology Science Centre that initiated and created the Danish National Birth Cohort. The cohort is furthermore a result of a major grant from this Foundation. Additional support for the Danish National Birth Cohort is obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation and the Augustinus Foundation. This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Abbreviations
- CI
Confidence interval
- GDM
Gestational diabetes mellitus
- ICD-10
International Classification of Diseases, tenth revision
- RR
risk ratio
Footnotes
Disclosure of interests: None.
Contribution to authorship: SNH, SKL and JMC designed the study (project conception, development of overall research plan, and study oversight). JO and BHB were responsible for data collection and provided the data. SNH and SKL analyzed data and performed statistical analysis. SNH, SKL and JMC wrote the paper. SNH and SKL had primary responsibility for final content; and all authors participated in the interpretation of the data, editing, and provided critical revision of the manuscript for important intellectual content and all authors approved the final version.
Details of ethics approval: Not required.
References
- 1.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105(1):110–3. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169(22):2053–63. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 3.Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91(4):950–7. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 4.Williams CJ, Fargnoli JL, Hwang JJ, van Dam RM, Blackburn GL, Hu FB, et al. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes Care. 2008;31(3):504–7. doi: 10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294(1):97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Tunnicliffe JM, Shearer J. Coffee, glucose homeostasis, and insulin resistance: physiological mechanisms and mediators. Appl Physiol Nutr Metab. 2008;33(6):1290–300. doi: 10.1139/H08-123. [DOI] [PubMed] [Google Scholar]
- 7.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 8.Tea consumption and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. PLoS One. 2012;7(5):e36910. doi: 10.1371/journal.pone.0036910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosso LM, Bracken MB. Caffeine metabolism, genetics, and perinatal outcomes: a review of exposure assessment considerations during pregnancy. Ann Epidemiol. 2005;15(6):460–6. doi: 10.1016/j.annepidem.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Laughon SK, Powers RW, Roberts JM, Parana S, Catov J. Caffeine and insulin resistance in pregnancy. Am J Perinatol. 2011;28(7):571–8. doi: 10.1055/s-0031-1274511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–48. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 12.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50(4):972–9. doi: 10.1097/GRF.0b013e31815a61d6. [DOI] [PubMed] [Google Scholar]
- 13.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes--a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons WD, Neims AH. Effect of smoking on caffeine clearance. Clin Pharmacol Ther. 1978;24(1):40–5. doi: 10.1002/cpt197824140. [DOI] [PubMed] [Google Scholar]
- 15.Kalow W, Tang BK. Caffeine as a metabolic probe: exploration of the enzyme-inducing effect of cigarette smoking. Clin Pharmacol Ther. 1991;49(1):44–8. doi: 10.1038/clpt.1991.8. [DOI] [PubMed] [Google Scholar]
- 16.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2007;298(22):2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 Suppl):1975s–9s. doi: 10.3945/ajcn.110.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health. 2001;29(4):300–7. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 19.Danish National Birth Cohort. Statens Serum Institut; Copenhagen, DK: [cited 2014]. Internet. Available from: www.dnbc.dk/ [Google Scholar]
- 20.Bech BH, Nohr EA, Vaeth M, Henriksen TB, Olsen J. Coffee and fetal death: a cohort study with prospective data. Am J Epidemiol. 2005;162(10):983–90. doi: 10.1093/aje/kwi317. [DOI] [PubMed] [Google Scholar]
- 21.Bunker ML, McWilliams M. Caffeine content of common beverages. J Am Diet Assoc. 1979;74(1):28–32. [PubMed] [Google Scholar]
- 22.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American Journal of Epidemiology. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 23.Hertzmark E, Li R, Hong B, Spiegelman D. The SAS GLMCURV9 Macro. Boston, MA: Channing Laboratory; 2012. [Google Scholar]
- 24.Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012;29(7):844–54. doi: 10.1111/j.1464-5491.2011.03541.x. [DOI] [PubMed] [Google Scholar]
- 25.Jensen DM, Molsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Ovesen P, Damm P. Screening for gestational diabetes mellitus by a model based on risk indicators: a prospective study. Am J Obstet Gynecol. 2003;189(5):1383–8. doi: 10.1067/s0002-9378(03)00601-x. [DOI] [PubMed] [Google Scholar]
- 26.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawson CC, LeMasters GK, Wilson KA. Changes in caffeine consumption as a signal of pregnancy. Reprod Toxicol. 2004;18(5):625–33. doi: 10.1016/j.reprotox.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology. 2006;17(4):413–8. doi: 10.1097/01.ede.0000220549.14177.60. [DOI] [PubMed] [Google Scholar]
- 29.Elwood JM. Commentary: On representativeness. Int J Epidemiol. 2013;42(4):1014–5. doi: 10.1093/ije/dyt101. [DOI] [PubMed] [Google Scholar]
- 30.Adeney KL, Williams MA, Schiff MA, Qiu C, Sorensen TK. Coffee consumption and the risk of gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2007;86(2):161–6. doi: 10.1080/00016340600994992. [DOI] [PubMed] [Google Scholar]
- 31.Robinson LE, Spafford C, Graham TE, Smith GN. Acute caffeine ingestion and glucose tolerance in women with or without gestational diabetes mellitus. J Obstet Gynaecol Can. 2009;31(4):304–12. doi: 10.1016/S1701-2163(16)34147-0. [DOI] [PubMed] [Google Scholar]
- 32.Aldridge A, Bailey J, Neims AH. The disposition of caffeine during and after pregnancy. Semin Perinatol. 1981;5(4):310–4. [PubMed] [Google Scholar]
- 33.ACOG Committee Opinion No. 462: Moderate caffeine consumption during pregnancy. Obstet Gynecol. 2010;116(2 Pt 1):467–8. doi: 10.1097/AOG.0b013e3181eeb2a1. [DOI] [PubMed] [Google Scholar]
- 34.Bech BH, Obel C, Henriksen TB, Olsen J. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. Bmj. 2007;334(7590):409. doi: 10.1136/bmj.39062.520648.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klebanoff MA, Levine RJ, DerSimonian R, Clemens JD, Wilkins DG. Maternal serum paraxanthine, a caffeine metabolite, and the risk of spontaneous abortion. N Engl J Med. 1999;341(22):1639–44. doi: 10.1056/NEJM199911253412202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


